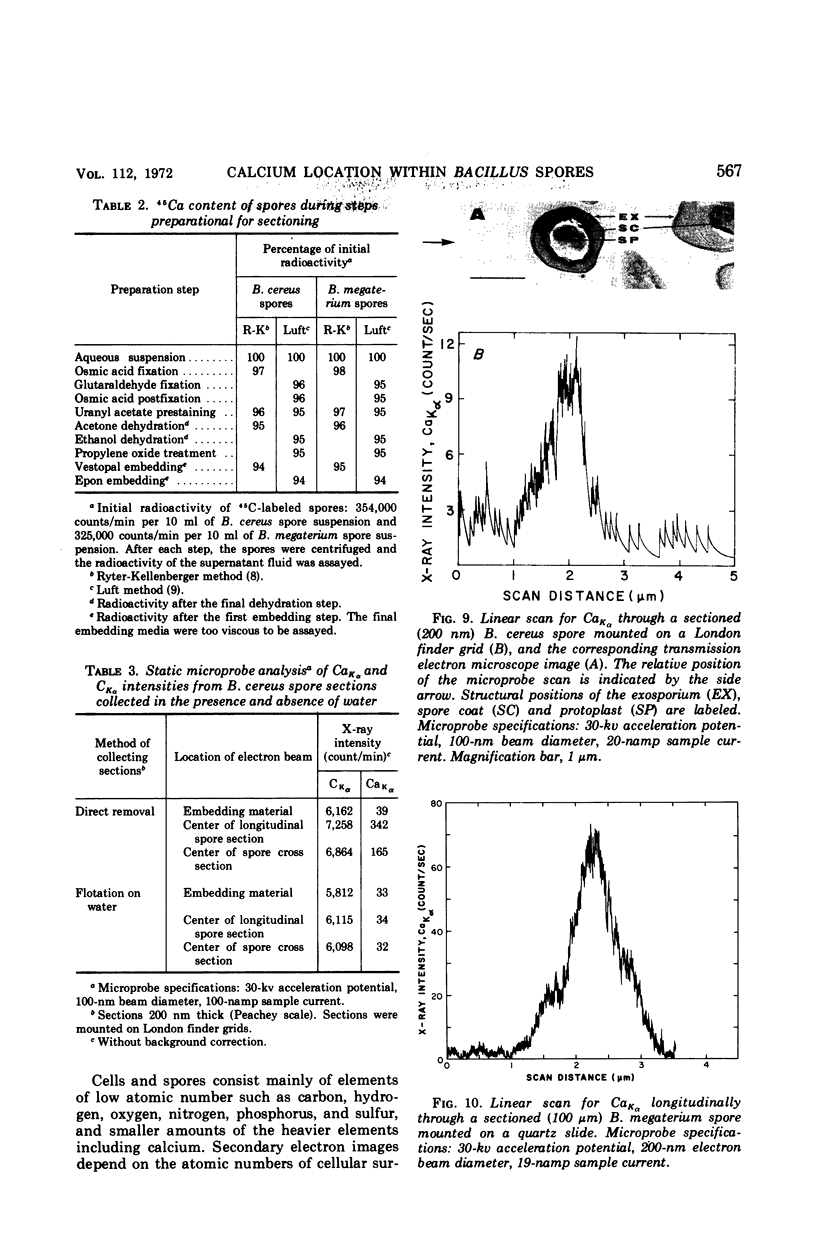
Abstract
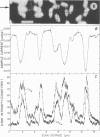
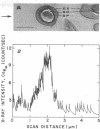
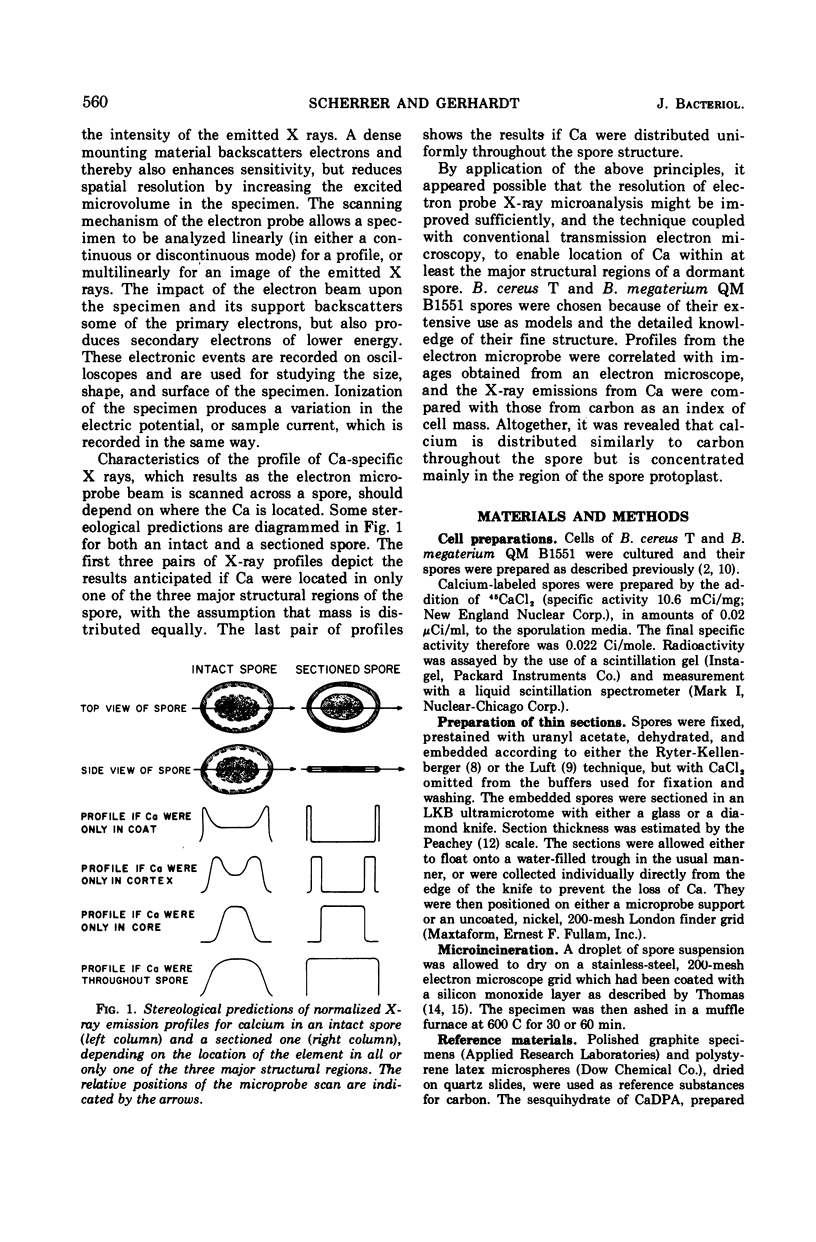
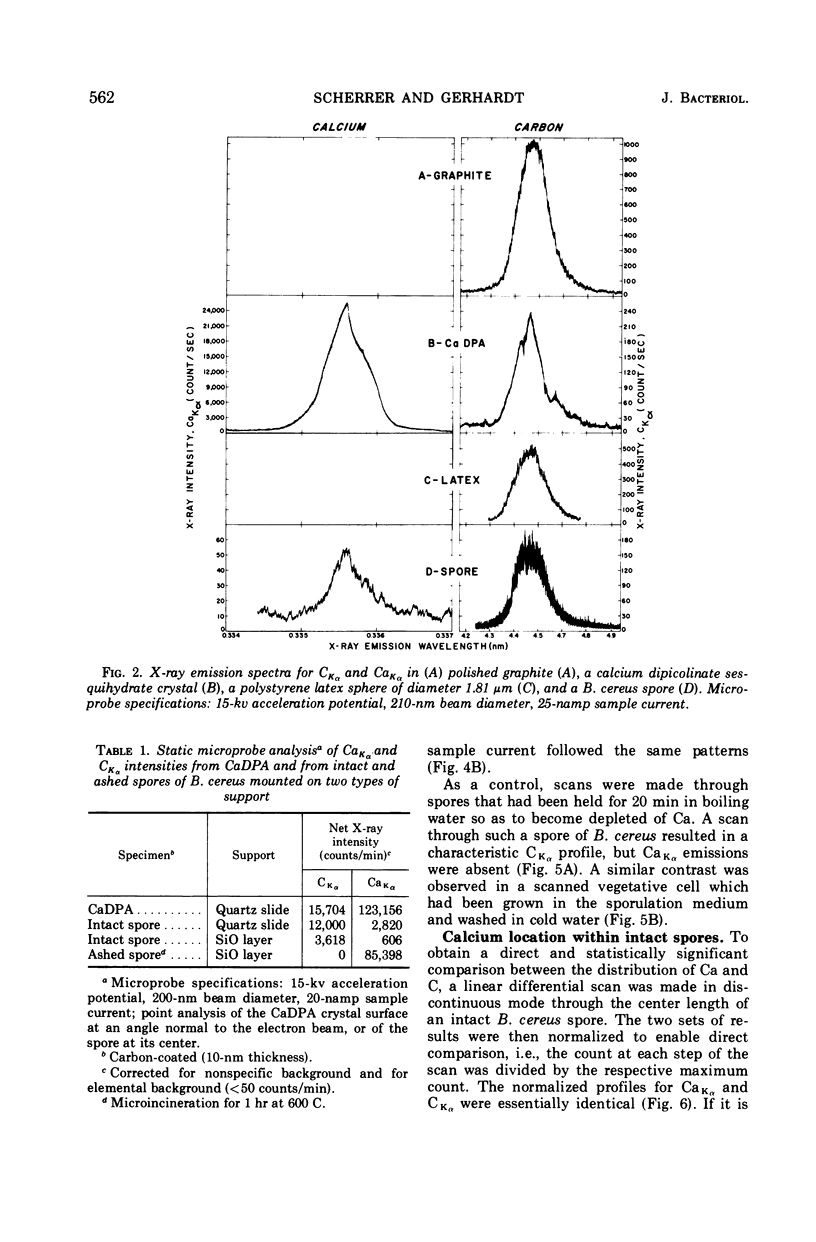
Spectroscopic microanalysis of the element-characteristic X rays produced by a scanning electron microprobe was employed to detect calcium and carbon in both intact and thin-sectioned spores of Bacillus cereus T and B. megaterium QM B1551. Linear scan profiles and multilinear scan images of the X-ray emissions for calcium (CaKα) were compared with those for carbon (CKα) as an index of mass. Location was accomplished by stereological comparisons with secondary electron images and conventional transmission electron micrographs. Although the elements could be detected at the attogram level theoretically, spatial resolution was limited to ∼500 to 1,000 nm in an intact spore, e.g., by the primary electron beam diameter, the electron-excited spore microvolume, and the type of specimen support. The resolution was improved to ∼100 to 200 nm by use of thin-sectioned spores, with precautions to prevent calcium leakage from the specimen during preparations. In both intact and sectioned spores, calcium was distributed throughout the spore, similarly to carbon, and concentrated mainly in a central region corresponding to the spore protoplast.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen C. A. An introduction to the electron probe microanalyzer and its application to biochemistry. Methods Biochem Anal. 1967;15:147–270. doi: 10.1002/9780470110331.ch4. [DOI] [PubMed] [Google Scholar]
- Beaman T. C., Pankratz H. S., Gerhardt P. Ultrastructure of the exosporium and underlying inclusions in spores of Bacillus megaterium strains. J Bacteriol. 1972 Mar;109(3):1198–1209. doi: 10.1128/jb.109.3.1198-1209.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz L. L., Beaman T. C., Gerhardt P. Chemical composition of exosporium from spores of Bacillus cereus. J Bacteriol. 1970 Jan;101(1):196–201. doi: 10.1128/jb.101.1.196-201.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEACHEY L. D. Thin sections. I. A study of section thickness and physical distortion produced during microtomy. J Biophys Biochem Cytol. 1958 May 25;4(3):233–242. doi: 10.1083/jcb.4.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahs G., Dickerson R. E. The crystal structure of calcium dipicolinate trihydrate (a bacterial spore metabolite). Acta Crystallogr B. 1968 Apr 15;24(4):571–578. doi: 10.1107/s0567740868002852. [DOI] [PubMed] [Google Scholar]
- THOMAS R. S. ULTRASTRUCTURAL LOCALIZATION OF MINERAL MATTER IN BACTERIAL SPORES BY MICRONINCINERATION. J Cell Biol. 1964 Oct;23:113–133. doi: 10.1083/jcb.23.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]