Abstract
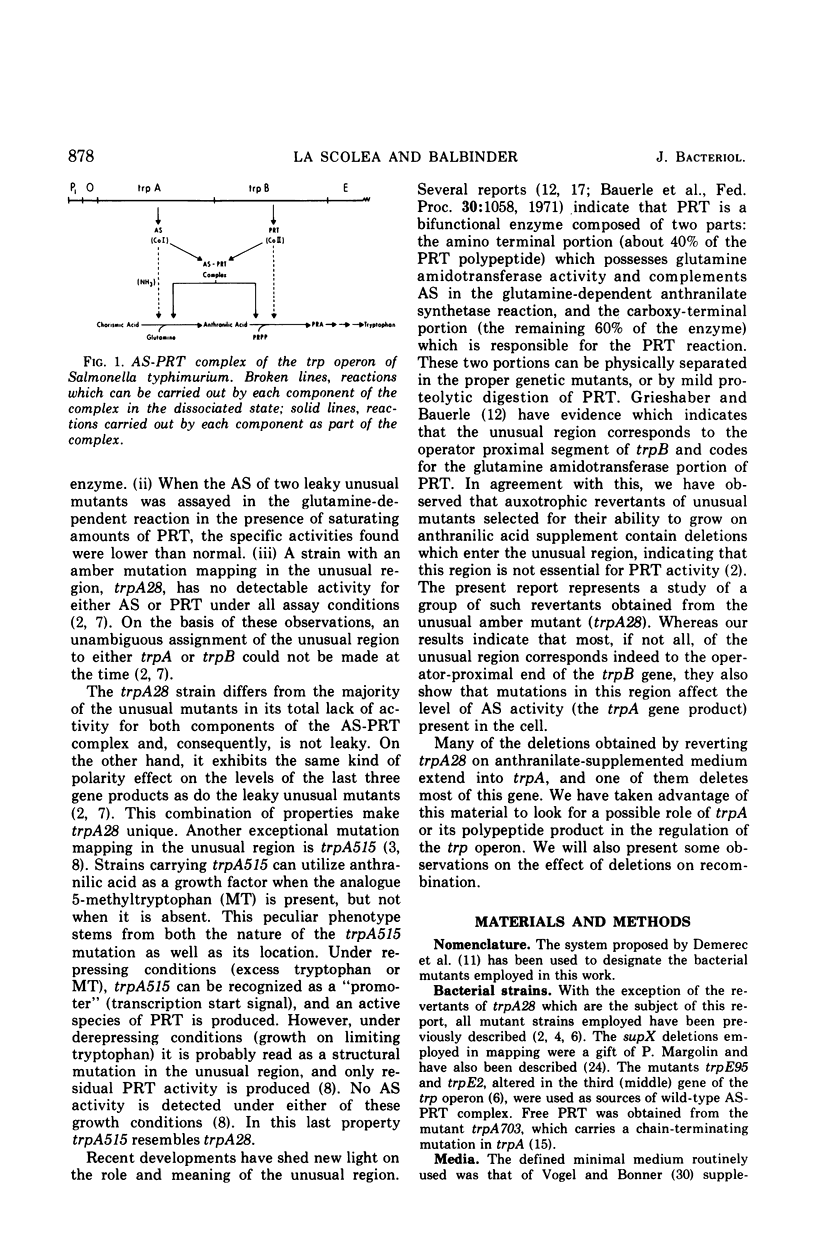
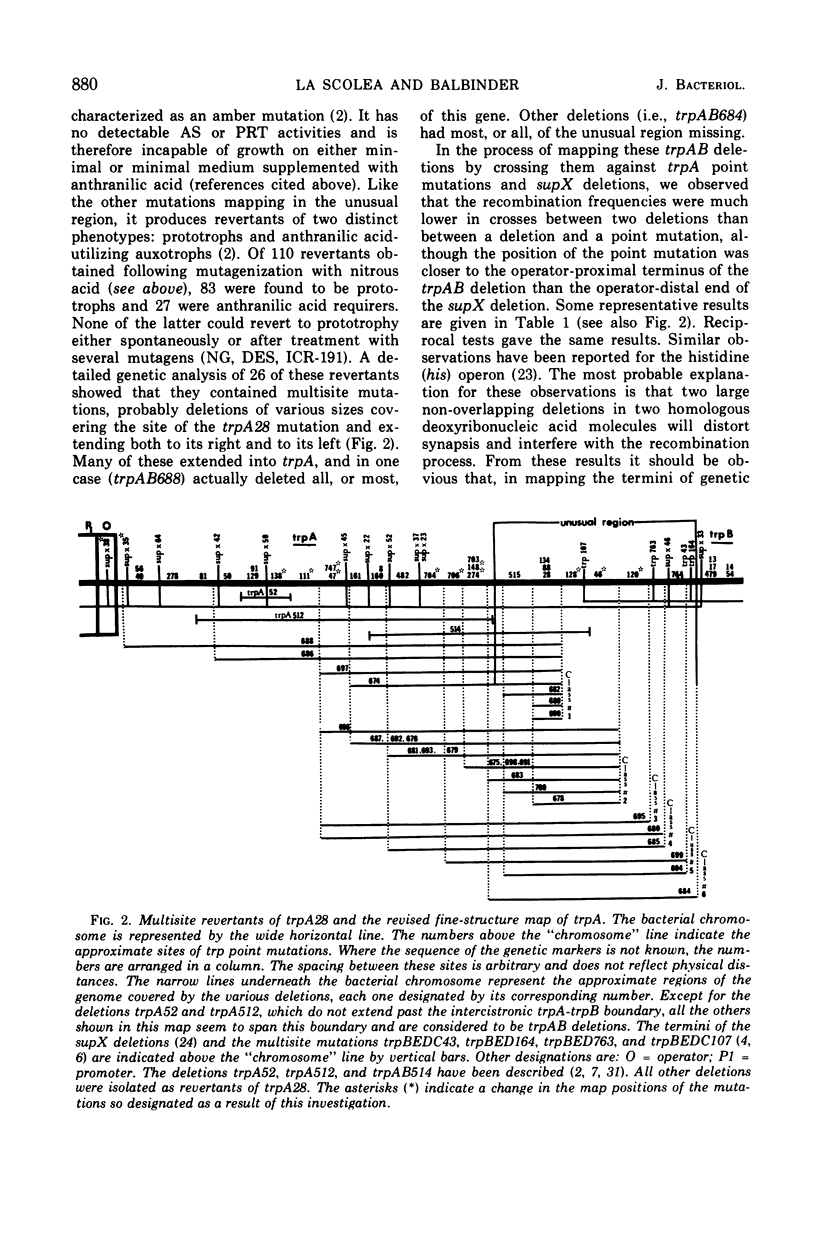
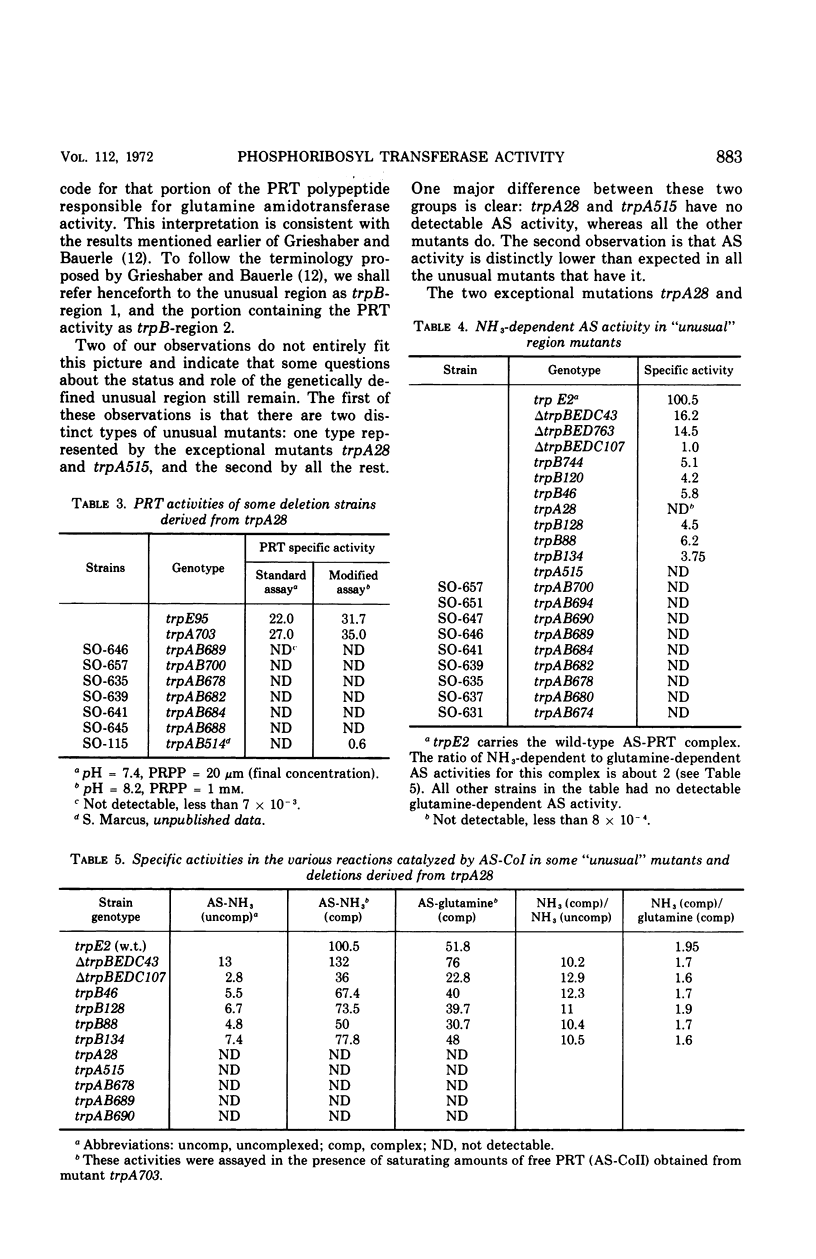
The amber mutant trpA28, which contains a mutation mapping within the so-called “unusual” region of the tryptophan (trp) operon of Salmonella typhimurium (between the genes trpA and trpB), lacks both components of the anthranilate synthetase (AS)-phosphoribosyl transferase (PRT) enzyme complex, the products of the genes trpA and trpB, respectively. Twenty-six revertants of this mutant selected on minimal medium supplemented with anthranilic acid, a substrate of PRT, contain deletions of various segments of the “unusual” region and make a species of PRT different in every respect from the wild-type, dissociated form of this enzyme. The results indicate that the unusual region corresponds to the operator proximal end of the trpB gene. Mutants in the unusual region, however, show unexpectedly low levels of AS activity and in two cases (trpA515 and trpA28) no detectable activity of this enzyme component.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balbinder E., Blume A. J., Weber A., Tamaki H. Polar and antipolar mutants in the tryptophan operon of Salmonella typhimurium. J Bacteriol. 1968 Jun;95(6):2217–2229. doi: 10.1128/jb.95.6.2217-2229.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbinder E., Callahan R., 3rd, McCann P. P., Cordaro J. C., Weber A. R., Smith A. M., Angelosanto F. Regulatory mutants of the tryptophan operon of Salmonella typhimurium. Genetics. 1970 Sep;66(1):31–53. doi: 10.1093/genetics/66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbinder E. The Fine Structure of the Loci Tryc and Tryd of Salmonella Typhimurium. II. Studies of Reversion Patterns and the Behavior of Specific Alleles during Recombination. Genetics. 1962 May;47(5):545–559. doi: 10.1093/genetics/47.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Bautz F. A., Bautz E. K. Mapping of deletions in a non-essential region of the phage T4 genome. J Mol Biol. 1967 Sep 14;28(2):345–355. doi: 10.1016/s0022-2836(67)80014-7. [DOI] [PubMed] [Google Scholar]
- Blume A. J., Balbinder E. The tryptophan operon of Salmonella typhimurium. Fine structure analysis by deletion mapping and abortive transduction. Genetics. 1966 Mar;53(3):577–592. doi: 10.1093/genetics/53.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J., Weber A., Balbinder E. Analysis of polar and nonpolar tryptophan mutants by derepression kinetics. J Bacteriol. 1968 Jun;95(6):2230–2241. doi: 10.1128/jb.95.6.2230-2241.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., 3rd, Balbinder E. Tryptophan operon: structural gene mutation creating a "promoter" and leading to 5-methyltryptophan dependence. Science. 1970 Jun 26;168(3939):1586–1589. doi: 10.1126/science.168.3939.1586. [DOI] [PubMed] [Google Scholar]
- Callahan R., Blume A. J., Balbinder E. Evidence for the order promoter-operator-first structural gene in the tryptophan operon of Salmonella. J Mol Biol. 1970 Aug;51(3):709–715. doi: 10.1016/0022-2836(70)90019-7. [DOI] [PubMed] [Google Scholar]
- Cordaro J. C., Balbinder E. Evidence for the separability of the operator from the first structural gene in the tryptophan operon of Salmonella typhimurium. Genetics. 1971 Feb;67(2):151–169. doi: 10.1093/genetics/67.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. J., Nagano H., Zalkin H., Hwang L. H. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Purification of the aggregate and regulatory properties of anthranilate synthetase. J Biol Chem. 1970 Mar 25;245(6):1416–1423. [PubMed] [Google Scholar]
- Henderson E. J., Zalkin H., Hwang L. H. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Catalytic and regulatory properties of aggregated and unaggregated forms of anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase. J Biol Chem. 1970 Mar 25;245(6):1424–1431. [PubMed] [Google Scholar]
- Hiraga S., Yanofsky C. Normal repression in a deletion mutant lacking almost the entire operator-proximal gene of the tryptophan operon of E. coli. Nat New Biol. 1972 May 10;237(71):47–49. doi: 10.1038/newbio237047a0. [DOI] [PubMed] [Google Scholar]
- Hwang L. H., Zalkin H. Multiple forms of anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase from Salmonella typhimurium. J Biol Chem. 1971 Apr 25;246(8):2338–2345. [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. The nature of the anthranilic acid synthetase complex of Escherichia coli. J Biol Chem. 1966 Sep 10;241(17):4112–4114. [PubMed] [Google Scholar]
- Kovach J. S., Phang J. M., Blasi F., Barton R. W., Ballesteros-Olmo A., Goldberger R. F. Interaction between histidyl transfer ribonucleic acid and the first enzyme for histidine biosynthesis of Salmonella typhimurium. J Bacteriol. 1970 Nov;104(2):787–792. doi: 10.1128/jb.104.2.787-792.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J. S., Phang J. M., Ference M., Goldberger R. F. Studies on repression of the histidine operon. II. The role of the first enzyme in control of the histidine system. Proc Natl Acad Sci U S A. 1969 Jun;63(2):481–488. doi: 10.1073/pnas.63.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPER J. C., GRABNAR M., STAHL R. C., HARTMAN Z., HARTMAN P. E. GENES AND PROTEINS INVOLVED IN HISTIDINE BIOSYNTHESIS IN SALMONELLA. Brookhaven Symp Biol. 1964 Dec;17:15–52. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Margolin P., Bauerle R. H. Determinants for regulation and initiation of expression of tryptophan genes. Cold Spring Harb Symp Quant Biol. 1966;31:311–320. doi: 10.1101/sqb.1966.031.01.041. [DOI] [PubMed] [Google Scholar]
- Sarabhai A., Brenner S. A mutant which reinitiates the polypeptide chain after chain termination. J Mol Biol. 1967 Jul 14;27(1):145–162. doi: 10.1016/0022-2836(67)90357-9. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- TESSMAN I. The induction of large deletions by nitrous acid. J Mol Biol. 1962 Oct;5:442–445. doi: 10.1016/s0022-2836(62)80033-3. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wuesthoff G., Bauerle R. H. Mutations creating internal promoter elements in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1970 Apr 14;49(1):171–196. doi: 10.1016/0022-2836(70)90384-0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Kling D. Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium. Biochemistry. 1968 Oct;7(10):3566–3573. doi: 10.1021/bi00850a034. [DOI] [PubMed] [Google Scholar]


