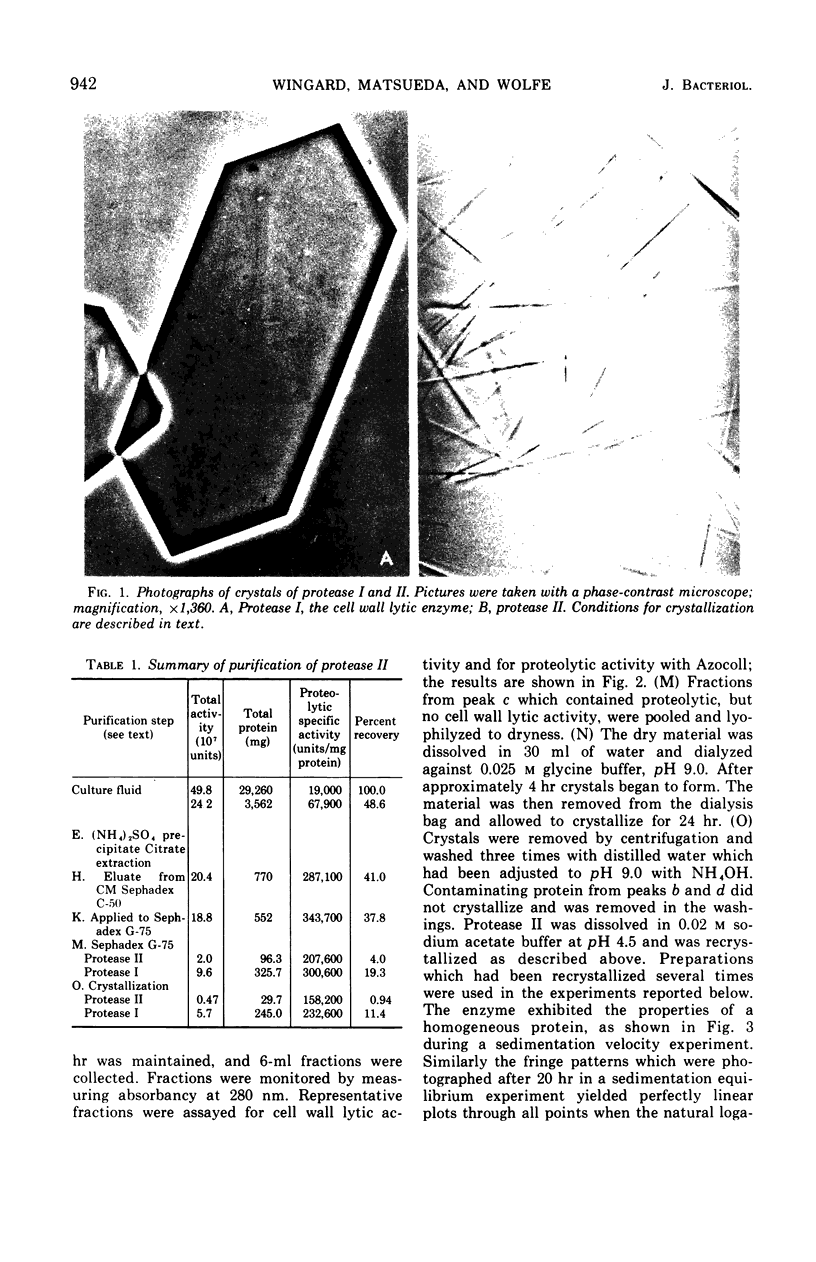
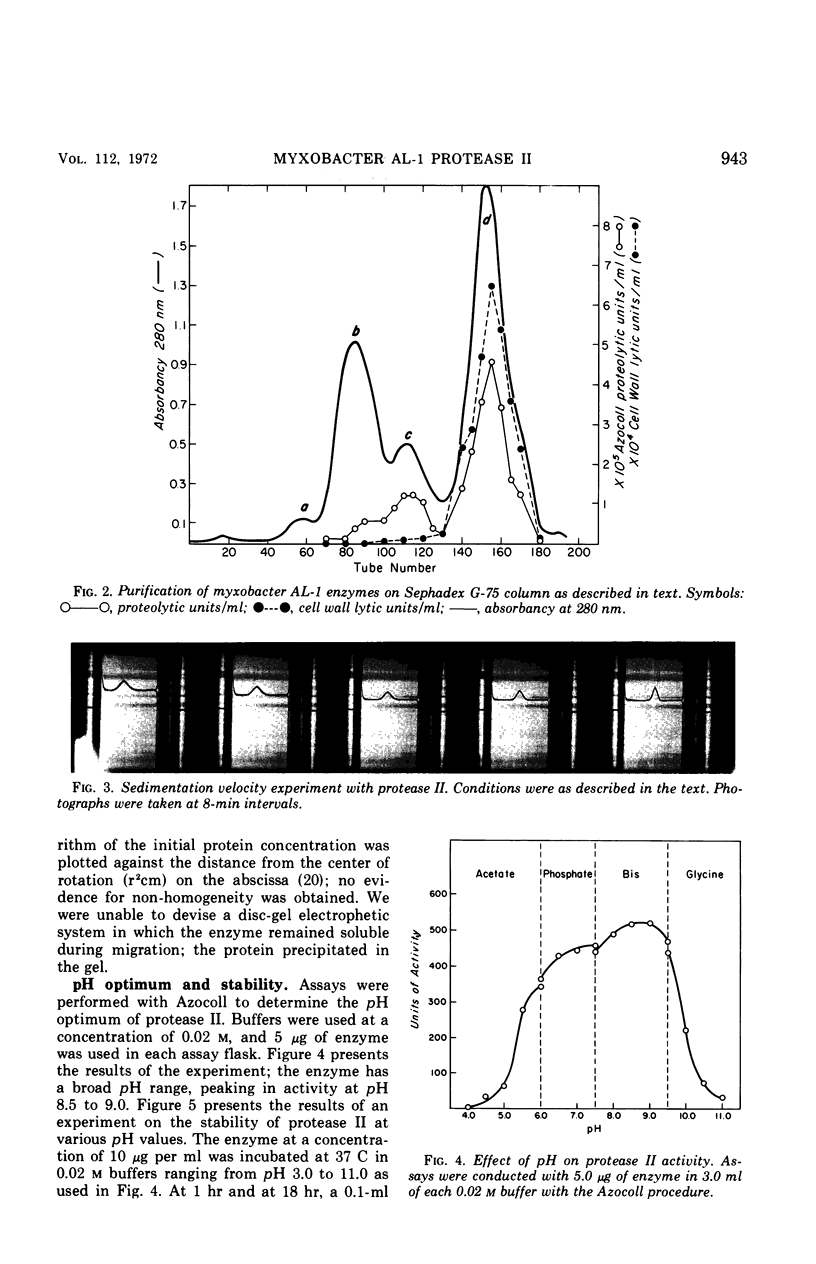
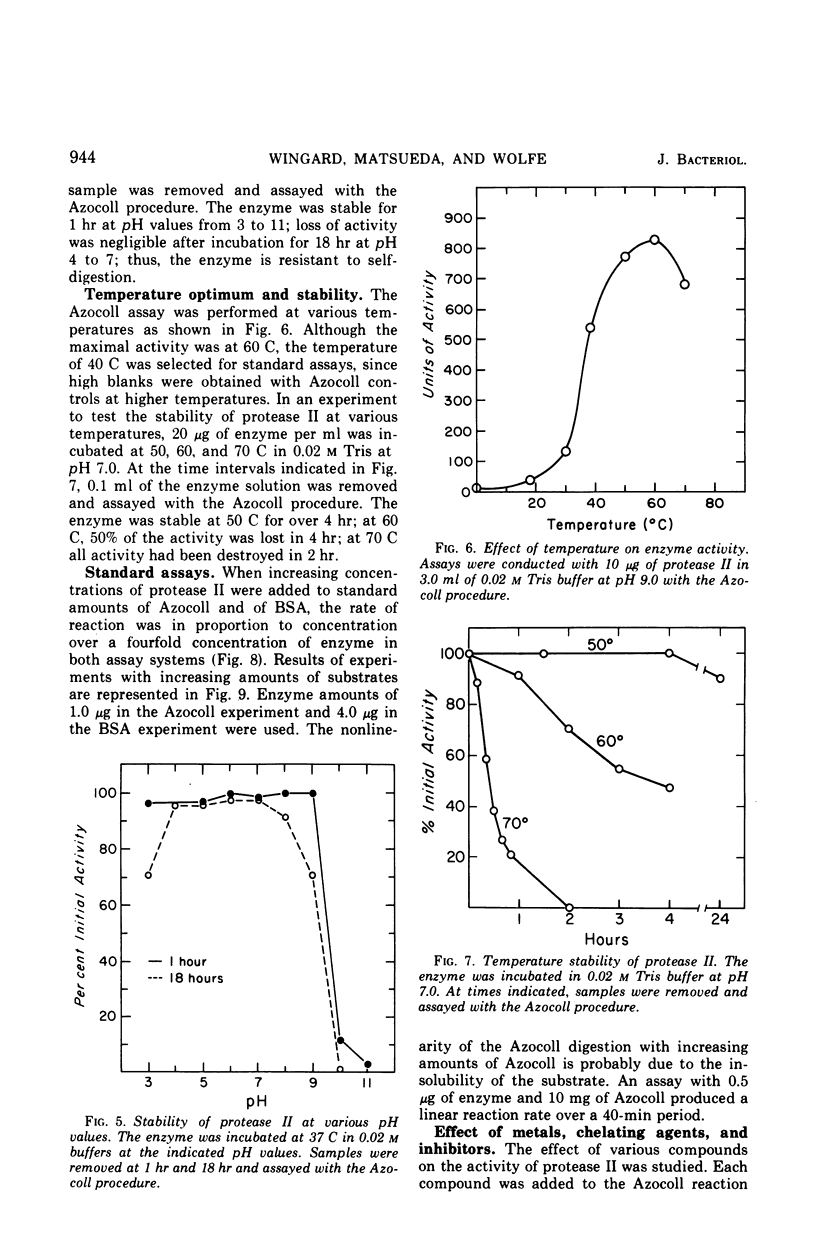
Abstract
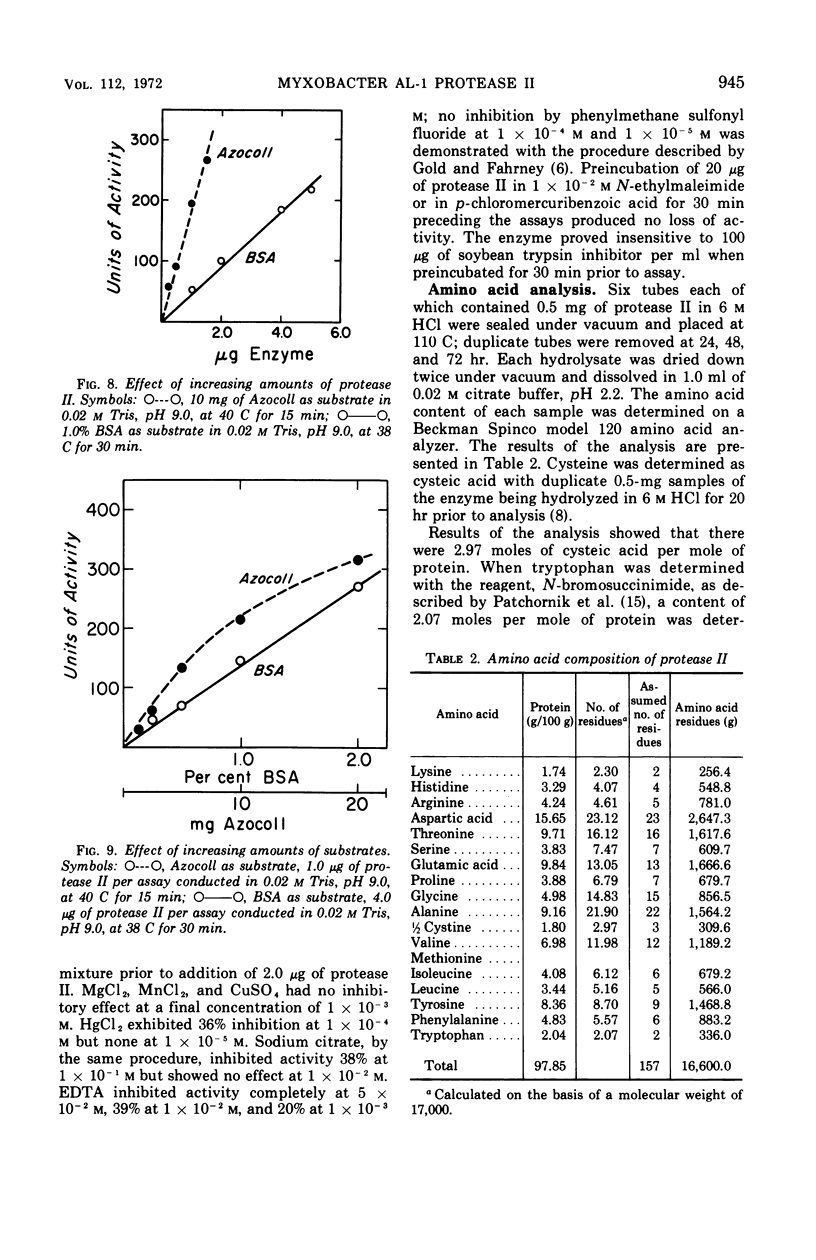
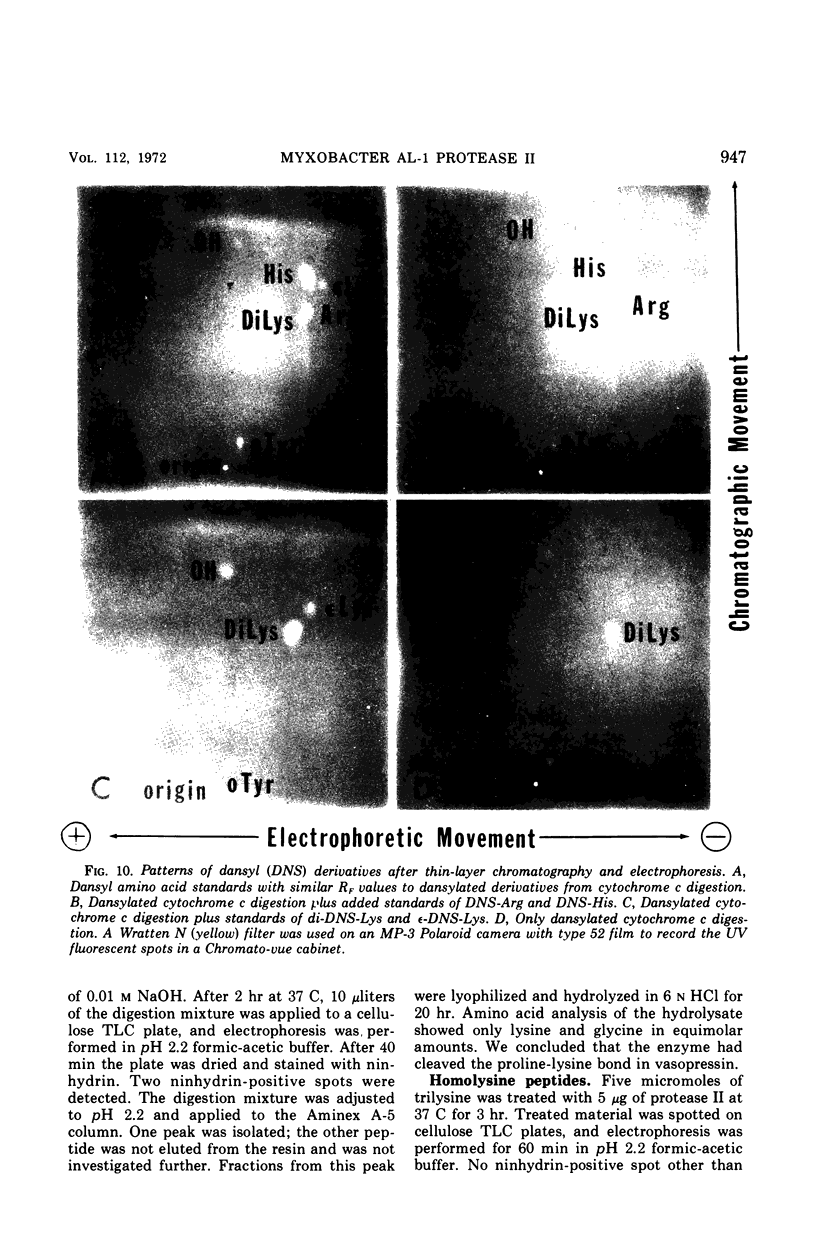
A second extracellular protease from myxobacter strain AL-1 has been purified to homogeneity and named protease II; the enzyme crystallizes as fine needles. The extracellular, cell wall lytic protease reported previously from the same organism is now designated protease I. Protease II exhibits a pH optimum of 8.5 to 9.0 and is stable from pH 3.0 to 9.0. The enzyme is heat stable at 50 C for 18 hr. Results of sedimentation equilibrium studies yielded a molecular weight of 17,000, and amino acid analysis revealed 157 residues with a minimal molecular weight of 16,660. Cleavage of peptide bonds in the oxidized B-chain of insulin, cytochrome c (horse heart). lysozyme, and vasopressin is restricted to the amino side of lysine. Dilysine and trilysine were not hydrolyzed. Products from digestions of polylysine were lysine and dilysine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott M. S., Ward D. N. Separation of dansyl amino acids in a single analysis. Anal Biochem. 1967 Oct;21(1):50–56. doi: 10.1016/0003-2697(67)90082-6. [DOI] [PubMed] [Google Scholar]
- CANFIELD R. E. THE AMINO ACID SEQUENCE OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2698–2707. [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. LYSIS OF BACTERIAL CELL WALLS BY AN ENZYME ISOLATED FROM A MYXOBACTER. J Bacteriol. 1965 Aug;90:395–402. doi: 10.1128/jb.90.2.395-402.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD A. M., FAHRNEY D. SULFONYL FLUORIDES AS INHIBITORS OF ESTERASES. II. FORMATION AND REACTIONS OF PHENYLMETHANESULFONYL ALPHA-CHYMOTRYPSIN. Biochemistry. 1964 Jun;3:783–791. doi: 10.1021/bi00894a009. [DOI] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Jackson R. L., Wolfe R. S. Composition, properties, and substrate specificities of Myxobacter AL-1 protease. J Biol Chem. 1968 Mar 10;243(5):879–888. [PubMed] [Google Scholar]
- Jones R. T. Automatic peptide chromatography. Methods Biochem Anal. 1970;18:205–258. doi: 10.1002/9780470110362.ch4. [DOI] [PubMed] [Google Scholar]
- Katz W., Strominger J. L. Structure of the cell wall of Micrococcus lysodeikticus. II. Study of the structure of the peptides produced after lysis with the Myxobacterium enzyme. Biochemistry. 1967 Mar;6(3):930–937. doi: 10.1021/bi00855a037. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- Sanger F. Fractionation of oxidized insulin. Biochem J. 1949;44(1):126–128. doi: 10.1042/bj0440126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]