Abstract
Background
Mohr-Tranebjærg syndrome (MTS) is an X-linked, recessive, syndromic, sensorineural hearing loss characterized by onset of deafness in childhood followed later in adult life by progressive neural degeneration affecting the brain and optic nerves. MTS is caused by mutations in the DDP/TIMM8A gene which encodes for a 97 amino acid polypeptide; this polypeptide is a translocase of the inner mitochrondrial membrane.
Objectives
To describe the otologic presentation and temporal bone histopathology in 4 affected individuals with MTS.
Material and Methods
All 4 subjects belonged to a large, multigeneration, Norwegian family and were known to carry a frame shift mutation in the TIMM8A gene. Temporal bones were removed at autopsy and studied by light microscopy. Cytocochleograms were constructed for hair cells, stria vascularis and cochlear neuronal cells. Vestibular neurons were also counted.
Results
All 4 subjects developed progressive hearing loss in early childhood, becoming profoundly deaf by the age of 10 years. All 4 developed language and at least one subject used amplification in early life. Audiometric evaluation in two subjects showed 80-100 dB hearing loss by the age of 10 years. The subjects died between the ages of 49 and 67. The otopathology was strikingly similar, in that all bones examined showed near-total loss of cochlear neuronal cells and severe loss of vestibular neurons. When compared to age-matched controls, there was 90 to 95% loss of cochlear neurons and 75 to 85% loss of vestibular neurons.
Conclusions
We infer that the hearing loss in MTS is likely to be the result of a postnatal and progressive degeneration of cochlear neurons, and that MTS constitutes a true auditory neuropathy. Our findings have implications for clinical diagnosis of patients with MTS, and management of the hearing loss.
Keywords: temporal bone, histopathology, hereditary hearing loss, Mohr-Tranebjærg syndrome
INTRODUCTION
Mohr-Tranebjærg syndrome (MTS) is an X-linked, recessive, syndromic hearing loss characterized by deafness in childhood, followed later in life by progressive neural degeneration affecting the brain and optic nerves 1-3. The typical initial manifestation is a progressive sensorineural hearing loss (SNHL) in childhood, with profound hearing loss usually occurring by the age of 10 years. Other features of the syndrome become evident later in life, including progressive visual loss leading to blindness, dystonia, spasticity, aggressive behavior, paranoia, dysphagia and dementia.
MTS is caused by mutations in a gene on Xq22 that was initially named “DDP” for deafness/dystonia peptide 4. The gene encodes a small polypeptide containing 97 amino acids. Investigations using yeast cells showed that DDP is a mitochondrial protein, and is part of a transport complex that mediates protein transport5. Mutations in the gene are believed to result in a defect in mitochondrial protein import, leading to defects in mitochondrial function. The DDP gene was renamed TIMM8A to reflect the function of its encoded polypeptide, which is a translocase of the inner mitochondrial membrane.
Examination of the temporal bone from an affected individual with MTS showed loss of ganglion cells within the cochlear and vestibular nerves 6. Since that report, we have had the opportunity to study the temporal bones from three additional affected individuals with MTS. We describe our findings in all four subjects in the present study.
MATERIAL AND METHODS
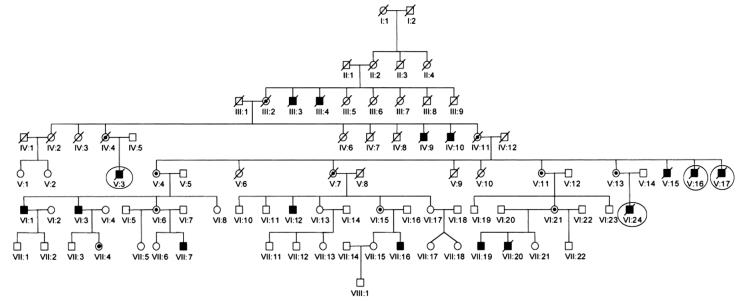
All subjects of this report belong to a large, multigenerational, Norwegian family with 16 affected members 3. All affected individuals are male. The four subjects that we studied are designated V:3, V:16, V:17 and VI:24 in the pedigree shown in Figure 1. All four individuals had been shown to carry a frame shift mutation characterized by a 1 base pair deletion of T at nucleotide position 151 in axon 1 of the TIMM8A gene, 151delT (Q38fsX64). According to revised terminology, the mutation is now called 116delT (Q38fsX64).
Fig. 1.
Pedigree of large, multigenerational, Norwegian family with the Mohr- Tranebjærg syndrome. There are 16 affected members, all male. Subjects V:3, V:16, V:17 and VI:24 are the focus of this report, and are marked by circles.
Temporal bones were removed at autopsy and processed for light microscopy in the standard manner, including fixation using 10% neutral buffered formalin, decalcification using ethylenediaminetetraacetate, embedment in celloidin, serial sectioning in the axial plane at a thickness of 20 microns, and hematoxylin and eosin staining of every tenth section 7. All stained sections were examined by light microscopy. Graphic reconstruction of the cochlea was performed according to the method described Schuknecht 7 to determine loss of various neurosensory elements such as hair cells, stria vascularis and cochlear neuronal cells. Cochlear neuronal cell counts were compared with normative data reported by Schuknecht 7. Counts of vestibular neurons (Scarpa's ganglion) were also done, using the method described by Richter 8 and compared with normative data reported by Velázquez et al 9.
RESULTS
Case V:16
History
This case was previously reported 6, and hence, only a brief description is provided. This patient was known to be “deaf” from about the age of 4 years. He developed language and enjoyed singing. He started to lose vision at age 48 and developed typical neurologic symptoms of dystonia and dysphagia in his late 50s. He died at age 67 from pneumonia, and the right temporal bone was removed at 29.5 hours postmortem.
Histopathology
Figure 2A shows the cytocochleogram. The inner ear shows near-total loss of cochlear neurons. When compared to normative data from age-matched controls, 92% of the neurons are missing. The total cochlear neuronal count is 1,765. Except for some slight losses in the apical turn, other structures within the cochlea are preserved, including outer and inner hair cells and supporting cells of the organ of Corti, stria vascularis, tectorial membrane, spiral limbus and the spiral ligament. Within the vestibular system, there is a severe loss of vestibular ganglion cells (total count = 4,638, which is a 76% loss compared to age-matched controls). The sensory and supporting cells of the vestibular sense organs, and the vestibular membranous labyrinth appear normal. Cells of the geniculate ganglion of the facial nerve appear healthy and are present in normal numbers. The specimen also contains a part of the trigeminal ganglion, whose neurons are present in normal numbers. The external and middle ears, as well as the mastoid and otic capsule appear normal.
Fig. 2.
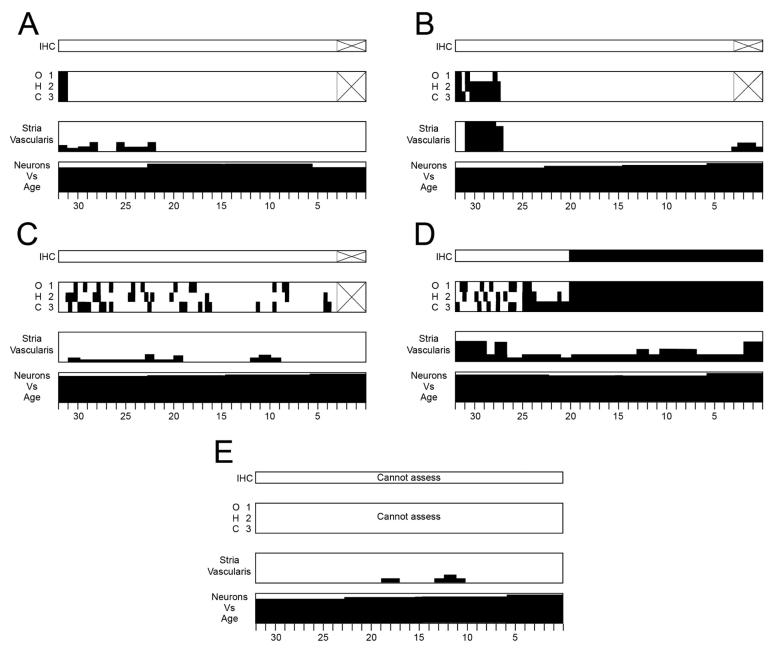
Cytocochleograms of histologic changes within the cochlea in the four individuals in this report. Graphic reconstruction of the cochlea was done in each ear according to the method described by Schuknecht 7. Black filling represents missing or abnormal elements. Vertical axes of cytocochleogram boxes for the stria and neurons show percentage of loss. IHC=inner hair cell; OHC=outer hair cell. Areas within the hair cell histograms marked by ‘X’ represent regions where cytologic evaluations could not be performed. Note that the consistent feature across all five temporal bones is near-total loss of cochlear neurons.
A. Subject V:16, right ear
B. Subject V:3, left ear
C. Subject VI:24, right ear
D. Subject VI:24, left ear
E. Subject V:17, right ear
Case V:3
History
This patient developed hearing loss around the age of 3 years and was known to be “deaf” from about the age of 7. He developed some language and attended a school for the deaf. No formal audiometric studies were done. He had normal mental function in early life with good social skills. He developed the typical features of the MTS syndrome later in adult life including spasticity, dystonia, aggressive behavior, paranoia and dementia. He had ataxia of hand movements and of gait, as well as photophobia and visual loss. He suffered from a fracture of the femur at age 51. His terminal illness was precipitated by a bout of esophagitis leading to bloody vomiting and progressive deterioration of his general health. He died at the age of 63. The cause of death is not known. His body was refrigerated until autopsy was performed at 72 hours postmortem. The left ear is available for study.
Histopathology
There is a large central perforation of the tympanic membrane along with tympanosclerosis and thickening of the pars propria of the surrounding drum remnant. The submucosa of the middle ear and mastoid is thickened and infiltrated with lymphocytes and round cells. There are fibrocystic changes involving the round window niche and parts of the epitympanum, mesotympanum and mastoid. The ossicles show small areas of resorption of bone surrounding vascular spaces but the ossicular chain is in continuity. The otic capsule appears normal. These changes in the middle ear and mastoid are consistent with a diagnosis of inactive chronic otitis media.
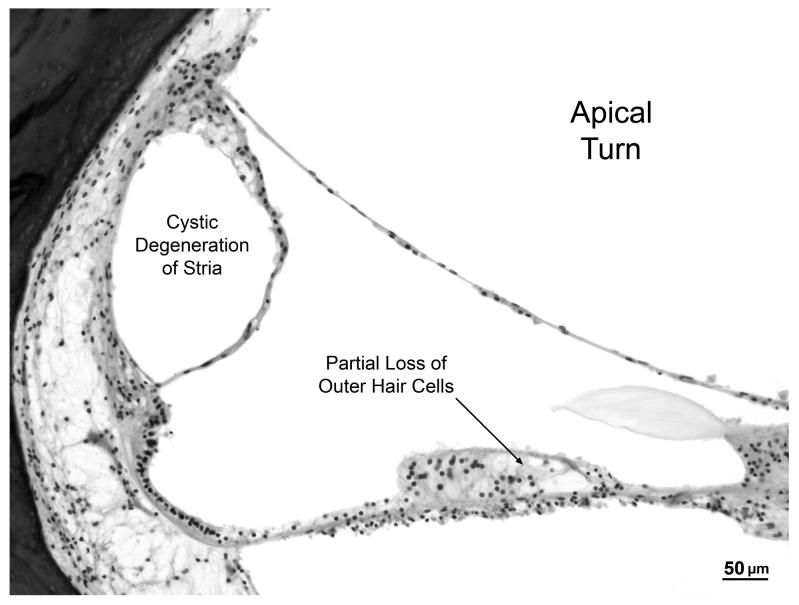
A cytocochleogram of the ear is shown in Fig. 2B and photomicrographs of the cochlea are shown in Fig. 3. The most striking abnormality within the cochlea is near-total loss of cochlear neurons in all turns, with severe atrophy of both peripheral dendrites and central axons. Both afferent and efferent nerve fibers are missing. The total spiral ganglion count is 2,151, which represents a 91% loss compared to age-matched controls. The organ of Corti including hair cells and supporting cells, as well as the stria vascularis are normal, except in the apical 5 mm where there is cystic degeneration of the stria vascularis and partial loss of outer hair cells. Reissner's membrane is in normal position without endolymphatic hydrops. The saccule, utricle and all three semicircular canals show normal appearing membranous walls, and their respective maculae and cristae show good populations of hair cells and supporting cells. The vestibular nerve has been partly been avulsed from the internal auditory canal, but portions that are present show moderate to severe loss of the vestibular ganglion cells. The geniculate ganglion shows a normal population of cells, although many of them are pyknotic. The trigeminal ganglion appears normal.
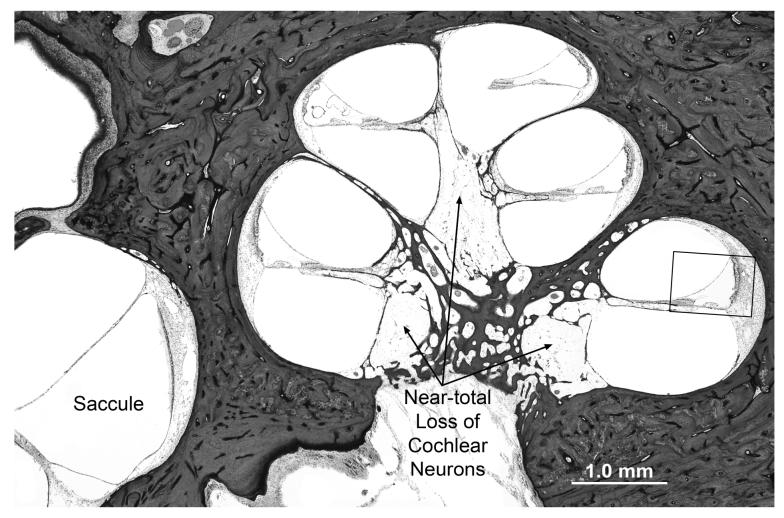
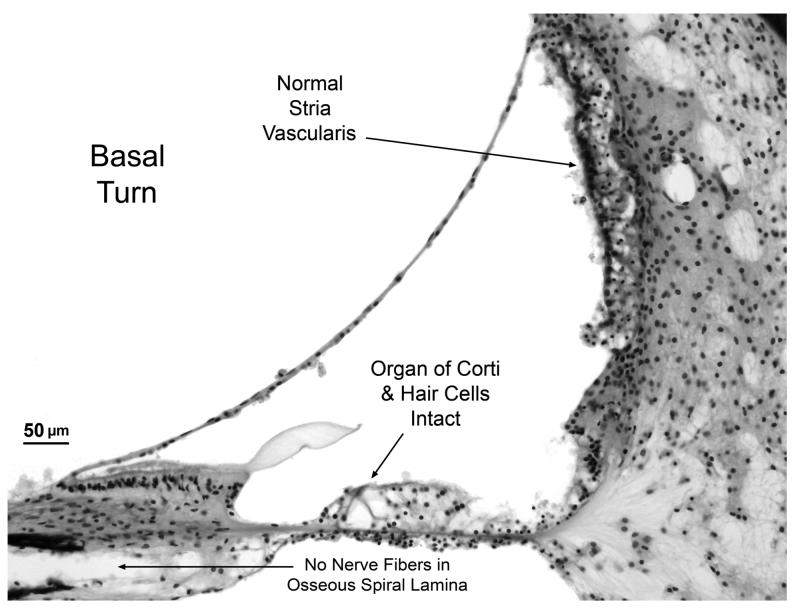
Fig. 3.
Photomicrographs of cochlea from subject V:3.
A. Lower power view showing cochlear turns and saccule. There is near total loss of spiral ganglion cells with severe atrophy of both peripheral and central axonal fibers. The saccule appears normal. The boxed area of the basal turn is shown at higher magnification in B.
B. Higher power view of scala media from basal turn showing that the organ of Corti (including hair cells) is intact. Other structures of the cochlear duct such as stria vascularis, spiral limbus and tectorial membrane appear normal. Note the absence of nerve fibers in osseous spiral lamina.
C. High power view of apical turn showing cystic degeneration of the stria vascularis and partial loss of outer hair cells within the organ of Corti.
Case VI:24
History
This subject was known to be “deaf” from about the age of 3 or 4 years. He developed language and enjoyed singing. An audiogram at age 7 showed an 80 to 100 dB bilateral hearing loss. He was fitted with hearing aids at age 10 and attended a school for the deaf at age 12. Another audiogram at age 21 also showed an 80 to 100 dB bilateral hearing loss. Electronystagmogram at age 21 was reported to be normal. He developed the typical neurologic symptoms of the MTS syndrome in his 20s and 30s, beginning with an ataxic gait at age 25, followed by dystonia, spasticity, involuntary movements and hyper-reflexia. He developed photophobia at age 40 followed by progressive visual loss. He had a foot fracture at age 33. He died from pneumonia at age 49 and both temporal bones were removed at 18.5 hours postmortem.
Histopathology
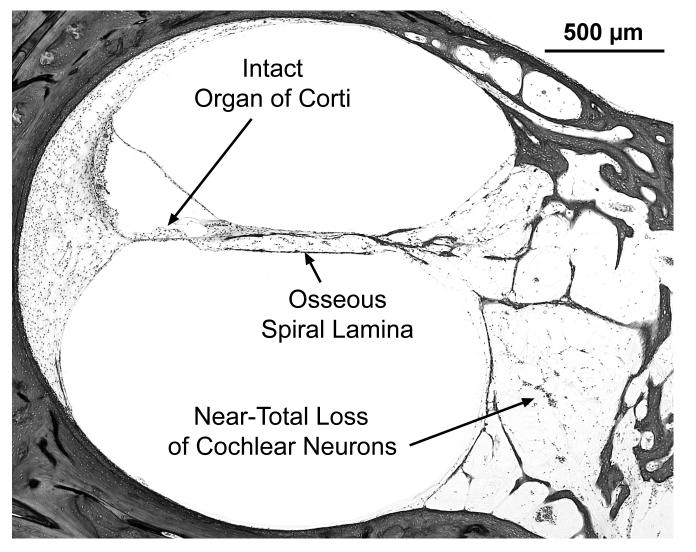
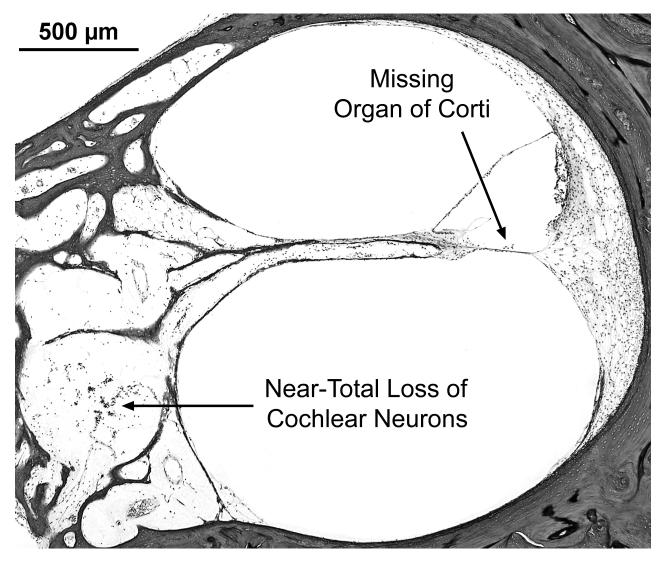
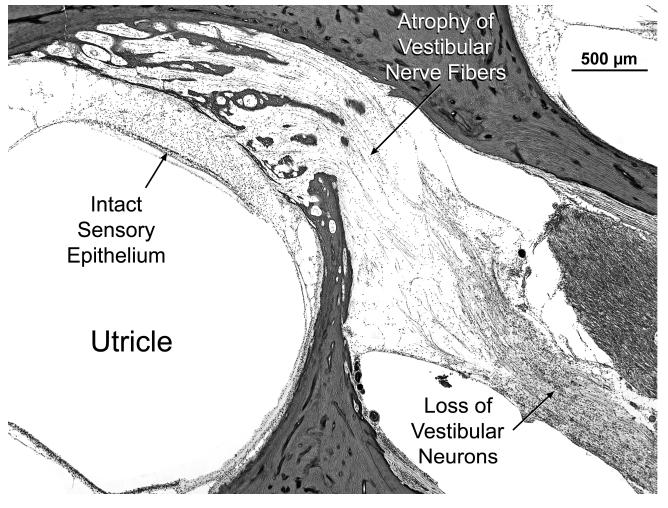
Many findings are similar between the two ears, and the temporal bones are described together. There are also a few differences between the two sides, as noted below. The external auditory canal, middle ear, mastoid and otic capsule are normal on both sides. The cytocochleograms are shown in Fig. 2C and 2D. There is near-total loss of cochlear neuronal cells (total counts: right ear-1,431, a 95% loss vs age-matched counts; left ear-2,826 , a 90% loss vs age matched). There is severe atrophy of the peripheral dendrites and central axonal processes of cochlear neuronal cells as well as loss of the intra-ganglionic spiral bundle (efferent innervation). The organ of Corti shows scattered loss of outer hair cells on the right, whereas on the left, it is atrophic in the 0 to 20 mm region with loss of both outer and inner hair cells (Fig. 4). There is mild atrophy of the stria vascularis in the left ear in all turns. Both ears show apical endolymphatic hydrops, mild atrophy of the spiral ligament in the more apical portions, normal spiral limbus and normal tectorial membrane. The vestibular sense organs show good populations of hair cells and supporting cells on both sides (Fig. 5). The vestibular membranous labyrinth is normal on both sides. The vestibular ganglion (of Scarpa) shows severe loss on both sides (total counts: right-3,669, a 82% loss vs age-matched controls; left-4,346, a 78% loss vs age-matched). Geniculate ganglion cells are present in good numbers on both sides. The left temporal bone shows a part of the trigeminal ganglion, which appears to be normal. Blood vessels supplying the auditory and vestibular sense organs are normal bilaterally. There is no inflammation or deposition of new bone or fibrous tissue within the inner ears.
Fig. 4.
Subject VI:24. Views of scala media from basal turn in right (A) and left (B) ears of this subject. On the left, the organ of Corti is missing, whereas, it is intact and normal on the right. Both sides show near-total loss of cochlear neurons and loss of nerve fibers in the osseous spiral lamina.
Fig. 5.
Subject VI:24. Vestibular system showing normal appearance of the macula of the utricle. There is a loss of vestibular neuronal cells and atrophy of vestibular nerve fibers.
Case V:17
History
This patient was known to be “deaf” from about the age of 3 years. He did develop language and an audiogram at age 13 showed a bilateral hearing loss greater than 80 dB. He attended a boarding school for the deaf between the ages of 10 and 15 years. He was noted to have no intelligible speech at age 19. He was a fisherman and continued this occupation until age 41 when he started to lose vision. He was nearly completely blind by age 49 and developed progressive neurologic symptoms in his 50s characterized by dystonia, spasticity, contractures and an ataxic gait. He died at age 60 from bilateral bronchopneumonia and the right temporal bone was removed 96 hours after death.
Histopathology
The external auditory canal, middle ear, mastoid and otic capsule appear normal. A cytocochleogram of the ear is shown in Fig. 2E. There is near-total loss of cochlear neurons, with loss of nearly all peripheral and central axonal processes. The total cochlear neuronal count is 1,485, which represents a 94% loss vs age-matched normals. The atrophy includes loss of the intra-ganglionic spiral bundle. Accurate assessments of the organ of Corti cannot be made because of postmortem autolysis, but it appears that hair cells are present. The stria vascularis, spiral ligament, tectorial membrane and spiral limbus appear normal. Reissner's membrane shows apical endolymphatic hydrops. The vestibular sense organs show advanced autolysis, but it appears that hair cells are present in the respective cristae and maculae. There is severe loss of cells of Scarpa's ganglion with atrophy of the peripheral nerve fibers. The total Scarpa's ganglion count was 2,692 which represents an 85% loss compared to age-matched controls. Blood vessels supplying the auditory and vestibular end organs are present without any evidence of vasculitis or occlusion. There is also no deposition of connective tissue or new bone within the inner ear. Cells of the geniculate and trigeminal ganglia are pyknotic, but are present in normal numbers.
DISCUSSION
MTS is an X-linked syndrome characterized by progressive hearing loss in childhood, followed later in life by progressive neural degeneration affecting the brain and optic nerves. There is phenotypic variability, both within and between families affected with MTS1-3, 10. The initial manifestation of post-lingual progressive SNHL in the first decade of life is shared by the majority of cases. However, congenital hearing loss has also been reported11,12. Dystonia is also a common clinical manifestation, but its onset is more variable, ranging from the first to the fifth decade of life2,3,10,12. Mild dystonia may be present in female carriers who are heterozygous for TIMM8A mutations2,3,10,12. Although many different mutations in the TIMM8A gene have been reported in families with MTS, it appears that differences in the mutations cannot account for the phenotypic variability. The latter has been hypothesized to be due to modifier genes, environmental factors or both3,10.
We examined 5 temporal bones from four individuals belonging to the same family with MTS, all of whom had an identical frame shift mutation in the TIMM8A gene. All four subjects developed progressive hearing loss in early childhood, becoming profoundly deaf by the age of 10 years. All four developed language and at least one subject used amplification in early life. Audiometric evaluation in two subjects showed 80 to 100 dB threshold shifts around the age of 10 years. All four developed neurodegeneration and vision loss that is typical of MTS later in adult life, and died between the ages of 49 and 67. Therefore, all four subjects were profoundly hearing impaired for a long period of time, between 40 and 60 years, depending on the case.
The otopathology was strikingly similar, in that all bones examined showed near-total loss of cochlear neuronal cells and severe loss of vestibular neurons. When compared to age-matched controls, there was 90-95% loss of cochlear neurons and 75-85% loss of vestibular neurons. It is also of note that the geniculate and trigeminal ganglia were unaffected in all specimens examined. The similarity of findings in these five temporal bones reinforces the conclusion that was reached in our earlier report based on examination of a single temporal bone6, that the otopathologic hallmark of MTS is a severe neuropathy affecting the cochlear and vestibular nerves. Thus, MTS constitutes a good example of a true auditory neuropathy, of which there are few well-documented examples in the literature with histopathologic confirmation13,14.
It is clear from our case series that the profound hearing loss experienced by these individuals can be correlated with the massive loss of cochlear neurons in the presence of preserved hair cells and other structures of the cochlear duct. Although audiometric data are not available from early childhood, all four individuals had enough hearing to be able to develop language. Therefore, we infer that the SNHL in MTS is likely to be the result of a post-natal, progressive, degeneration of cochlear neurons.
In addition to the severe loss of auditory and vestibular ganglion cells, other histopathology abnormalities were observed in some temporal bones such as chronic otitis media (one ear), partial atrophy of the organ of Corti (two ears), and mild atrophy of the stria vascularis (one ear). Some of these changes were asymmetric, for example, atrophy of the organ of Corti and strial loss between the two ears of subject VI:24. The histopathologic changes in any given temporal bone specimen represent the end result of insults accumulated over the lifespan of an individual. These insults include, but are not restricted to, the effects of genetic mutations, exposure to noise, potentially ototoxic medications, trauma, infections and systemic disorders. It is difficult to ascertain the precise causes for these other histopathological abnormalities that were observed in our cases.
The molecular basis for neural degeneration in MTS is under investigation 15,16. Neuronal cell loss has been shown to occur within the optic nerve, retina, striate cortex, basal ganglia and dorsal roots of the spinal cord in different individuals with MTS 3. It has been shown that expression of mitochondrial proteins is not uniform within the central nervous system 16. The DDP/TIMM8A protein is prominently expressed in the soma and dendritic portion of Purkinje cells within the brain. It has been shown in mammals that the DDP/TIMM8A protein partners with another TIMM protein (called TIMM13) to form a 70 kDa complex in the mitochondrial intermembrane space, and that this complex is part of a translocation apparatus for the import and assembly of inner membrane proteins. MTS is believed to result from defects in the assembly of the DDP/TIMM8A-TIMM13 complex, leading to mitochondrial dysfunction in specific subsets of cells where this protein is critical. Future work involving immunolocalization of these proteins in various parts of the brain and ear, as well as development of a suitable animal model may shed more light on the molecular pathogenesis of MTS.
The observed histopathologic changes in our temporal bones may be used to predict clinical features that may alert a clinician to the occurrence of MTS in a child with progressive SNHL. We would predict an audiometric finding of loss of speech discrimination ability that is out of proportion to the loss of pure tone audiometric thresholds. While cochlear neuronal cells are critical for understanding speech, loss of up to 80% of cochlear neurons has been shown to be consistent with normal pure tone thresholds and losses of 90% create only moderate threshold elevations 7. One would also predict poor morphology of auditory brainstem responses (ABRs), as ABRs are critically dependent on a functioning cochlear nerve and central auditory pathways. Otoacoustic emissions (OAEs) would be preserved if the outer hair cells are intact, as was clearly the case in two of our specimens. The presence of a progressive SNHL in a male child with poor speech discrimination scores, poor morphology of ABRs, intact OAEs and no other systemic abnormalities should prompt a suspicion of MTS and appropriate genetic analysis.
There is a dearth of data in the literature concerning the audiological findings in MTS. Richter et al 17 described 3 children with X-linked agammaglobulinemia who had large deletions within the Bruton tyrosine kinase gene as well as a deletion of the whole TIMM8A gene. These children suffered from progressive SNHL. Audiological testing in two of these cases showed presence of OAEs and absence of ABRs, which is consistent with what one would predict from the otopathology observed in our cases. On the other hand, Aguirre et al10 described two brothers with SNHL and MTS, one of whom had absent ABRs and absent OAEs. One of the temporal bones in the present study also showed loss of a large number of outer hair cells, which could explain the absent OAEs. Additional well documented audiological and temporal bone histopathologic studies are necessary to define the complete spectrum of abnormalities in MTS.
All five temporal bones examined showed a severe loss of vestibular ganglion cells, consistent with a progressive vestibular neuropathy due to the MTS mutation. Such degeneration probably evolved slowly over time, which may allow for central compensation and may explain the absence of significant vestibular complaints in the medical histories of our subjects. However, it is likely that the vestibular degeneration resulted in bilateral peripheral vestibular hypofunction, and it probably contributed to the imbalance and ataxia experienced by these patients later in life.
Our histopathologic findings also have implications for management of hearing loss in MTS. The severity of auditory neuropathy in our cases suggests that amplification may provide limited benefit. It is more difficult to speculate whether cochlear implantation may be beneficial. Although it is traditionally assumed that implant performance is related to the number of surviving cochlear neuronal cells, temporal bone histopathologic studies have failed to show a positive correlation between implant performance and numbers of cochlear neuronal cells 18,19. It is possible that implantation early in life while stimulable auditory nerve fibers are still available may be of benefit in children with MTS. Indeed, cochlear implantation has been successful in other cases of auditory neuropathy 20-22. Another potential avenue for future rehabilitation and treatment is regeneration of cochlear neurons and of the auditory nerve using therapies based on stem cells. The histopathologic finding that the organ of Corti and other end-organ structures of the cochlear duct are generally intact makes this a particularly attractive avenue for future research. Discovery of molecular targets within mitochondrial cochlear neuronal cells may also provide the basis for future novel therapeutic interventions.
SUMMARY AND CONCLUSIONS
We described the otopathology in 4 individuals affected with the Mohr-Tranebjærg syndrome (MTS), an X-linked, recessive, syndromic, sensorineural hearing loss caused by a mutation in the DDP/TIMM8A gene. The otopathology was strikingly similar, in that all temporal bones examined showed near-total loss of cochlear neuronal cells and severe loss of vestibular neurons. We infer that the hearing loss in MTS is likely to be the result of a postnatal and progressive degeneration of cochlear neurons, and that MTS constitutes a true auditory neuropathy. Our findings have implications for clinical diagnosis of patients with MTS, and management of the hearing loss.
ACKNOWLEDGEMENTS
Supported in part by NIH grant 1U24DC008559 (to SNM) and by grants (to LT) from the Oticon Foundation, Denmark. We are also grateful to Mr. Axel Eliasen and Mr. Lakshmi Mittal for support of our work.
REFERENCES
- 1.Mohr J, Magerøy K. Sex-linked deafness of a possibly new type. Acta Genet Stat Med (Basel) 1960;10:54–62. doi: 10.1159/000151118. [DOI] [PubMed] [Google Scholar]
- 2.Tranebjærg L, Schwartz C, Eriksen H, et al. A new X linked recessive deafness syndrome with blindness, dystonia, fractures, and mental deficiency is linked to Xq22. J Med Genet. 1995;32:257–263. doi: 10.1136/jmg.32.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tranebjærg L, Jensen PKA, van Ghelue M, Vnencak-Jones CL, Sund S, Elgjo K, Jakobsen J, Lindal S, Warburg M, Fuglsang-Frederiksen A, Skullerud K. Neuronal cell death in the visual cortex is a prominent feature of the X-linked recessive mitochondrial deafness-dystonia syndrome caused by mutations in the TIMM8A gene. Ophthal Genet. 2001;22:207–223. doi: 10.1076/opge.22.4.207.2220. [DOI] [PubMed] [Google Scholar]
- 4.Jin H, May M, Tranebjærg L, Kendall E, Fontan G, Jackson J, Subramony SH, Arena F, Lubs H, Smith S, Stevenson R, Schwatz C, Vetrie D. A novel X-linked gene, DDP, shows mutations in families with deafness (DFN-1), dystonia, mental deficiency and blindness. Nat Genet. 1996;14:177–180. doi: 10.1038/ng1096-177. [DOI] [PubMed] [Google Scholar]
- 5.Koehler CM, Leuenberger D, Merchant S, Renold A, Junne T, Schatz G. Human deafness dystonia syndrome is a mitochondrial disease. Proc Natl Acad Sci. 1999;96:2141–2146. doi: 10.1073/pnas.96.5.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchant SN, McKenna MJ, Nadol JB, Jr, Kristiansen A, Tropitzsch A, Lindal S, Tranebjærg L. Temporal bone histopathology and genetic studies in Mohr-Tranebjærg syndrome (DFN-1) Otol Neurotol. 2001;22:506–511. doi: 10.1097/00129492-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Schuknecht HF. Pathology of the Ear. 2nd edition. Lea and Febiger; Philadelphia: 1993. pp. 7–30. 417-419. [Google Scholar]
- 8.Richter E. Quantitative study of human Scarpa's ganglion and vestibular sensory epithelia. Acta Otolarngol (Stockh) 1980;90:199–208. doi: 10.3109/00016488009131716. [DOI] [PubMed] [Google Scholar]
- 9.Velasquez L, Merchant SN, Tsuji K, Glynn B, Wall C, Rauch SD. Temporal bone studies of the human peripheral system. II. Normative Scarpa's ganglion cell data. Ann Otol Rhinol Laryngol. 2000;109(suppl 181):14–19. doi: 10.1177/00034894001090s503. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre LA, del Castillo I, Macaya A, Meda C, Villamar M, Moreno-Pelayo MA, Moreno F. A novel mutation in the gene encoding TIMM8a, a component of the mitochondrial protein translocase complexes, in a Spanish familial case of deafness-dystonia (Mohr-Tranebjaerg) syndrome. Am J Med Genet. 2006;140:392–397. doi: 10.1002/ajmg.a.31079. [DOI] [PubMed] [Google Scholar]
- 11.Ujike H, Tanabe Y, Takehisa Y, Hayabara T, Kuroda S. A family with X- linked dystonia-deafness syndrome with a novel mutation of the DDP gene. Arch Neurol. 2001;58:1004–1007. doi: 10.1001/archneur.58.6.1004. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow RH, Wooten GF. A novel deafness/dystonia peptide gene mutation that causes dystonia in female carriers of Mohr-Tranebjaerg syndrome. Ann Neurol. 2001;50:537–540. doi: 10.1002/ana.1160. [DOI] [PubMed] [Google Scholar]
- 13.Nadol JB., Jr . Primary cochlear neuronal degeneration. In: Sininger Y, Starr A, editors. Auditory Neuropathy: A New Perspective On Hearing Disorders. Singular-Thomson Learning; Australia: 2001. pp. 99–140. [Google Scholar]
- 14.Rapin I, Gravel J. “Auditory neuropathy”: physiologic and pathologic evidence calls for more diagnostic specificity. Int J Pediatr Otorhinolaryngol. 2003;67:707–728. doi: 10.1016/s0165-5876(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 15.Roesch K, Curran SP, Tranebjærg L, Koehler CM. Human deafness dystonia syndrome is caused by a defect in assembly of the DDP1/TIMM8A-TIMM13 complex. Hum Molec Genet. 2002;11:477–486. doi: 10.1093/hmg/11.5.477. [DOI] [PubMed] [Google Scholar]
- 16.Roesch K, Hynds PJ, Varga R, Tranebjærg L, Koehler CM. The calcium-binding aspartate/glutamate carriers, cirin and aralar1, are new substrates for the DDP1/TIMM8ATIMM13 complex. Hum Molec Genet. 2004;13:2101–2111. doi: 10.1093/hmg/ddh217. [DOI] [PubMed] [Google Scholar]
- 17.Richter D, Conley ME, Rohrer J, Myers LA, Zahradka K, Kelecic J, Sertic J, Stavlenic-Rukavina A. A contiguous deletion syndrome of X-linked agammaglobulinemia and sensorineural deafness. Pediatr Allergy Immunol. 2001;12:107–111. doi: 10.1034/j.1399-3038.2001.0129999107.x. [DOI] [PubMed] [Google Scholar]
- 18.Nadol JB, Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- 19.Fayad JN, Linthicum FH. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 20.Buss E, Labadie RF, Brown CJ, Gross AJ, Grose JH, Pillsbury HC. Outcome of cochlear implantation in pediatric auditory neuropathy. Otol Neurotol. 2002;23(3):328–32. doi: 10.1097/00129492-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Madden C, Hilbert L, Rutter M, Greinwald J, Choo D. Pediatric cochlear implantation in auditory neuropathy. Otol Neurotol. 2002;23(2):163–8. doi: 10.1097/00129492-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Peterson A, Shallop J, Driscoll C, Breneman A, Babb J, Stoeckel R, Fabry L. Outcomes of cochlear implantation in children with auditory neuropathy. J Am Acad Audiol. 2003 May-Jun;14(4):188–201. [PubMed] [Google Scholar]