Abstract
Broth cultures of Bacillus pumilus NRRL B-3275 (BpB1) grown at 25, 30, or 37 C contain 1 to 2% spontaneous auxotrophic mutants in both the exponential and stationary phases of growth. Of 70 such mutants isolated from cultures grown at 37 C, approximately two-thirds reverted at such a high frequency as to preclude their study. Of the remaining 22 mutants, 18 required a single amino acid, 1 required adenine, and 1 required uracil. Two of the auxotrophs each required two unrelated amino acids resulting from two independent mutations. All of the mutations reverted spontaneously. Enhanced reversion of approximately one-third of the mutations was obtained with nitrosoguanidine, ethyl methane sulfonate, or diethyl sulfate, or with more than one of these mutagens. The reversion of one mutation was enhanced by 2-aminopurine. The reversion of the remaining mutations was not enhanced by the above mutagens, nor by mutagens known to induce (and revert) frameshift mutations in other bacterial systems. Nine of 10 mutants examined did not show a selective growth advantage over the parents. All but three of the mutations could be linked by PBS1 transduction to one of the previously described auxotrophic markers in strain BpB1. No evidence was obtained for clustering of the mutations on the BpB1 genome. Six of the mutations conferred a requirement for serine. One linked by transduction to trp-2, three linked to argA1, and two (ser-2, -3) linked to argO1. Pigmented mutants (containing a carotenoid-like pigment), which occur spontaneously in BpB1 cultures at a frequency on the order of 1 to 5 mutants per 104 cells, link by transduction to ser-2, -3. Spontaneous mutants of strain BpB1 resistant to rifampin, streptomycin, erythromycin, 5-fluorouracil, or 5-methyltryptophan occur at a frequency similar to that of strains of B. pumilus which do not exhibit a high rate of spontaneous mutation to auxotrophy. It is suggested that certain sites or regions of the BpB1 genome exhibit a high rate of spontaneous mutation.
Full text
PDF


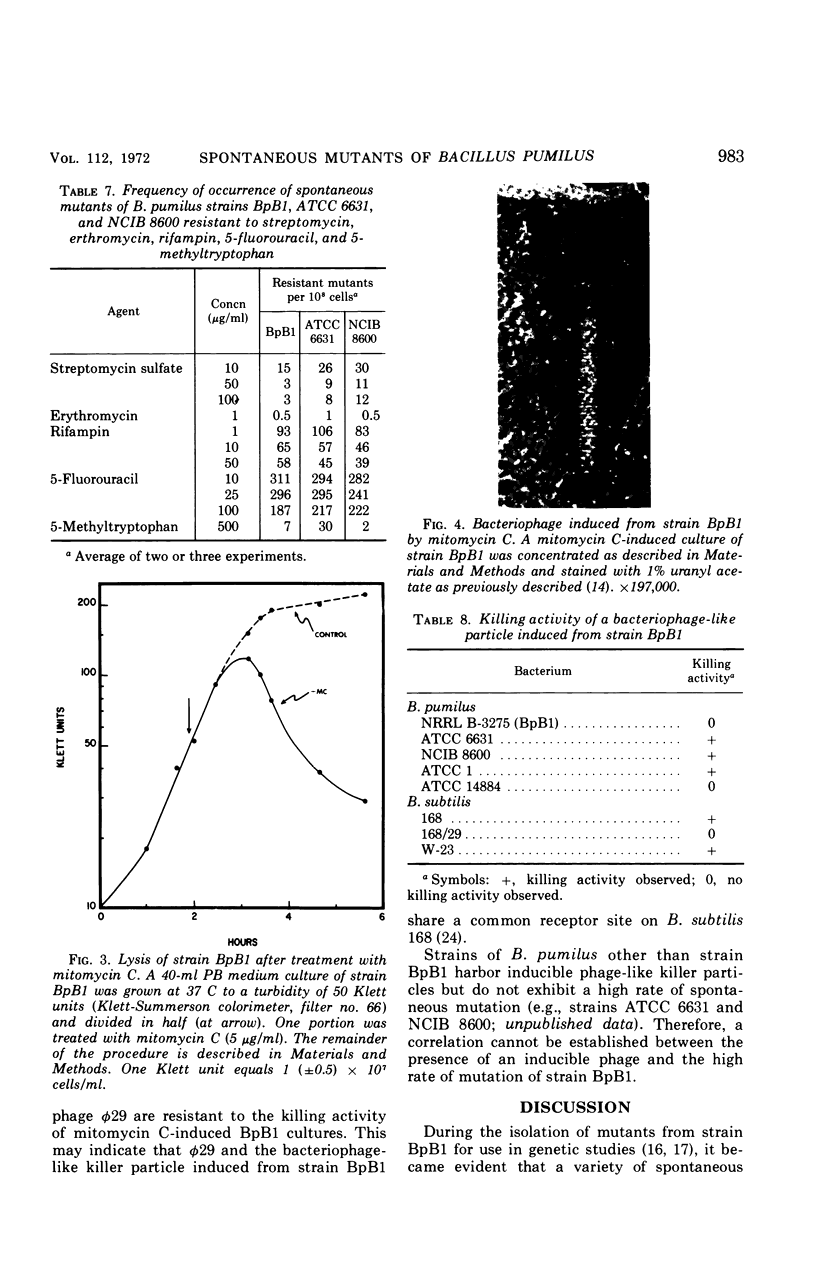


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg C. M. Auxotroph accumulation in deoxyribonucleic acid polymeraseless strains of Escherichia coli K-12. J Bacteriol. 1971 Jun;106(3):797–801. doi: 10.1128/jb.106.3.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Brammar W. J., Yanofsky C. Spontaneous and ICR191-A-induced frameshift mutations in the A gene of Escherichia coli tryptophan synthetase. J Bacteriol. 1968 Nov;96(5):1672–1679. doi: 10.1128/jb.96.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boram W., Abelson J. Bacteriophage Mu integration: on the mechanism of Mu-induced mutations. J Mol Biol. 1971 Nov 28;62(1):171–178. doi: 10.1016/0022-2836(71)90137-9. [DOI] [PubMed] [Google Scholar]
- Burmeister H. R., Hesseltine C. W. Induction and propagation of a Bacillus subtilis L form in natural and synthetic media. J Bacteriol. 1968 May;95(5):1857–1861. doi: 10.1128/jb.95.5.1857-1861.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coukell M. B., Yanofsky C. Increased frequency of deletions in DNA polymerase mutants of Escherichia coli. Nature. 1970 Nov 14;228(5272):633–635. doi: 10.1038/228633a0. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Allen E. F., Forsberg S. A., Preparata R. M., Greening E. O. Genetic control of mutation rates in bacteriophageT4. Nature. 1969 Mar 22;221(5186):1128–1132. [PubMed] [Google Scholar]
- GOLDSTEIN A., SMOOT J. S. A strain of Escherichia coli with an unusually high rate of auxotrophic mutation. J Bacteriol. 1955 Nov;70(5):588–595. doi: 10.1128/jb.70.5.588-595.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D., Karamata D., Hempstead P. G. Temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:307–312. doi: 10.1101/sqb.1968.033.01.034. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Levine K., Hartman Z., Berger H. Hycanthone: a frameshift mutagen. Science. 1971 Jun 4;172(3987):1058–1060. doi: 10.1126/science.172.3987.1058. [DOI] [PubMed] [Google Scholar]
- Huang W., Marmur J. Characterization of inducible bacteriophages in Bacillus licheniformis. J Virol. 1970 Feb;5(2):237–246. doi: 10.1128/jvi.5.2.237-246.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberfarb R. M., Bryson V. Isolation, characterization, and genetic analysis of mutator genes in Escherichia coli B and K-12. J Bacteriol. 1970 Oct;104(1):363–375. doi: 10.1128/jb.104.1.363-375.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. PBPI: a flagella specific bacteriophage mediating transduction in Bacillus pumilus. Virology. 1972 Mar;47(3):743–752. doi: 10.1016/0042-6822(72)90564-8. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Genetic analysis in Bacillus pumilus by PBSI-mediated transduction. J Bacteriol. 1970 Feb;101(2):603–608. doi: 10.1128/jb.101.2.603-608.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Identification of Bacillus subtilis NRRL B-3275 as a strain of Bacillus pumilus. J Bacteriol. 1969 Nov;100(2):658–661. doi: 10.1128/jb.100.2.658-661.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Linkage groups in Bacillus pumilus determined by bacteriophage PBS1-mediated transduction. J Bacteriol. 1971 May;106(2):697–699. doi: 10.1128/jb.106.2.697-699.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeschger N. S., Hartman P. E. ICR-induced frameshift mutations in the histidine operon of Salmonella. J Bacteriol. 1970 Feb;101(2):490–504. doi: 10.1128/jb.101.2.490-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffers H. P., Spinelli V., Belser N. O. A Factor (or Mutator Gene) Influencing Mutation Rates in Escherichia Coli. Proc Natl Acad Sci U S A. 1954 Nov;40(11):1064–1071. doi: 10.1073/pnas.40.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Cox E. C., Horn V. The unusual mutagenic specificity of an E. Coli mutator gene. Proc Natl Acad Sci U S A. 1966 Feb;55(2):274–281. doi: 10.1073/pnas.55.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Haywood P., Pollock M. Isolation of L-forms of Bacillus subtilis which grow in liquid medium. J Bacteriol. 1970 Jun;102(3):867–870. doi: 10.1128/jb.102.3.867-870.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]