Abstract
The three-dimensional NMR structures of six octapeptide agonist analogues of somatostatin (SRIF) in the free form are described. These analogues, with the basic sequence H-DPhe/Phe2-c[Cys3-Xxx7-DTrp8-Lys9-Thr10-Cys14]-Thr-NH2 (the numbering refers to the position in native SRIF), with Xxx7 being Ala/Aph, exhibit potent and highly selective binding to human SRIF type 2 (sst2) receptors. The backbone of these sst2-selective analogues have the usual type-II’ β-turn reported in the literature for sst2/3/5-subtype-selective analogues. Correlating biological results and NMR studies led to the identification of the side chains of DPhe2, DTrp8 and Lys9 as the necessary components of the sst2 pharmacophore. This is the first study to show that the aromatic ring at position 7 (Phe7) is not critical for sst2 binding and that it plays an important role in sst3 and sst5 binding. This pharmacophore is therefore different from that proposed by others for sst2/3/5 analogues.
Introduction
Somatostatin (SRIF, H-Ala1-Gly2-c[Cys3-Lys4-Asn5-Phe6-Phe7-Trp8-Lys9-Thr10-Phe11-Thr12-Ser13-Cys14]-OH), a cyclic tetradecapeptide, isolated from the hypothalamus as a growth hormone inhibitor, is now known to be a multifunctional peptide located in most of the brain regions and in peripheral organs.1,2 Cells containing SRIF are typically neurons or endocrine-like cells which are found in high density throughout the central and peripheral nervous systems, in the endocrine pancreas, and in the gut and in small numbers in the thyroid, adrenals, submandibular glands, kidneys, prostrate and placenta.1,2 The activities of SRIF are mediated through a family of five different high affinity membrane receptors, sst1-sst5 (ssts). Due to its broad spectrum of physiological activities and its short duration of action due to rapid proteolytic degradation in vivo,3 SRIF continues to be a target for the development of subtype-specific analogues.4-7 (and references therein)
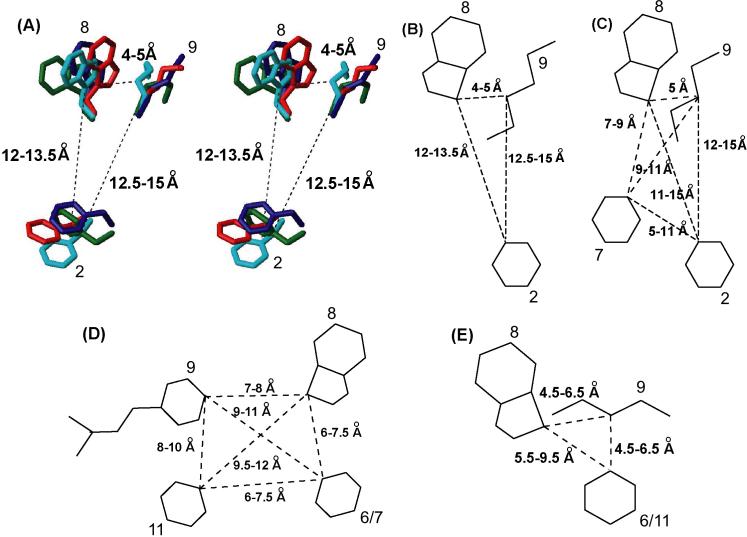
Indeed, over the last three decades, hundreds of SRIF analogues have been reported and tested in different biological systems including affinity and selectivity towards the five receptor-subtypes.8-10 Correspondingly, early structure-activity relationship (SAR) studies ruled out the specific involvement of the side chains of all residues but Phe7-DTrp8-Lys9 for biological recognition.11-13 (Note: the numbering of residues follows that of native SRIF.) Extensive structural studies including NMR and x-ray diffraction14 were carried out to elucidate the pharmacophore and the consensus structural motif of analogues binding predominantly to sst2/5 (and sst3) receptors. On the basis of these 3D structures of the free peptides, a pharmacophore model was proposed for SRIF analogues binding to sst2/5 (and sst3).15-18 In this model, the side chains and the relative spatial arrangement of DPhe2, Phe7, DTrp8 and Lys9 constitute the most essential elements necessary for binding (Figure 3C). The side chain of DTrp8 is in close proximity to the side chain of Lys9 (∼5 Å), whereas the side chain of Phe7 is about 7 − 9 Å away from the side chain of DTrp8 and 9 − 11 Å from the side chain of Lys9. The aromatic side chain at position 2 outside the disulfide bond was not conserved in its position in most of these analogues. The distance between DPhe2 and DTrp8, Lys9, Phe7 were 11−15 Å, 12−15 Å, 5−11 Å respectively.19 In this model of the pharmacophore, the rotamer of the side chains of DTrp8 and Phe7 are in the trans configuration and that of Lys9 is in the gauche configuration.
Figure 3.
Consensus structural motif of sst2-selective SRIF analogues. (A) Stereo view of the consensus structural motif for the sst2-selective analogues 1 (red), 2 (green), 3 (navy blue) and 4 (cyan). Only the side chains of the residues DPhe2, DTrp8 and Lys9 for 1, 2, 3 and 4 are shown. The distances between the Cγ atoms of DTrp8, Lys9 and DPhe2 are displayed. For each analogue, the conformer with the lowest target function is displayed. (B) Schematic drawing of the pharmacophore for the sst2-selective SRIF analogues. (C) Schematic drawing of the pharmacophore for the sst2/3/5-selective SRIF analogues.16 (D) Schematic drawing of the pharmacophore for the sst1-selective SRIF analogues.62 (E) Schematic drawing of the pharmacophore for the sst4-selective SRIF analogues.20 In all of the pharmacophores, the distance between the Cγ atoms of the respective residues is displayed.
Recently, we have proposed models of the sst4 and the sst1 pharmacophores derived from the NMR consensus structures of several receptor selective analogues.20,21 These models of the pharmacophores are different from that of the reported sst2/3/5 selective pharmacophore in the following manner. The sst4 pharmacophore has one aromatic side chain, either Phe6 or Phe11 involved in binding in addition to the DTrp8 and Lys9 pair. The distance between DTrp8 and Lys9 is 4.5−6.5 Å, between DTrp8 and Phe6/11 is 5.5−9.5 Å and between Lys9 and Phe6/11 is 4.5−6.5 Å. The aromatic side chain of Phe6/11 is closer to the side chain of Lys9 than that of DTrp8 (Figure 3E). On the other hand, the sst1 pharmacophore has two aromatic side chains, at position 6 or 7 and 11, involved in binding in addition to the DTrp8 and IAmp9 pair. The positioning of the two aromatic side chains at the back of the peptide plane has to be noted, which is opposite to that of the sst4 and sst2/3/5 pharmacophores. The sst1 receptor selectivity is mainly achieved by the amino acid IAmp, which replaces Lys at position 9. The distance between DTrp8 and IAmp9 is 7−8 Å, between DTrp8 and Phe6/7 is 6−7.5 Å and between IAmp9 and Phe11 is 8−10 Å (Figure 3D).
In a similar manner, we present a model of the sst2 pharmacophore based on the 3D NMR structures of the free peptides. The three-dimensional structures of six analogues in DMSO-d6 are presented including the structure of octreotide amide. These analogues have the typical octreotide scaffold with a type-II’ β-turn backbone conformation similar to that of the reported sst2/3/5 selective analogues, yet their pharmacophore has one aromatic side chain (Phe7) less than that of the reported sst2/3/5 selective analogues.
Results
Peptide synthesis and characterization are described in Table 1 and experimental section. The proton resonance assignment and the structure determination by NMR for each of the sst2-selective analogues 1 − 6 (Table 1) are presented in Tables 2 and 3.
Table I.
Physico-chemical properties and sst1−5 binding affinities (IC50s, nM) of analogues studied by NMR.
| ID # | Compound | Purity (%) | MSc | IC50 nMd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HPLCa | CZEb | M(mono) calc. | MH+(mono) obs. | sst1 | sst2 | sst3 | sst4 | sst5 | ||
| 1 | H-DPhe-c[Cys-Ala-DTrp-Lys-Thr-Cys]-Thr-NH2 | 99 | 99 | 955.40 | 956.38 | >1K (3) | 8.6;4.5;3.6 | 772;∼1472;543 | >1K (3) | 158;807;226 |
| 2 | H2N-CO-DPhe-c[Cys-Aph(CONH2)-DTrp-Lys-Thr-Cys]-Thr-NH2 | 98 | 97 | 1132.45 | 1133.17 | >1K (3) | 6.4;9;6.2 | 210;725;394 | >1K;905;494 | 400;77;100 |
| 3 | H2N-CO-Phe-c[Cys-Aph(CONH2)-DTrp-Lys-Thr-Cys]-Thr-NH2 | 98 | 99 | 1132.46 | 1133.44 | >1K (2) | 7.5;20 | 942;1094 | 957;872 | 109;260 |
| 4 | H2N-CO-DPhe-c[Cys-Aph(CONHOCH3)-DTrp-Lys-Thr-Cys]-Thr-NH2 | 99 | 99 | 1119.46 | 1120.42 | >1K (2) | 3.1;4.4 | >1K;958 | >1K (2) | 808;539 |
| 5 | H-c[Cys-Phe-DTrp-Lys-Thr-Cys]-OH | 97 | 98 | 78430 | 785.27 | >1K | 378 | >1K | >1K | >1K |
| 6 | H-DPhe-c[Cys-Phe-DTrp-Lys-Thr-Cys]-Thr-NH2 | 95 | 99 | 1031.43 | 1032.00 | >1K | 2.7;2.2 | 80;38 | >10K | 2.7;3.9 |
Percent purity determined by HPLC (Hewlett-Packard Series II 1090 Liquid Chromatograph) using buffer system: A = TEAP (pH 2.5) and B = 60% CH3CN/40% A with a gradient slope of 1% B/min, at flow rate of 0.2 mL/min on a Vydac C18 column (0.21 × 15 cm, 5-μm particle size, 300 Å pore size). Detection at 214 nm.
Capillary zone electrophoresis (CZE) was done using a Beckman P/ACE System 2050 controlled by an IBM Personal System/2 Model 50Z and using a ChromJet integrator. Field strength of 15 kV at 30 °C, mobile phase: 100 mM sodium phosphate (85:15, H2O:CH3CN) pH 2.50, on a Supelco P175 capillary (363 μm OD x 75 μm ID X 50 cm length). Detection at 214 nm.
Mass spectra (MALDI-MS) were measured on an ABI-Perseptive DE-STR instrument. The instrument employs a nitrogen laser (337 nm) at a repetition rate of 20 Hz. The applied accelerating voltage was 20 kV. Spectra were recorded in delayed extraction mode (300 ns delay). All spectra were recorded in the positive reflector mode. Spectra were sums of 100 laser shots. Matrix α-cyano-4-hydroxycinnamic acid was prepared as saturated solutions in 0.3% trifluoroacetic acid and 50% acetonitrile. The observed monoisotopic (M + H)+ values of each peptide corresponded with the calculated (M + H)+ values.
The IC50 values (nM) were derived from competitive radioligand displacement assays reflect the affinities of the analogues for the cloned somatostatin receptors using the non-selective 125I-[Leu8,DTrp22,Tyr25]SRIF-28, as the radioligand.
Table 2.
Proton chemical shifts of analogues 1−6*
|
Residue |
1H |
Analogues |
|||||
|---|---|---|---|---|---|---|---|
|
1 |
2 |
3 |
4 |
5 |
6 |
||
| H2N-CO |
|
|
5.42 |
5.66 |
7.59 |
|
|
| dPhe/Phe2 | NH | 7.99 | 6.33 | 6.42 | 8.01 | ||
| αH | 4.20 | 4.55 | 4.53 | 4.19 | 4.15 | ||
| βH | 3.22,2.96 | 3.08,2.72 | 3.00,2.80 | 3.25,2.95 | 3.24,2.95 | ||
| H2,6 | 7.38 | 7.28 | 7.14 | 7.38 | 7.38 | ||
| |
H3,5 |
7.29 |
7.23 |
7.21 |
7.30 |
|
7.29 |
| Cys3 | NH | 9.22 | 8.72 | 8.59 | 9.23 | 9.22 | |
| αH | 5.27 | 5.22 | 5.16 | 5.27 | 4.14 | 5.28 | |
| |
βH |
2.76,2.82 |
2.77,2.77 |
2.81,2.81 |
2.82,2.82 |
3.07,2.98 |
2.81,2.81 |
| |
|
Ala |
Aph(CONH2) |
Aph(CONH2) |
Aph(CONHOCH3) |
Phe |
Phe |
| Aph/Ala7 | NH | 8.44 | 8.49 | 8.45 | 8.52 | 8.36 | 8.55 |
| αH | 4.47 | 4.58 | 4.60 | 4.65 | 4.67 | 4.66 | |
| βCH3 | 1.17 | ||||||
| βH | 2.77,2.77 | 2.81,2.81 | 2.79,2.79 | 2.81,2.81 | 2.84,2.84 | ||
| H2,6 | 6.96 | 6.99 | 7.03 | 7.03 | 7.08 | ||
| H3,5 | 7.22 | 7.28 | 7.44 | 7.16 | 7.17 | ||
| HT | 8.46 | 8.54 | 8.84 | ||||
| NH2 | 5.86 | 5.92 | 9.55 | ||||
| |
CH3 |
|
|
|
3.62 |
|
|
| dTrp8 | NH | 8.92 | 8.82 | 8.80 | 8.81 | 8.74 | 8.77 |
| αH | 4.30 | 4.20 | 4.19 | 4.19 | 4.58 | 4.19 | |
| βH | 3.07,2.99 | 2.98,2.73 | 2.98,2.72 | 2.98,2.73 | 3.14,2.86 | 2.96,2.71 | |
| HD1 | 7.19 | 6.93 | 6.91 | 6.97 | 6.99 | 6.95 | |
| HE1 | 10.87 | 10.76 | 10.77 | 10.74 | 10.80 | 10.81 | |
| HZ2 | 7.32 | 7.29 | 7.30 | 7.29 | 7.32 | 7.32 | |
| HH2 | 7.06 | 7.04 | 7.06 | 7.04 | 7.08 | 7.06 | |
| HZ3 | 6.99 | 6.98 | 7.00 | 6.99 | 7.00 | 6.99 | |
| |
HE3 |
7.54 |
7.42 |
7.41 |
7.41 |
7.47 |
7.43 |
| Lys9 | NH | 8.43 | 8.36 | 8.39 | 8.40 | 8.50 | 8.46 |
| αH | 3.99 | 4.01 | 4.00 | 3.97 | 3.89 | 3.99 | |
| βH | 1.70,1.25 | 1.72,1.27 | 1.73,1.29 | 1.70,1.26 | 1.69,1.37 | 1.72,1.30 | |
| γH | 0.72,0.72 | 0.77,0.74 | 0.80,0.76 | 0.76,0.72 | 0.86,0.86 | 0.79,0.79 | |
| δH | 1.29,1.29 | 1.30,1.30 | 1.32,1.32 | 1.32,1.32 | 1.35,1.35 | 1.35,1.35 | |
| εH | 2.55,2.55 | 2.56,2.56 | 2.58,2.58 | 2.55,2.55 | 2.60,2.60 | 2.57,2.57 | |
| εNH | 7.60 | 7.56 | 7.62 | 7.58 | 7.63 | 7.62 | |
|
Residue |
1H |
Analogues |
|||||
|---|---|---|---|---|---|---|---|
|
1 |
2 |
3 |
4 |
5 |
6 |
||
| Thr10 | NH | 7.68 | 7.58 | 7.58 | 7.63 | 7.72 | 7.62 |
| αH | 4.48 | 4.49 | 4.51 | 4.48 | 4.29 | 4.50 | |
| βH | 3.99 | 3.97 | 3.97 | 4.02 | 4.18 | 4.00 | |
| γH | 1.05 | 1.06 | 1.06 | 1.08 | 1.09 | 1.07 | |
| |
OH |
4.88 |
4.80 |
4.83 |
4.87 |
|
|
| Cys14 | NH | 8.42 | 8.46 | 8.47 | 8.49 | 8.08 | 8.49 |
| αH | 5.15 | 5.30 | 5.21 | 5.15 | 4.58 | 5.17 | |
| |
βH |
2.86,2.86 |
2.85,2.85 |
2.84,2.84 |
2.86,2.86 |
3.14,2.86 |
2.86,2.86 |
| Thr15 | NH | 8.06 | 8.17 | 8.13 | 8.05 | 8.07 | |
| αH | 4.22 | 4.21 | 4.23 | 4.23 | 4.21 | ||
| βH | 4.02 | 4.06 | 3.93 | 4.03 | 4.03 | ||
| γH | 1.05 | 1.07 | 0.99 | 1.07 | 1.06 | ||
| |
OH |
5.15 |
5.13 |
5.07 |
5.15 |
|
|
| NH2 | 7.53,7.36 | 7.42,7.26 | 7.43,7.35 | 7.53,7.36 | 7.35,7.52 | ||
The chemical shifts were measured at 298K in DMSO in ppm with the internal reference of the DMSO signal at 2.49 ppm.
Table 3.
Characterization of the NMR structures of analogues 1−6*
| ID# | NOE distance restraints | Angle restraints*** | CYANA Target function** | Backbone RMSD (Å) | Overall RMSD (Å) | CFF91 energies (Kcal/mol) | Residual restraint violations on | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total energy | Van der Waals | Electrostatic | Distances | Dihedral Angles | ||||||||
| No. ≥ 0.1 Å | Max (Å) | No. ≥ 1.5 deg | Max (deg) | |||||||||
| 1 | 131 | 24 | 0.005 | 0.52 ± 0.09 | 1.03 ± 0.20 | 178 ± 7 | 89 ± 4 | 89 ± 7 | 0.7 ± 0.1 | 0.11 ± 0.01 | 0 ± 0 | 0 ± 0 |
| 2 | 135 | 17 | 0.001 | 0.44 ± 0.24 | 0.96 ± 0.30 | 211 ± 10 | 118 ± 5 | 93 ± 8 | 0.2 ± 0.1 | 0.05 ± 0.01 | 0 ± 0 | 0 ± 0 |
| 3 | 130 | 25 | 0.003 | 0.47 ± 0.28 | 1.07 ± 0.45 | 204 ± 14 | 124 ± 11 | 81 ± 7 | 0.3 ± 0.1 | 0.07 ± 0.03 | 0 ± 0 | 0 ± 0 |
| 4 | 130 | 19 | 0.008 | 0.73 ± 0.25 | 1.21 ± 0.31 | 188 ± 11 | 116 ± 7 | 72 ± 6 | 0.5 ± 0.1 | 0.10 ± 0.02 | 0 ± 0 | 0 ± 0 |
| 5 | 80 | 14 | 0.0002 | 0.08 ± 0.09 | 0.62 ± 0.19 | 158 ± 10 | 86 ± 7 | 73 ± 13 | 0.1 ± 0.0 | 0.03 ± 0.00 | 0 ± 0 | 0 ± 0 |
| 6 | 115 | 24 | 0.002 | 0.56 ± 0.14 | 1.06 ± 0.28 | 210 ± 14 | 121 ± 10 | 89 ± 8 | 0.4 ± 0.1 | 0.11 ± 0.02 | 0 ± 0 | 0 ± 0 |
The bundle of 20 conformers with the lowest residual target function was used to represent the NMR structures of each analogue.
The target function is zero only if all the experimental distance and torsion angle constraints are fulfilled and all non-bonded atom pairs satisfy a check for the absence of steric overlap. The target function is proportional to the sum of the square of the difference between calculated distance and isolated constraint or van der Waals restraints and similarly isolated angular restraints are included in the target function. For the exact definition see reference.32
Meaningful NOE distance restraints may include intra-residual and sequential NOEs.32
Assignment of proton resonances, collection of structural restraints, and structure determination
The nearly complete chemical shift assignment of proton resonances (Table 2) for analogues 1 − 6 (Table 1) has been carried out using two-dimensional (2D) NMR experiments applying the standard procedure described in the Experimental Section. The N-terminal amino protons for analogues 5 and 6 were not observed due to fast exchange with the solvent. The amide proton of DPhe2/Phe for analogues 2 and 3 were observed at high field due to the carbamoylation of the N-terminus. Chemical shifts can provide insight into the structure of the peptides, which is particularly true for SRIF analogues. The ring current of the indole ring leads to a distinct shift of the Cγ protons of the sequential Lys side chain to lower frequencies when these groups are closer in space, as in a β-turn. The downfield shift of Cγ protons has been observed in all of these sst2-selective analogues, which is similar to the observations found in the non-selective analogues binding to sst2/3/5.17,22-30 This indicates the presence of a β-turn around these residues in these analogues.
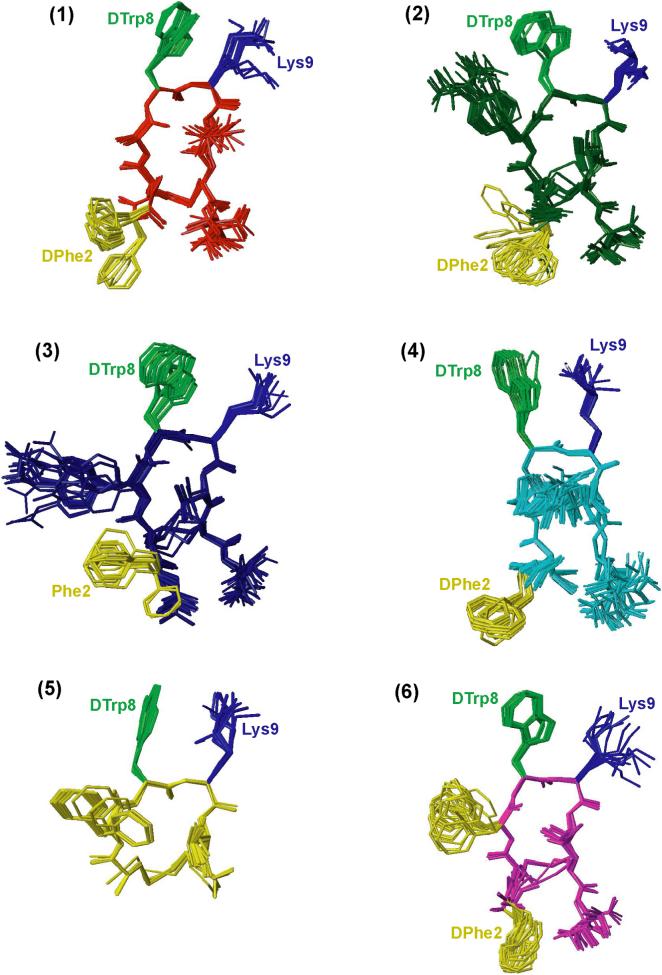
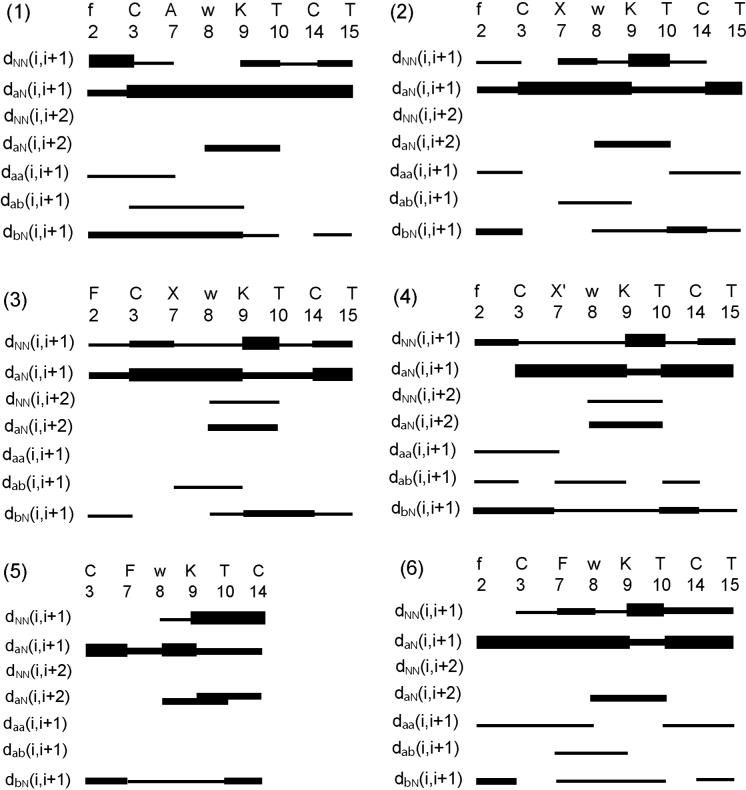
A large number of experimental NOEs is observed for all of the six analogues in the NOESY spectrum measured with a mixing time of 100 ms, leading to over 100 to 120 meaningful distance restraints per analogue and concomitantly ∼15 restraints per residue, which is a typical number for folded proteins (Table 3). These structural restraints are used as input for the structure calculation with the program CYANA31 followed by restrained energy minimization using the program DISCOVER.32 The resulting bundle of 20 conformers per analogue represents the 3D structure of each analogue in solution. For each analogue, the small residual constraint violations in the distances for the 20 refined conformers (Table 3) and the coincidence of the experimental NOEs and short interatomic distances (data not shown) indicate that the input data represent a self-consistent set, and that the restraints are well satisfied in the calculated conformers (Table 3). The deviations from ideal geometry are minimal, and similar energy values are obtained for all of the 20 conformers for each analogue. The quality of the structures determined is reflected by the small backbone RMSD values relative to the mean coordinates of ∼0.5 Å (see Table 3 and Figure 2).
Figure 2.
NMR structures of analogues 1−6. For each analogue, twenty energy-minimized conformers with the lowest target function are used to represent the 3D NMR structure. The bundle is obtained by overlapping the Cα atoms of the residues 2−9. The backbone and the side chains are displayed including the disulfide bridge. The following color code is used: red (1) H-DPhe-c[Cys-Ala-DTrp-Lys-Thr-Cys]-Thr-NH2, dark green (2) H2NCO-DPhe-c[Cys-Aph(CONH2)-DTrp-Lys-Thr-Cys]-Thr-NH2, navy blue (3) H2NCO-Phe-c[Cys-Aph(CONH2)-DTrp-Lys-Thr-Cys]-Thr-NH2, cyan (4) H2NCO-DPhe-c[Cys-Aph(CONHOCH3)-DTrp-Lys-Thr-Cys]-Thr-NH2, yellow (5) H-c[Cys-Phe-DTrp-Lys-Thr-Cys]-OH, magenta (6) H-DPhe-c[Cys-Phe-DTrp-Lys-Thr-Cys]-Thr-NH2. The side chains that are involved in sst2-binding are highlighted; these are DTrp at position 8 in light green, Lys at position 9 in blue and DPhe or Phe at position 2 in yellow.
Three-dimensional structure of H-DPhe2- c[Cys3-Ala7-DTrp8-Lys9-Thr10-Cys14]-Thr-NH2(1)
Analogue 1 binds to receptor 2 with high affinity (IC50 < 10 nM) and has an Ala at position 7. The quality of the structure is reflected by the small RMSD (Table 3), which can also be visually depicted from Figure 2 showing the bundle of 20 conformers representing the 3D structure. From the backbone torsion angles (Table 4), it can be seen that the backbone contains a β-turn of type-II’ around DTrp8-Lys9. The β-turn is supported by the strong sequential dαN(i,i+1) NOEs and medium-range dαN(i,i+2) NOE (Figure 1) between DTrp8 and Thr10, as well as the hydrogen bond between Thr10NH-O’Ala7 in all the 20 structures. The low temperature coefficient observed for the amide proton of Thr10 (−1.1 ppb/K) confirms the presence of the hydrogen bond.33 From the torsion angles listed in Table 4, it can be seen that the side chain of DTrp8 is in the trans rotamer, Lys9 is in the gauche+ rotamer and that of DPhe2 is in the gauche− rotamer.
Table 4.
Torsion angles ϕ, ψ and χ1 (in degrees) of the bundle of 20 energy minimized conformers.
|
Analogue |
Angle |
DPhe2 |
Cys3 |
Ala7 |
DTrp8 |
Lys9 |
Thr10 |
Cys14 |
Thr15 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ϕ | - | −33 ± 60 | −156 ± 13 | 72 ± 3 | −91 ± 0 | −140 ± 4 | −156 ± 16 | −11 ± 46 |
| ψ | 67 ± 17 | 87 ± 6 | 132 ± 3 | −146 ± 2 | 26 ± 1 | −149 ± 23 | 87 ± 8 | 121 ± 56 | |
| |
χ1 |
108 ± 6 |
132 ± 3 |
170 ± 95 |
−151 ± 3 |
−128 ± 2 |
−22 ± 86 |
176 ± 3 |
173 ± 54 |
|
2 |
|
|
|
Aph(CONH2)7 |
|
|
|
|
|
| ϕ | 121 ± 45 | −84 ± 35 | −178 ± 19 | 97 ± 14 | −103 ± 7 | −74 ± 5 | 87 ± 2 | −19 ± 37 | |
| ψ | 172 ± 9 | 117 ± 16 | 88 ± 9 | −113 ± 14 | 2 ± 6 | −137 ± 8 | 81 ± 8 | 110 ± 76 | |
| |
χ1 |
115 ± 32 |
−121 ± 40 |
−175 ± 14 |
150 ± 6 |
−83 ± 7 |
−43 ± 7 |
−143 ± 24 |
−163 ± 58 |
|
3 |
|
Phe2 |
|
Aph(CONH2)7 |
|
|
|
|
|
| ϕ | −149 ± 9 | 174 ± 38 | 179 ± 22 | 95 ± 22 | −103 ± 12 | −63 ± 5 | 83 ± 28 | −109 ± 59 | |
| ψ | −145 ± 51 | 110 ± 11 | 98 ± 20 | −108 ± 7 | −8 ± 1 | −140 ± 5 | 78 ± 8 | 48 ± 87 | |
| |
χ1 |
−132 ± 30 |
−144 ± 24 |
−126 ± 18 |
−171 ± 6 |
−105 ± 1 |
−39 ± 2 |
−110 ± 34 |
−129 ± 76 |
|
4 |
|
DPhe2 |
|
Aph(CONHOCH3)7 |
|
|
|
|
|
| ϕ | 175 ± 6 | 43 ± 2 | −111 ± 8 | 44 ± 5 | −141 ± 4 | −143 ± 10 | 167 ± 71 | −83 ± 83 | |
| ψ | 41 ± 4 | 53 ± 5 | 59 ± 5 | −104 ± 3 | 45 ± 8 | 165 ± 52 | 78 ± 14 | 105 ± 78 | |
| |
χ1 |
98 ± 6 |
−76 ± 34 |
−177 ± 5 |
160 ± 5 |
−45 ± 8 |
−52 ± 7 |
−155 ± 13 |
118 ± 49 |
|
5 |
|
|
|
Phe7 |
|
|
|
|
|
| ϕ | − | −154 ± 8 | 156 ± 3 | −93 ± 3 | −93 ± 3 | −54 ± 5 | |||
| ψ | 168 ± 2 | 23 ± 6 | −128 ± 3 | 12 ± 0 | 2 ± 4 | − | |||
| |
χ1 |
|
−28 ± 3 |
160 ± 38 |
166 ± 1 |
−116 ± 0 |
−62 ± 8 |
−156 ± 4 |
|
|
6 |
|
|
|
Phe7 |
|
|
|
|
|
| ϕ | − | −71 ± 28 | −173 ± 11 | 102 ± 2 | −93 ± 3 | −104 ± 5 | −168 ± 5 | −89 ± 64 | |
| ψ | −160 ± 0 | −153 ± 66 | 98 ± 6 | −121 ± 0 | 1 ± 3 | 45 ± 5 | 81 ± 3 | 98 ± 70 | |
| χ1 | 32 ± 0 | −117 ± 5 | −150 ± 29 | −168 ± 1 | −126 ± 4 | 140 ± 8 | −148 ± 12 | 175 ± 4 |
Figure 1.
Survey of characteristic NOEs used in CYANA for structure calculation for analogues 1-6. Thin, medium and thick bars represent weak (4.5 to 6 Å), medium (3 to 4.5 Å) and strong (< 3 Å) NOEs observed in the NOESY spectrum. The medium-range connectivities dNN(i,i+2), dαN(i,i+2), and dβN(i,i+2) are shown by lines starting and ending at the positions of the residues related by the NOE. Residues designated with X and X’ correspond to the amino acid Aph(CONH2) and Aph(CONHOCH3), respectively.
Three-dimensional structure of H2N-CO-DPhe2-c[Cys3-Aph(CONH2)7-DTrp8-Lys9-Thr10-Cys14]-Thr-NH2 (2)
Analogue 2 differs from analogue 1 at position 7 by the Aph(CONH2) group as well as the N-terminal carbamoylation (Table 1). The backbone torsion angles (Table 4) indicate a β-turn of type-II’ conformation around DTrp8-Lys9, which is supported by the medium range dαN(i,i+2) NOE between DTrp8-Thr10 (Figure 1). The turn structure is further stabilized by the observed hydrogen bond between Thr10NH-O’Aph7 in most of the structures. The presence of the hydrogen bond is further confirmed by the unshifted amide proton resonance of Thr10 when the temperature was raised from 298K to 313K. The long range medium NOE observed between DPhe2(NH) and Thr10(HN) as well as the strong αH NOE observed between the cysteins stabilize the structure. The side chain of DPhe2 is in the gauche+ rotamer, Aph7 and DTrp8 are in the trans rotamer and that of Lys9 is in the gauche− rotamer (Table 4).
Three-dimensional structure of H2N-CO-Phe2-c[Cys3-Aph(CONH2)7-DTrp8-Lys9-Thr10-Cys14]-Thr-NH2(3)
Analogue 3 binds selectively with high affinity to the sst2 receptor and is different from analogue 2, with a Phe at position 2. From the backbone torsion angles (Table 4), it can be seen that the backbone contains a β-turn of type-II’ around DTrp8-Lys9. The β-turn is supported by the medium-range dαN(i,i+2) NOE and the weak dNN(i,i+2) NOE (Figure 1) between DTrp8 and Thr10, as well as the hydrogen bond Thr10NH-O’Aph7 observed in most of the 20 structures. The low temperature coefficient observed for the amide proton of Thr10 (−0.4 ppb/K) confirms the presence of the hydrogen bond.33 In addition long range medium NOEs are observed between the amide protons of Phe2 and Thr10 and the cysteins, which stabilizes the structure. The side chains of Phe2, Aph7 and Lys9 are in the gauche+ rotamer and that of DTrp8 is in the trans rotamer (Table 4).
Three-dimensional structure of H2N-CO-DPhe2-c[Cys3-Aph(CONHOCH3)7-DTrp8-Lys9-Thr10-Cys14]-Thr-NH2(4)
Analogue 4 binds selectively with nanomolar affinity to the sst2 receptor. It differs from analogues 2 and 3 by the longer chain of Aph(CONHOCH3) at position 7, which inhibits the binding to receptors 3 and 5 completely (Table 1). The backbone torsion angles indicate a β-turn of type-II’ conformation around DTrp8-Lys9 (Table 4), which is supported by the medium-range dαN(i,i+2) NOE and the weak dNN(i,i+2) NOE observed between DTrp8 and Thr10 (Figure 1). The unshifted amide proton resonance of Thr10 at 7.63 ppm (from 298K to 313K) suggests the presence of a hydrogen bond involving this amide proton, which has been observed in all the 20 structures. The side chain of DPhe2 is in the gauche+ rotamer, Aph7 and DTrp8 are in the trans rotamer and that of Lys9 is in the gauche− rotamer (Table 4). The other long range NOEs, which stabilize the structure, are observed between the amide proton of DPhe2 and αH proton of Cys14 and the αH protons of the cysteins.
Three-dimensional structure of H-c[Cys3-Phe7-DTrp8-Lys9-Thr10-Cys14]-OH (5)
Analogue 5 is the shortest analogue, a cyclic hexapeptide containing only the core residues. Analogue 5 was synthesized to identify the role of DPhe2 in sst2 binding. Indeed, it did not bind to all the five receptors (Table 1). From the backbone torsion angles it can be seen that it has a type-II’ β-turn around DTrp8 and Lys9(Table 4) and the turn is supported by the presence of the medium range dαN(i,i+2) NOE observed between DTrp8 and Thr10 (Figure 1) as well as the hydrogen bond observed between Thr10NH-O’Phe7 in all of the 20 structures. The low temperature coefficient observed for the amide proton of Thr10 (−1.4 ppb/K) confirms the presence of the hydrogen bond.33 The side chain of Phe7 and DTrp8 are in the trans rotamer and that of Lys9 is in the gauche+ rotamer (Table 4).
Three-dimensional structure of H-DPhe2-c[Cys3-Phe7-DTrp8-Lys9-Thr10-Cys14]-Thr-NH2 (6)
Analogue 6, which is very similar to octreotide (Thr-ol is substituted by Thr-NH2), binds to the sst2/3/5 receptors with moderately high affinity (Table 1). The 3D structure of this analogue was obtained in order to compare the structure of octreotide with the other analogues under identical conditions. Melacini et al. reported that the backbone of Sandostatin (octreotide) could be in a conformational equilibrium between β-turn and helical structures in solvents different from DMSO.19,28 From the torsion angles, we observe a type-II’ β-turn conformation for the backbone, which is supported by the presence of the medium range dαN(i,i+2) NOE observed between DTrp8 and Thr10 (Figure 1) as well as the hydrogen bond observed between Thr10NH-O’Phe7 in all of the 20 structures. The unshifted amide proton resonance of Thr10 at 7.58 ppm (from 298K to 313K) confirms that this amide proton is involved in a hydrogen bond. The side chain of Phe7 and DTrp8 are in the trans rotamer and that of Lys9 is in the gauche+ rotamer (Table 4).
Discussion
We have recently proposed models of the sst1 and sst4 pharmacophores based on the 3D NMR structures of several receptor selective analogues. The sst1 and sst4 receptors have maximum sequence similarity and hence were assumed to be part of one close family, different from the family of sst2/3/5 receptors. Yet, the proposed sst1 and sst4 pharmacophores are completely distinct from each other. For example, the position and the number of aromatic rings involved in binding are different for the two ligands, suggesting that these hydrophobic residues interact with different regions of the sst1 and sst4 receptors, respectively (Figure 3D and 3E). On the other hand, most of the previously published analogues binding with high affinity to sst2 receptor were also binding to sst5 receptors with nM affinity and sometimes to sst3 receptors as well. Melacini et al., proposed a pharmacophore model for these analogues binding non-selectively to all of the three receptors (Figure 3C).19 Here, we have identified the crucial residues involved in selective sst2 binding, revealing subtle differences in the pharmacophores between the only sst2-selective analogues and the less selective sst2/3/5-selective analogues. In the sst2/3/5-selective pharmacophore found in octreotide and homologues, we identified two aromatic side chains (at position 2 and 7) in addition to the DTrp8 and Lys9 residues. Removal of the aromatic amino acid at position 2 (analogue 5) resulted in loss of binding to all of the three receptors (compare with analogue 6). This clearly confirms that residue 2 is important for binding to sst2/3/5 receptors (Table 1). On the other hand, replacement of Phe at position 7 by Ala (analogue 1) resulted in selective binding (nM) to receptor 2. Hence, it can be concluded that this aromatic ring is not crucial for sst2 binding, and is crucial for sst3 and sst5 binding, as the binding to receptors 3 and 5 decreased (Table 1). Replacement of Phe at position 7 by an amino acid having a longer side chain, such as Aph has also decreased the binding to sst3 and sst5 receptors, retaining high binding affinity to sst2 receptors.
Three-dimensional structures of the sst2-selective analogues and the pharmacophore model
Most of the bioactive analogues of SRIF reported so far have a β-turn type either of type II’ or type VI for the backbone conformation.13,15,17,18,24,34-39 The structures of all of the presented four sst2-selective analogues have also a β-turn of type-II’ (Figure 2, Table 4). In these analogues, the side chain of DTrp8 is in the trans conformer and the side chain of Lys9 is either in the gauche+ or gauche− conformer, bringing the two side chains adjacent to each other in a close proximity. The side chain of Phe or DPhe at position 2 is either in the gauche+ or gauche−conformer, far from the DTrp-Lys pair and their position is conserved in all of the four analogues studied here (Figure 3A). Hence, we propose a pharmacaphore model for sst2-selective analogues involving the three side chains, namely, the indole (DTrp) at position 8, the amino-alkyl (Lys) at position 9 and an aromatic ring at position 2 (Figure 3B). In addition, the right spatial arrangement of the indole ring, the lysine side chain and the aromatic ring of a phenylalanine is also important for sst2-selective binding. The proposed distances between the Cγ of residue 8 and Cγ of Phe2 is 12−13.5 Å; Cγ of residue 8 and Cγ of Lys 9 is 4−5 Å and Cγ of Phe2 and Cγ of Lys9 is 12.5−15 Å. Conservative replacements of these residues do not change the binding affinities and receptor selectivity evidently.
Comparison of the sst2-selective versus sst2/3/5-selective pharmacophore
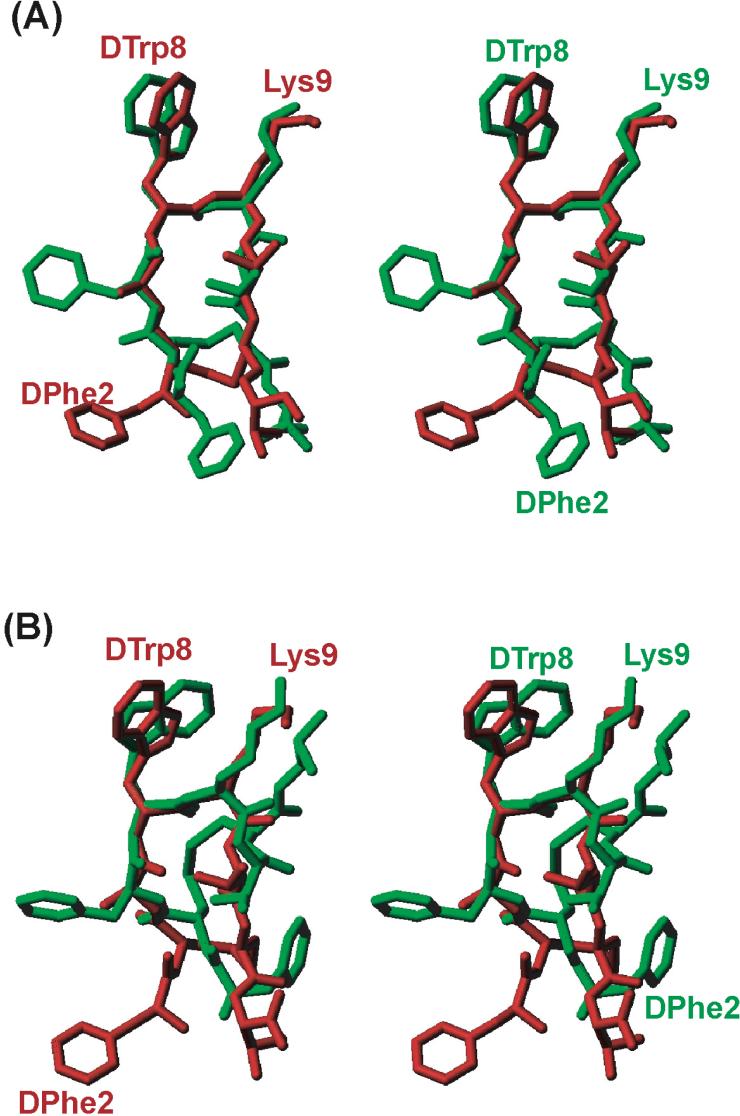
With the consensus structural motif for the sst2-selective SRIF analogues (Figures 3A and B), comparison between the sst2 and the pharmacophore for sst2/3/5-selective analogues (Figure 3C) is possible. Interestingly, Goodman and coworkers illustrated that in SRIF analogues, which bind selectively to sst2 2/3/5, the side chains of DTrp8, Lys9, Phe7 and Phe2 constitute the most essential elements necessary for binding.15,16 In their pharmacophore model, DTrp8 and Lys9 were at a close proximity of ∼5 Å, which is similar to the sst2 pharmacophore. The aromatic ring at position 7, Phe7 is farther away from DTrp8 (7 to 9 Å) and Lys9 (9 to 11 Å), and is not present in the sst2 pharmacophore. Hence Phe7 is not crucial for sst2 receptor binding. The position of DPhe2 in the sst2 pharmacophore is highly conserved whereas it varies in the sst2/3/5 pharmacophore. This difference can easily be seen from the superposition of the sst2-selective analogue 1 and the sst2/3/5-selective octreotide (Figure 4).16 They differ mostly in the position of the phenylalanine at position 2. Based on our studies reported here and the observation of Melacini et al.,19 octreotide undergoes a conformational exchange between β-turn and helical backbone conformation, it can be proposed that octreotide undergoes conformational change to fit into both the sst2 and sst5 pharmacophores. It should prefer the β-turn conformation, where the DPhe2 is close to the sst2 pharmacophore (Figure 4A) and must prefer the helical conformation to fit the sst5 pharmacophore (Figure 4B), where DPhe2 is further away from the sst2 pharmacophore (this hypothesis is further supported by unpublished results of sst5 selective analogues). This conformational flexibility is therefore necessary to explain the non-selective binding of the octreotide type of analogues to sst2/3/5 receptors simultaneously.
Figure 4.
Comparison of the 3D structure of sst2-selective (analogue 1) and sst2/3/5-selective (octreotide and analogue 6) SRIF analogues. Stereo view of the superposition of the 3D structure of the sst2-selective analogue 1 (red) with the 3D structure of the sst2/3/5-selective octreotide (green)16 (A) in β-turn conformation and (B) in helical conformation. It must be noted that DPhe2 labeled, in both of the analogues is important for selective binding, but differs in its spatial orientation relative to the DTrp8-Lys9 pair.
Comparison of the sst2-selective versus sst1- and sst4-selective pharmacophores
Since three different pharmacophores are available for the subtype-selective analogues, namely, 1, 2 and 4, the differences among these subtype-selective pharmacophores can be discussed with respect to sst2 pharmacophore (Figure 3B). The sst1 pharmacophore has two aromatic side chains important for binding and they are closer to DTrp-IAmp pair and are present on the back side of the peptide, compared to the sst2 pharmacophore, where one aromatic ring important for binding is far from DTrp-Lys pair. Also IAmp at position 9 crucial for sst1 selectivity is further away from DTrp8, compared to the other pharmacophores, where Lys at position 9 is closer to DTrp8. The sst4 pharmacophore is somewhat similar to sst2 pharmacophore in the number of interacting residues with the receptors, namely DTrp8, Lys9 and one aromatic side chain either at position 6 or 11. But the position of the aromatic side chain is closer to the DTrp-Lys pair in the sst4 pharmacophore, whereas it is farther away in the sst2 pharmacophore. Hence, these subtype-selective pharmacophores explain why the sst2-selective analogues did not bind to other receptors of SRIF, specifically to 1 and 4.
Conclusions
The 3D conformations of four cyclic SRIF octapeptide analogues with a hexapeptide core having high binding affinity and selectivity to the sst2 receptor have been presented. These studies indicate that these analogues have a β-turn of type-II’ for the backbone conformation which orients the side chains of the essentially important residues, namely indole at position 8, amino alkyl group at position 9 and an aromatic ring outside the cycle, in their respective positions for effective receptor-ligand binding. Based on this, we have proposed the SRIF binding motif for the sst2 receptor consisting of these three side chains, namely DTrp8, Lys9 and DPhe2. This binding motif differs from the binding motif for sst2/sst3/sst5-selective receptors by one less aromatic side chain at position 7 (Figure 3). Furthermore, the model proposed also explains the selective binding of the non-peptoid analogues of SRIF agonists.40-43 The pharmacophore models proposed so far enable us to understand the binding of several other SRIF analogues to somatostatin receptors and will play an important role in designing highly selective peptides as well as non-peptide ligands of SRIF.
Experimental Section
Peptide synthesis, purification and characterization
Starting Materials
4-methylbenzhydrylamine resin (MBHA) with the capacity of 0.4 mmol/g and Boc-Cys(Mob)-CM resin with a capacity of 0.3−0.4 mmol/g were used. All Boc-Nα–protected amino acids were commercially available (Chem Impex, Wood Dale, IL, Reanal Finechemical Co., Budapest, Hungary), except Boc-Aph(Fmoc) which was synthesized in our laboratory. 44
Synthesis and Purification
Peptides were synthesized by SPPS methodology following the Boc strategy and purified as we published earlier.45 The -CO-NH2 (Cbm) ureido group at the 4-amino function of Aph and at the N-terminus of the peptides was introduced simultaneously on the resin. The 4-amino function of Aph was freed with 20% piperidine in NMP and the N-terminus Boc was deprotected with 50% TFA in DCM after completion of the synthesis, then the carbamoylation was carried out with NaOCN (200 mg, 1.3 mmol) in NMP (8 mL) and glacial acetic acid (6 mL) per gram of initial resin. The ureido group -CO-NHOCH3 (Cbm-OMe) at the 4-amino function of Aph (analogue 4) was also introduced on the resin. The Nα-Boc protected resin-bound hexapeptide Boc-Aph(Fmoc)-DTrp-Lys[Z(2Cl)]-Thr(Bzl)-Cys(Mob)-Thr(Bzl)-MBHA was treated with 20% piperidine in NMP to free the 4-amino function of Aph and CH3O-NH-COOC6H4-NO2 (10 fold excess) in the presence of DIPEA in NMP was added to the resin which was shaken at RT for 4 h as we described earlier.46 After completion of the synthesis, N-terminus Boc was deprotected with 50% TFA in DCM and the carbamoylation was carried out as described above. The completed peptides were cleaved from the resin by HF containing the scavengers anisole (10% v/v) and methyl sulfide (5% v/v) for 60 min at 0 °C. The diethyl ether precipitated crude peptides were cyclized in 75% acetic acid (200 mL) by addition of iodine (10% solution in methanol) until the appearance of a stable orange color. Forty minutes later, ascorbic acid was added to quench the excess of iodine. The crude, lyophilized peptides were purified by preparative RP-HPLC.47
Characterization
The purity of the final peptides was determined by analytical RP-HPLC, CZE analysis.48 (See legend of Table 1 for details.) Each peptide showed a purity of >95% by these methods. The observed monoisotopic (M + H)+ values of each peptide corresponded with the calculated (M + H)+ values.
Sample preparation and NMR Experiments
Peptides were synthesized on solid-phase with Boc chemistry on a CS-Bio Peptide Synthesizer (Model CS536).4-6 Anhydrous HF cleaved the products from the resin support with simultaneous side chain deprotection. The diethyl ether precipitated crude peptides were cyclized in 75% acetic acid by the addition of iodine (10% solution in methanol). All peptides were purified by preparative RP-HPLC. The purity of the final products was determined by analytical RP-HPLC and CZE. They were >95% pure by both methods. The products were also characterized by mass spectroscopy; the observed (M+H)+ values coincided with the calculated (M+H)+ values.
NMR samples were prepared by dissolving 2 mg of the analogue in 0.5 mL of DMSO-d6. The 1H NMR spectra were recorded on a Bruker 700 MHz spectrometer operating at proton frequency of 700 MHz. Chemical shifts were measured using DMSO (δ = 2.49 ppm) as an internal standard. The 1D spectra were also acquired at temperatures between 298 and 318 K and were utilized to measure the temperature coefficients of the amide resonances. All the 2D spectra were acquired at 298 K. Resonance assignments of the various proton resonances have been carried out using total correlation spectroscopy (TOCSY);49,50 double-quantum filtered spectroscopy (DQF-COSY)51 and nuclear Overhauser enhancement spectroscopy (NOESY).52-54 The TOCSY experiments employed the MLEV-17 spin-locking sequence suggested by Davis and Bax,49 applied for a mixing time of 50 ms. The NOESY experiments were carried out with a mixing time of 100 ms. The TOCSY and NOESY spectra were acquired using 800 complex data points in the ω1 dimension and 1024 complex data points in the ω2 dimension with t1max = 47 ms and a t2max = 120 ms and were subsequently zero-filled to 1024 × 2048 before Fourier transformation. The DQF-COSY spectra were acquired with 1024 × 4096 data points and were zero-filled to 2048 × 4096 before Fourier transformation. The TOCSY, DQF-COSY and NOESY spectra were acquired with 8, 8 and 16 scans, respectively, with a relaxation delay of 1 s. The signal from the residual water of the solvent was suppressed using pre-saturation during the relaxation delay and during the mixing time. The TOCSY and NOESY data were multiplied by 75° shifted sine-function in both dimensions. All the spectra were processed using the software PROSA.55 The spectra were analyzed using the software X-EASY.56
Structure Determination
The chemical shift assignment of the major conformer (the population of the minor conformer was < 10%) was obtained by the standard procedure using DQF-COSY and TOCSY spectra for intra-residual assignment and the NOESY spectrum was used for the sequential assignment.57 The collection of structural restraints was based on the NOEs and vicinal 3JNHα couplings. Dihedral angle constraints were obtained from the 3JNHα couplings, which were measured from the 1D 1H NMR spectra and from the intra-residual and sequential NOEs along with the macro GRIDSEARCH in the program CYANA.31 The calibration of NOE intensities versus 1H-1H distance restraints and appropriate pseudo-atom corrections to the non-stereo specifically assigned methylene, methyl and ring protons were performed using the program CYANA. On an average, approximately 100 NOE constraints and 20 angle constraints were utilized while calculating the conformers (Table 3). A total of 100 conformers were initially generated by CYANA and a bundle containing 20 CYANA conformers with the lowest target function values were utilized for further restrained energy minimization, using the CFF91 force field58 with the energy criteria fit 0.1 kCal/mol/Å59 in the program DISCOVER with steepest decent and conjugate gradient algorithms.60 The resulting energy minimized bundle of 20 conformers was used as a basis for discussing the solution conformation of the different SRIF analogues. The structures were analyzed using the program MOLMOL.61
Acknowledgments
This work was supported in part by NIH grants DK-50124 and DK-59953. We thank Drs. D. Hoyer, T. Reisine and S. Schulz for the gift of sst1−5 transfected CHO-K1, CCL39 or HEK293 cells. We thank Dr. W. Fisher and W. Low for mass spectrometric analyses, R. Kaiser, C. Miller, and B. Waser for technical assistance in the synthesis and characterization of some peptides and biological testing. We are indebted to D. Doan for manuscript preparation. J. R. is The Dr. Frederik Paulsen Chair in Neurosciences Professor. We thank the H. and J. Weinberg Foundation, the H. N. and F. C. Berger Foundation and the Auen Foundation for financial support. R. R. is the Pioneer Fund development Chair.
Additional abbreviations
- Amp
4-aminomethylphenylalanine
- Aph
4-amino-phenylalanine
- CYANA
Combined assignment and dynamics algorithm for NMR applications
- DMSO
dimethylsulfoxide
- DQF-COSY
double quantum filtered correlation spectroscopy
- IAmp
4-(N-Isopropyl)-aminomethyl-phenylalanine
- NMR
nuclear magnetic resonance
- NOESY
nuclear Overhauser enhancement spectroscopy
- 3D
three-dimensional
- PROSA
Processing algorithms
- RMSD
root mean square deviation
- SAR
structure activity relationships
- SRIF
somatostatin
- TOCSY
total correlation spectroscopy.
Footnotes
Abbreviations: The abbreviations for the common amino acids are in accordance with the recommendations of the IUPAC-IUB Joint Commission on Biochemical Nomenclature (Eur. J. Biochem. 1984, 138:9−37). The symbols represent the L-isomer except when indicated otherwise.
References
- 1.Reichlin S. Somatostatin. N. Engl. J. Med. 1983;309:1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- 2.Reichlin S. Somatostatin (second of two parts). N. Engl. J. Med. 1983;309:1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- 3.Patel YC, Wheatley T. In vivo and in vitro plasma disappearance and metabolism of somatostatin-28 and somatostatin-14 in the rat. Endocrinology. 1983;112:220–225. doi: 10.1210/endo-112-1-220. [DOI] [PubMed] [Google Scholar]
- 4.Rivier J, Erchegyi J, Hoeger C, Miller C, Low W, Wenger S, Waser B, Schaer J-C, Reubi JC. Novel sst4-selective somatostatin (SRIF) agonists. Part I: Lead identification using a betide scan. J. Med. Chem. 2003;46:5579–5586. doi: 10.1021/jm030243c. [DOI] [PubMed] [Google Scholar]
- 5.Erchegyi J, Penke B, Simon L, Michaelson S, Wenger S, Waser B, Cescato R, Schaer J-C, Reubi JC, Rivier J. Novel sst4-selective somatostatin (SRIF) agonists. Part II: Analogues with β-methyl-3-(2-naphthyl)-alanine substitutions at position 8. J. Med. Chem. 2003;46:5587–5596. doi: 10.1021/jm0302445. [DOI] [PubMed] [Google Scholar]
- 6.Erchegyi J, Waser B, Schaer J-C, Cescato R, Brazeau JF, Rivier J, Reubi JC. Novel sst4-selective somatostatin (SRIF) agonists. Part III: Analogues amenable to radiolabeling. J. Med. Chem. 2003;46:5597–5605. doi: 10.1021/jm030245x. [DOI] [PubMed] [Google Scholar]
- 7.Lewis I, Bauer W, Albert R, Chandramouli N, Pless J, Weckbecker G, Bruns C. A novel somatostatin mimic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potential. J. Med. Chem. 2003;46:2334–2344. doi: 10.1021/jm021093t. [DOI] [PubMed] [Google Scholar]
- 8.Moller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim. Biophys. Acta. 2003;1616:1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 9.Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J. Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 10.Reubi JC, Waser B, Schaer J-C, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001;28:836–846. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 11.Vale W, Rivier C, Brown M, Rivier J. Hypothalamic Peptide Hormones and Pituitary Regulation: Advances in Experimental Medicine and Biology. Plenum Press; New York: 1977. Pharmacology of TRF, LRF and somatostatin. pp. 123–156. [DOI] [PubMed] [Google Scholar]
- 12.Vale W, Rivier J, Ling N, Brown M. Biologic and immunologic activities and applications of somatostatin analogs. Metabolism. 1978;27:1391–1401. doi: 10.1016/0026-0495(78)90081-1. [DOI] [PubMed] [Google Scholar]
- 13.Veber DF, Freidinger RM, Perlow DS, Paleveda WJ, Jr., Holly FW, Strachan RG, Nutt RF, Arison BH, Homnick C, Randall WC, Glitzer MS, Saperstein R, Hirschmann R. A potent cyclic hexapeptide analogue of somatostatin. Nature (Lond) 1981;292:55–58. doi: 10.1038/292055a0. [DOI] [PubMed] [Google Scholar]
- 14.Pohl E, Heine A, Sheldrick GM, Dauter Z, Wilson KS, Kallen J, Huber W, Pfaffli PJ. Structure of octreotide, a somatostatin analogue. Acta Crystallogr. 1995;51:48–59. doi: 10.1107/S0907444994006104. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, He Y, Raynor K, Tallent M, Reisine T, Goodman M. Side chain chiral methylated somatostatin analog synthesis and conformational analysis. J. Am. Chem. Soc. 1992;114:9390–9401. [Google Scholar]
- 16.Melacini G, Zhu Q, Osapay G, Goodman M. A refined model for the somatostatin pharmacophore: Conformational analysis of lanthionine-sandostatin analogs. J. Med. Chem. 1997;40:2252–2258. doi: 10.1021/jm960851a. [DOI] [PubMed] [Google Scholar]
- 17.Mattern R-H, Tran T-A, Goodman M. Conformational analyses by 1H NMR and computer simulations of cyclic hexapeptides related to somatostatin containing acidic and basic peptoid residues. J. Pept. Res. 1999;53:146–160. doi: 10.1034/j.1399-3011.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Mattern RH, Tran TA, Goodman M. Conformational analyses of cyclic hexapeptide analogs of somatostatin containing arylalkyl peptoid and naphthylalanine residues. J. Pept. Sci. 1999;5:161–175. doi: 10.1002/(SICI)1099-1387(199904)5:4<161::AID-PSC177>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Melacini G, Zhu Q, Goodman M. Multiconformational NMR analysis of sandostatin (octreotide): Equilibrium between β-sheet and partially helical structures. Biochemistry. 1997;36:1233–1241. doi: 10.1021/bi962497o. [DOI] [PubMed] [Google Scholar]
- 20.Grace CRR, Erchegyi J, Koerber SC, Reubi JC, Rivier J, Riek R. Novel sst4-selective somatostatin (SRIF) agonists. Part IV: Three-dimensional consensus structure by NMR. J. Med. Chem. 2003;46:5606–5618. doi: 10.1021/jm030246p. [DOI] [PubMed] [Google Scholar]
- 21.Erchegyi J, Kirby D, Hoeger C, Koerber SC, Low W, Waser B, Eltschinger V, Schaer J-C, Cescato R, Grace CRR, Reubi JC, Riek R, Rivier JE. Design of somatostatin (SRIF) receptor 1- and 4-selective ligands.. Peptides 2004 - Proceedings of the 3rd International and 28th European Peptide Symposium; Kenes International: Prague, Czech Republic. 2004. pp. 180–181. [Google Scholar]
- 22.Wynants C, Van Binst G, Loosli HR. SMS 201−995, a very potent analogue of somatostatin. Assignment of the 1H 500 MHz n.m.r. spectra and conformational analysis in aqueous solution. Int. J. Pept. Prot. Res. 1985;25:608–614. doi: 10.1111/j.1399-3011.1985.tb02217.x. [DOI] [PubMed] [Google Scholar]
- 23.Kessler H, Haupt A, Schudok M, Ziegler K, Frimmer M. Peptide conformations. 49(1): synthesis and structure-activity relationships of side chain modified peptides of cyclo(-d-Pro-Phe-Thr-Lys-Trp-Phe-). International Journal of Peptide and Protein Research. 1988;32:183–193. doi: 10.1111/j.1399-3011.1988.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 24.Mierke DF, Pattaroni C, Delaet N, Toy A, Goodman M, Tancredi T, Motta A, Temussi PA, Moroder L, Bovermann G, Wünsch E. Cyclic hexapeptides related to somatostatin. Int. J. Pept. Prot. Res. 1990;36:418–432. doi: 10.1111/j.1399-3011.1990.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 25.He Y-B, Huang Z, Raynor K, Reisine T, Goodman M. Syntheses and conformations of somatostatin-related cyclic hexapeptides incorporating specific alpha and beta-methylated residues. J. Am. Chem. Soc. 1993;115:8066–8072. [Google Scholar]
- 26.Jaspers H, Horváth A, Mezö I, Kéri G, Van Binst G. Conformational study of a series of somatostatin analogues with antitumor and/or GH inhibitory activity. Int. J. Pept. Prot. Res. 1994;43:271–276. doi: 10.1111/j.1399-3011.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 27.Gilon C, Huenges M, Mathä B, Gellerman G, Hornik V, Afargan M, Amitay O, Ziv O, Feller E, Gamliel A, Shohat D, Wanger M, Arad O, Kessler H. A backbone-cyclic, receptor 5-selective somatostatin analogue: Synthesis, bioactivity, and nuclear magnetic resonance conformational analysis. J. Med. Chem. 1998;41:919–929. doi: 10.1021/jm970633x. [DOI] [PubMed] [Google Scholar]
- 28.Mattern R-H, Zhang L, Rueter JK, Goodman M. Conformational analyses of sandostatin analogs containing stereochemical changes in positions 6 or 8. Biopolymers. 2000;53:506–522. doi: 10.1002/(SICI)1097-0282(200005)53:6<506::AID-BIP7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Cheng RP, Suich DJ, Cheng H, Roder H, DeGrado WF. Template-constrained somatostatin analogues: a biphenyl linker induces a type-V′ turn. J. Am. Chem. Soc. 2001;123:12710–12711. doi: 10.1021/ja0116932. [DOI] [PubMed] [Google Scholar]
- 30.Jiang S, Gazal S, Gelerman G, Ziv O, Karpov O, Litman P, Bracha M, Afargan M, Gilon C, Goodman M. A bioactive somatostatin analog without a type II′ beta-turn: synthesis and conformational analysis in solution. J. Pept. Sci. 2001;7:521–528. doi: 10.1002/psc.348. [DOI] [PubMed] [Google Scholar]
- 31.Güntert P, Mumenthaler C, Wüthrich K. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 1997;273:283–298. doi: 10.1006/jmbi.1997.1284. [DOI] [PubMed] [Google Scholar]
- 32.Hagler AT, Dauber P, Osguthorpe DJ, Hempel JC. Dynamics and conformational energetics of a peptide hormone: vasopressin. Science. 1985;227:1309–1315. doi: 10.1126/science.3975616. [DOI] [PubMed] [Google Scholar]
- 33.Andersen NH, Neidigh JW, Harris SM, Lee GM, Liu Z, Tong H. Extracting information from the temperature gradients of polypeptide NH chemical shifts. 1. The importance of conformational averaging. J. Am. Chem. Soc. 1997;119:8547–8561. [Google Scholar]
- 34.Arison BH, Hirschmann R, Veber DF. Inferences about the conformation of somatostatin at a biologic receptor based on NMR studies. Biorg. Chem. 1978;7 [Google Scholar]
- 35.Kessler H, Griesinger C, Lautz J, Muller A, van Gunsteren WF, Berendsen HJC. Conformational dynamics detected by nuclear magnetic resonance NOE values and J coupling constants. J. Am. Chem. Soc. 1988;110:3393–3396. [Google Scholar]
- 36.Huang A, Pröbstl A, Spencer JR, Yamazaki T, Goodman M. Cyclic hexapeptide analogs of somatostatin containing bridge modifications. Int. J. Pep. Prot. Res. 1993;42:352–365. doi: 10.1111/j.1399-3011.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 37.Mattern R-H, Tran T-A, Goodman M. Conformational analyses of somatostatin-related cyclic hexapeptides containing peptoid residues. J. Med. Chem. 1998;41:2686–2692. doi: 10.1021/jm970392t. [DOI] [PubMed] [Google Scholar]
- 38.Falb E, Salitra Y, Yechezkel T, Bracha M, Litman P, Olender R, Rosenfeld R, Senderowitz H, Jiang S, Goodman M. A bicyclic and hsst2 selective somatostatin analogue: design, synthesis, conformational analysis and binding. Bioorg. Med. Chem. 2001;9:3255–3264. doi: 10.1016/s0968-0896(01)00234-6. [DOI] [PubMed] [Google Scholar]
- 39.Veber DF. Design and discovery in the development of peptide analogs.. Twelfth American Peptide Symposium; Peptides: Chemistry and Biology: Cambridge, Mass. June 16−21, 1991.1991. pp. 1–14. [Google Scholar]
- 40.Ankersen M, Crider M, Liu S, Ho B, Andersen HS, Stidsen C. Discovery of a novel non-peptide somatostatin agonist with SST4 selectivity. J. Am. Chem. Soc. 1998;120:1368–1373. [Google Scholar]
- 41.Liu S, Tang C, Ho B, Ankersen M, Stidsen CE, Crider AM. Nonpeptide somatostatin agonists with sst4 selectivity: Synthesis and structure-activity relationships of thioureas. J. Med. Chem. 1998;41:4693–4705. doi: 10.1021/jm980118e. [DOI] [PubMed] [Google Scholar]
- 42.Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen D-M, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai S-J, Blake A, Chan WWS, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT, Schaeffer JM. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282:737–740. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- 43.Prasad V, Birzin ET, McVaugh CT, Van Rijn RD, Rohrer SP, Chicchi G, Underwood DJ, Thornton ER, Smith AB, 3rd, Hirschmann R. Effects of heterocyclic aromatic substituents on binding affinities at two distinct sites of somatostatin receptors. Correlation with the electrostatic potential of the substituents. J. Med. Chem. 2003;46:1858–1869. doi: 10.1021/jm0205088. [DOI] [PubMed] [Google Scholar]
- 44.Theobald P, Porter J, Rivier C, Corrigan A, Perrin M, Vale W, Rivier J. Novel gonadotropin releasing hormone antagonist: Peptides incorporating modified Nω-cyanoguanidino moieties. J. Med. Chem. 1991;34:2395–2402. doi: 10.1021/jm00112a013. [DOI] [PubMed] [Google Scholar]
- 45.Reubi JC, Schaer J-C, Wenger S, Hoeger C, Erchegyi J, Waser B, Rivier J. SST3-selective potent peptidic somatostatin receptor antagonists. Proc. Natl. Acad. Sci. USA. 2000;97:13973–13978. doi: 10.1073/pnas.250483897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samant MP, Grace CRR, Hong DJ, Croston G, Riek R, Rivier C, Rivier J. Novel analogues of degarelix incorporating hydroxy-, methoxy- and pegylated- urea moieties at residues 3, 5, 6 and the N-terminus. Part III. 2006. [DOI] [PMC free article] [PubMed]
- 47.Miller C, Rivier J. Peptide chemistry: Development of high-performance liquid chromatography and capillary zone electrophoresis. Biopolymers Pept. Sci. 1996;40:265–317. doi: 10.1002/(SICI)1097-0282(1996)40:3%3C265::AID-BIP2%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 48.Miller C, Rivier J. Analysis of synthetic peptides by capillary zone electrophoresis in organic/aqueous buffers. J. Pept. Res. 1998;51:444–451. doi: 10.1111/j.1399-3011.1998.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 49.Davis DG, Bax A. Assignment of complex 1H NMR spectra via two-dimensional homonuclear Hartmann-Hahn spectroscopy. J. Am. Chem. Soc. 1985;107:2820–2821. [Google Scholar]
- 50.Braunschweiler L, Ernst RR. Coherence transfer by isotropic mixing: Application to proton correlation spectroscopy. J. Magn. Reson. 1983;53:521–528. [Google Scholar]
- 51.Rance M, Sorensen OW, Bodenhausen B, Wagner G, Ernst RR, Wüthrich K. Improved spectral resolution in COSY1H NMR spectra of proteins via double quantum filtering. Biochem. Biophys. Res. Commun. 1983;117:479–485. doi: 10.1016/0006-291x(83)91225-1. [DOI] [PubMed] [Google Scholar]
- 52.Kumar A, Wagner G, Ernst RR, Wüthrich K. Buildup rates of the nuclear Overhauser effect measured by two-dimensional proton magnetic resonance spectroscopy: Implications for studies of protein conformation. J. Am. Chem. Soc. 1981;103:3654–3658. [Google Scholar]
- 53.Macura S, Ernst RR. Elucidation of cross-relaxation in liquids by two-dimensional NMR spectroscopy. Mol. Phys. 1980;41:95–117. [Google Scholar]
- 54.Macura S, Huang Y, Suter D, Ernst RR. Two-dimensional chemical exchange and cross-relaxation spectroscopy of coupled nuclear spins. J. Magn. Reson. 1981;43:259–281. [Google Scholar]
- 55.Güntert P, Dotsch V, Wider G, Wüthrich K. Processing of multi-dimensional NMR data with the new software PROSA. J. Biolmol. NMR. 1992;2:619–629. [Google Scholar]
- 56.Eccles C, Güntert P, Billeter M, Wüthrich K. Efficient analysis of protein 2D NMR spectra using the software package EASY. J. Biomol. NMR. 1991;1:111–130. doi: 10.1007/BF01877224. [DOI] [PubMed] [Google Scholar]
- 57.Wüthrich K. NMR of Proteins and Nucleic Acids. J. Wiley & Sons; New York: 1986. [Google Scholar]
- 58.Maple JR, Thacher TS, Dinur U, Hagler AT. Biosym force field research results in new techniques for the extraction of inter- and intramolecular forces. Chem. Design Auto. News. 1990;5:5–10. [Google Scholar]
- 59.Koerber SC, Rizo J, Struthers RS, Rivier JE. Consensus bioactive conformation of cyclic GnRH antagonists defined by NMR and molecular modeling. J. Med. Chem. 2000;43:819–828. doi: 10.1021/jm990118u. [DOI] [PubMed] [Google Scholar]
- 60.Hagler AT. The Peptides: Analysis, Synthesis, Biology. Academic Press; Orlando, FL: 1985. Theoretical simulation of conformation, energetics and dynamics of peptides. pp. 213–299. [Google Scholar]
- 61.Koradi R, Billeter M. MOLMOL: a program for display and analysis of macromolecular structures. PDB Newsletter. 1998;84:5–7. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 62.Grace CRR, Durrer L, Koerber SC, Erchegyi J, Reubi JC, Rivier JE, Riek R. Somatostatin receptor 1 selective analogues: 4. Three-dimensional consensus structure by NMR. J. Med. Chem. 2005;48:523–533. doi: 10.1021/jm049518u. [DOI] [PubMed] [Google Scholar]