Abstract
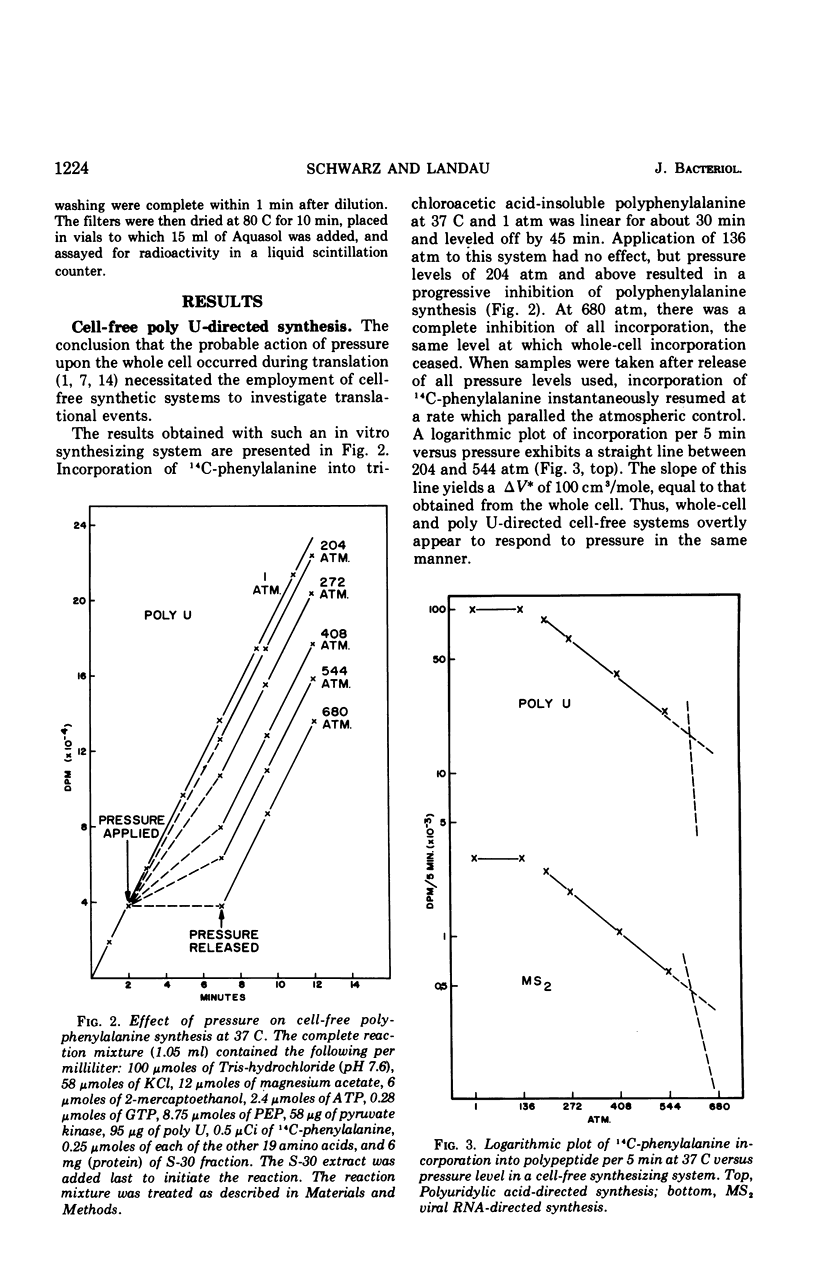
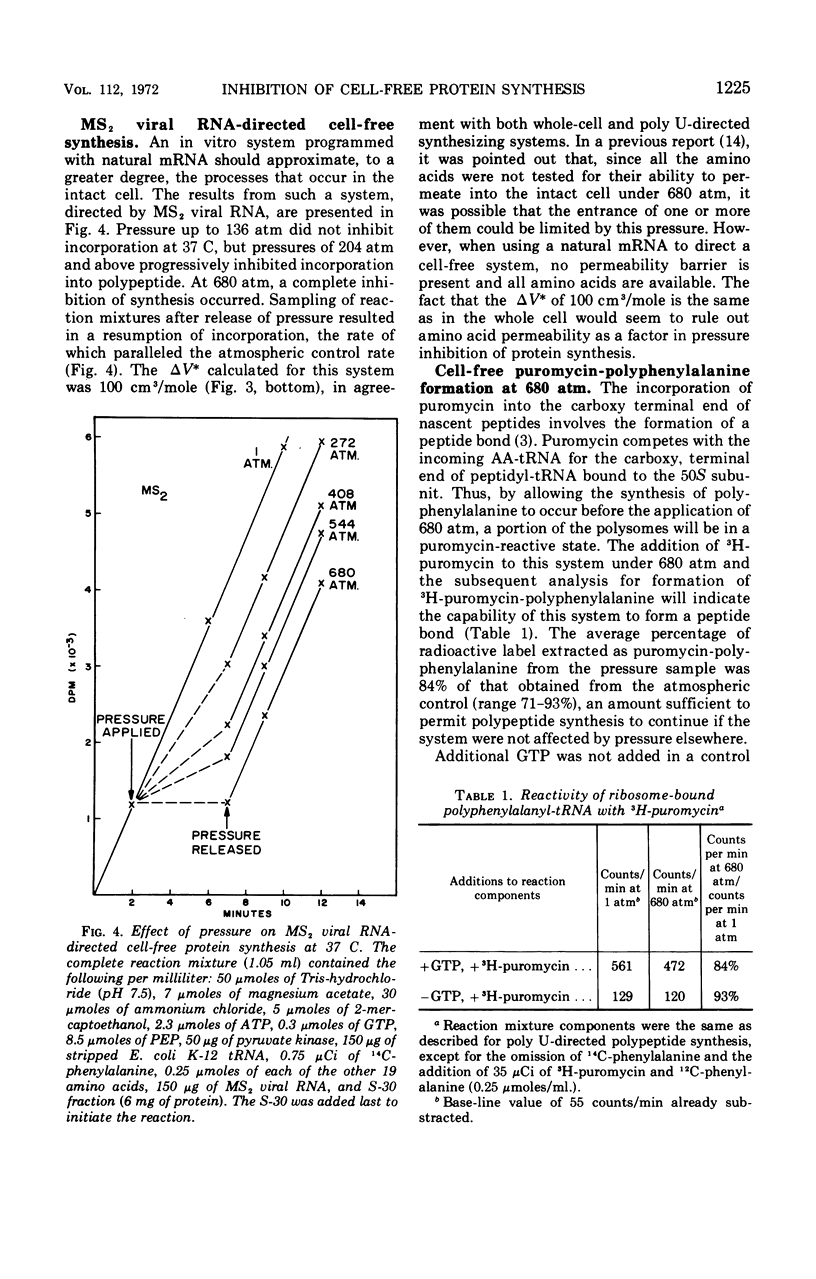
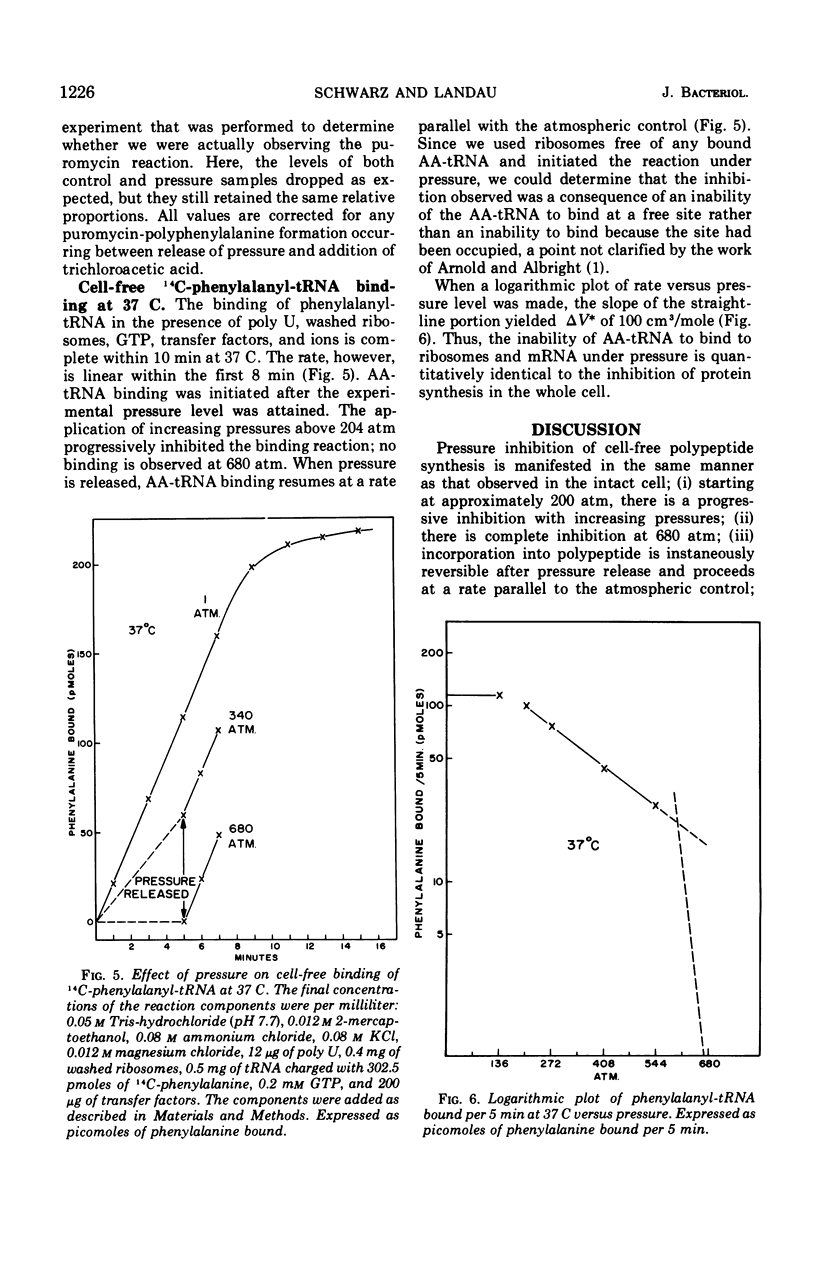
Pressure inhibition of cell-free polypeptide synthesis is manifested in the same manner as that observed in the intact cell: (i) starting at approximately 200 atm, there is a progressive inhibition with increasing pressures; (ii) there is complete inhibition at 680 atm; (iii) incorporation into polypeptide is instantaneously reversible after pressure release and proceeds at a rate parallel to an atmospheric control; and (iv) the volume change of activation (ΔV*) is 100 cm3/mole. Peptide bond formation per se can occur at a pressure level which is totally inhibitory to polypeptide synthesis. The one investigated step in translation that is inhibited in an identical manner is the binding of aminoacyl-transfer ribonucleic acid (AA-tRNA) to the ribosome-messenger RNA (mRNA) complex. The volume change of activation (ΔV*) calculated for the binding reaction is also 100 cm3/mole. Thus, the inability of AA-tRNA to bind to ribosomes and mRNA under pressure, possibly in conjunction with translocation, appears to be responsible for the observed inhibition of the translational mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. M., Albright L. J. Hydrostatic pressure effects on the translation stages of protein synthesis in a cell-free system from Escherichia coli. Biochim Biophys Acta. 1971 May 13;238(2):347–354. doi: 10.1016/0005-2787(71)90103-1. [DOI] [PubMed] [Google Scholar]
- Changchien L. M., Aronson J. N. Spermidine requirement for Bacillus thuringiensis ribosomes in cell-free phenylalanine incorporation. J Bacteriol. 1970 Sep;103(3):734–740. doi: 10.1128/jb.103.3.734-740.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E. Reaction of ribosome-bound peptidyl transfer ribonucleic acid with aminoacyl transfer ribonucleic acid or puromycin. J Biol Chem. 1967 Dec 10;242(23):5564–5571. [PubMed] [Google Scholar]
- Infante A. A., Baierlein R. Pressure-induced dissociation of sedimenting ribosomes: effect on sedimentation patterns. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1780–1785. doi: 10.1073/pnas.68.8.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. A., Graves P. N. Stability of free ribosomes, derived ribosomes and polysomes of the sea urchin. Biochim Biophys Acta. 1971 Aug 12;246(1):100–110. doi: 10.1016/0005-2787(71)90075-x. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Krauss M. Dissociation of ribosomes induced by centrifugation: evidence for doubting conformational changes in ribosomes. Biochim Biophys Acta. 1971 Aug 12;246(1):81–99. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landau J. V. Induction, transcription and translation in Escherichia coli: a hydrostatic pressure study. Biochim Biophys Acta. 1967 Dec 19;149(2):506–512. doi: 10.1016/0005-2787(67)90178-5. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Rolleston F. S., Low R. B., Wool I. G. Dissociation and reassociation of skeletal muscle ribosomes. J Mol Biol. 1969 Jul 14;43(1):135–149. doi: 10.1016/0022-2836(69)90084-9. [DOI] [PubMed] [Google Scholar]
- Ravel J. M. Demonstration of a guanosine triphosphate-dependent enzymatic binding of aminoacyl-ribonucleic acid to Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1811–1816. doi: 10.1073/pnas.57.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Landau J. V. Hydrostatic pressure effects on Escherichia coli: site of inhibition of protein synthesis. J Bacteriol. 1972 Feb;109(2):945–948. doi: 10.1128/jb.109.2.945-948.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D. Rapid exchange of subunits between free ribosomes in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2453–2457. doi: 10.1073/pnas.68.10.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson J., Sopori M. L., Gupta S. L., Lengyel P. Apparent changes in ribosome conformation during protein synthesis. Centrifugation at high speed distorts initiation, pretranslocaton, and posttranslocation complexes to a different extent. Biochemistry. 1972 Apr 11;11(8):1377–1382. doi: 10.1021/bi00758a008. [DOI] [PubMed] [Google Scholar]


