Abstract
Myocardial infarction is the leading cause of mortality in Western societies with annual expenditures of $431.8 billion spent on coronary artery disease in man. Therapeutics to combat infarction from myocardial injury, based on studies of ischemic preconditioning (IPC), are currently in progress. Hence, this review provides an update on IPC, including general and molecular mechanisms responsible for IPC and the effects of IPC in models of aging or disease. A summary of therapeutics shown to possess efficacy in preclinical and clinical trials and future directions of studies regarding cardiac IPC are also discussed.
Keywords: ischemia, reperfusion, infarct size, ischemic preconditioning, ischemic threshold, opioids, adenosine, bradykinin, dopamine, somatostatin, G protein, caveolin, COX, HETE, EET, LOX, JAK, STAT, PKC, PI3k, PTEN, glycogen synthase kinase, GSK, Bcl-2, p53, MDM2, MAPK, connexin 43, KATP, MPTP, reactive oxygen species
Introduction
Cardiovascular disease is the leading cause of death in Western societies. In 2007, the predicted United States incidence of myocardial infarction is 800,000 individuals with the estimated cost attributed to cardiovascular disease at $431.8 billion[1]. Therefore, there is continued interest in developing therapeutics to combat human injury sustained from a myocardial infarction.
Since the discovery of ischemic preconditioning (IPC)G by Murry, Jennings and Reimer in 1986[2], an ongoing investigation into the mechanism responsible for IPC has resulted in over 2,000 original published manuscripts regarding cardiac IPC, with the general mechanisms[3] now partially understood. These findings have formulated the role for endogenous triggers[3,4], signaling cascades[3,4] and the mitochondria[5] in regulating the IPC stimulus.
Hence, this review concisely summarizes the mechanisms involved in IPC-induced myocardial salvage, by concentrating on recent findings and referencing reviews on the subtopics discussed by others regarding IPC-induced cardioprotection.
The General IPC Mechanism([3]-for review)
Acute IPC-induced cardioprotection occurs in all species tested, including mouse, rat, rabbit, feline, canine, sheep, baboon and human[3]. Efficacy of the IPC stimulus is assessed by various techniques, including infarct size, cardiac contractility, incidence of arrhythmias, the electrocardiogram and biochemical assays, such as troponin or creatine kinase release. The gold standard to quantify myocardial recovery in pre-clinical studies is the measurement of infarct size reduction.
Although initial studies using canine and rabbit models suggest that IPC preserves the myocardium equally with a single 5 minute cycle in comparison to additional sequential 5 minute cycles, rat and porcine models suggest that a correlation exists between the number of IPC cycles and infarct size reduction[3]. Pre-clinical studies also suggest that the duration of the ischemia and reperfusion cycles, the reperfusion time between the acute IPC stimulus and index ischemia, and the length of index ischemia after acute IPC all contribute to the efficacy of the IPC cardioprotective stimulus.
In rabbits, the IPC stimulus duration requires at least one cycle of IPC with the ischemia lasting longer than 2 minutes to be effective. The length of reperfusion in the IPC cycle requires minimally between 30 seconds to 1 minute[3]. The protection afforded by acute IPC has a memory period of approximately 2 hours in anesthetized animals and 4 hours in conscious animals following the IPC stimulus[3].
The length of index ischemia following the acute IPC stimulus contributes to the efficacy of IPC in canines, with the initial report by Murry demonstrating four cycles of a 5 minute IPC stimulus failed to reduce infarct size after 3 hours of ischemia[2], while in a separate study, one cycle of IPC was ineffective after 1.5 hours[6]. Acute IPC preserves the myocardium from both ischemic and reperfusion-induced injury, with recent findings suggesting that IPC-induced cardioprotection initiates at least part of its salvage mechanism during the initial seconds and minutes of reperfusion[7]. It is unclear whether the loss of IPC-induced cardioprotection after an extended ischemic time is caused by unsalvageable myocardium from irreversible ischemia or whether the IPC stimulus is initiated too early prior to reperfusion to counteract reperfusion injury.
When the ischemia and reperfusion stimulus is initiated after index ischemia, termed ischemic post-conditioning (POC)G, the stimulus is usually as effective as IPC in reducing infarct size in most animal species except in rats, however, the duration of cycle pulses for POC (<30 seconds) is different compared to IPC. Variations of the acute IPC stimulus also salvage the myocardium, including delayed IPCG, intra-cardiac IPCG, transferred inter-cardiac IPCG and both acute and delayed remote IPCG.
Importantly, it is the mechanical stimulus that initiates the salvage pathways responsible for IPC and differentiates IPC from the stimulus of pharmacological-induced myocardial salvageG. While undergoing the IPC stimulus, the vasculature experiences large fluxes of shear stress, circumferential stretch and exchange of biomolecules. How the myocardium interprets this mechanical signal and generates a molecular cascade to initiate IPC-induced reperfusion salvage will be further discussed.
The Acute IPC Threshold
Myocytes possess an extensive network of cell surface membrane receptors whose activation by ligands contribute to and reciprocally mimic IPC-induced cardioprotection (Table 1). The current hypothesis for the trigger and receptor contribution in IPC-induced cardioprotection, named by the Downey group, is the IPC protective threshold[4],G. The triggers responsible for IPC include adenosine, bradykinin and opioids, which after binding to their respective G-protein coupled receptors, initiate a signaling cascade responsible for IPC-induced myocardial salvage. Additional trigger substances that activate receptor subtypes such as endothelin or adrenergic agonists, although found to mimic the effects of IPC, do not alter the protective threshold needed for IPC[4]. This indicates that these trigger substances are not released in large enough quantities after the IPC stimulus to contribute to myocardial salvage.
Table 1. Myocardial Triggers and Receptors Indicated In IPC-induced Myocardial SalvageA.
Plausible receptor ligands activated by the IPC stimulus
Numerous ligands are implicated as triggers of the IPC stimulus, including the more traditional agents such as adenosine, bradykinin and opioids. This table summarizes evidence for both the traditional ligand triggers and those slightly more controversial for their role in initiating the IPC stimulus by receptor and intracellular signaling cascade activation.
| Exogenous or Endogenous Trigger | Receptor Subtypes | What Are The Receptors Coupled To? | Does IPC Increase or Decrease The Production of The Endogenous Substance? | Does Genetic Alteration Of The Receptor By Transgene or Knockout Alter IPC-induced Myocardial Salvage? | Does Pharamcological Receptor Blockade Abrogate IPC-induced Myocardial Salvage? | Does Exogenous Administration of Trigger Agent Mimic IPC-induced Protection? | Reference |
|---|---|---|---|---|---|---|---|
| Adenosine | A1, A2A, A2B, A3 | G-Protein | Increase | Transgenic A1 and A3 mice improve salvage Knockout A1 and A3 mice abolish IPC salvage | Yes | Yes | [9, 41] |
| Bradykinin | B1,B2 | G-Protein | Increase | B2 knockouts block IPC-induced salvage | Yes | Yes | [9] |
| Opioids | δ,κ,μ | G-Protein | Increase | Unknown | Yes | Yes | [9] |
| Somatostatin | SSTR1-5 | G-Protein | Unknown | Unknown | No | Yes | [10] |
| Endothelin-1B | ETA, ETB | G-Protein | Decrease | Unknown | Unknown | Yes | [4,11] |
| Norepinephrine | α1, α2, β1 | G-Protein | Unknown | β2 knockout mice block IPC-induced salvage β2 transgenic overexpression in mice reduce salvage | Yes (α1 blockade) | Yes | [9] |
| Isoproterenol | β1, β2 | G-Protein | Not applicable | β2 knockout mice block IPC-induced salvage β2 transgenic overexpression in mice reduce salvage | No (β blockade) | Yes | [9] |
| β blockers | β1, β2 | G-Protein | Not applicable | β2 knockout mice block IPC-induced salvage β2 transgenic overexpression in mice reduce salvage | Not applicable | Yes | [9] |
| DopamineC | D1-D5, α1, α2, β1, β2 | G-Protein | Unknown | β2 knockout mice block IPC-induced salvage β2 transgenic overexpression in mice reduce salvage | Unknown | Yes, via α1 | [9,12] |
| Acetylcholine | M1-M5 | G-Protein | Increase | Unknown | No | Yes | [9] |
| InsulinD | IR | Tyrosine Kinase | Unknown | Unknown | Unknown | Yes | [13] |
| VEGF | Flt1, Flk | Tyrosine Kinase | Increase | Flt1 knockout mice partially abolish IPC salvage | Unknown | Unknown | [8] |
Endothelial growth factor receptor (EGFR), although involved in pharmacological-induced cardioprotection, is not involved in IPC-induced cardioprotection[23,24]
Findings regarding endothelin are controversial regarding cardioprotection and mixed findings are reported for the role regarding endothelin in IPC-induced myocardial salvage
At high doses, dopamine stimulates adrenergic receptors
Although insulin mimics IPC-induced infarct size reduction, this agent may have this effect via a mechanism that at least partially differs from IPC
However, sufficient evidence suggests that the trigger which contributes to the IPC stimulus may also activate tyrosine kinase receptors (TKR), such as FLT-1[8] (Table 1)[4,8-13,41], that are required for the IPC stimulus and perhaps contribute to IPC by altering its threshold, as well as other receptors, via receptor cross-talk/coupling[9],G. Even though a receptor is not directly activated by a ligand, it may indirectly be activated once a ligand binds to the receptor, since the receptor is coupled to the ligand receptor. In support of this hypothesis are the pre-clinical findings that demonstrate an interaction between opioids with adenosine receptors[9], adenosine with opioid receptors[9] and β-blockers with adenosine receptors[9] where the pharmacological-induced cardioprotective stimulus is abrogated by receptor blockade that is different from the exogenous trigger applied.
The Molecular Mechanisms of IPC
Upon mechanical stimuli and receptor activation by IPC, a number of cellular pathways are initiated, leading to a signaling complex that initiates mitochondrial survival components[5]. An IPC stimulus is presumed to either reduce or delay the death of myocytes by altering the myocyte initiation of either necrosisG, apoptosisG or both. Evidence would suggest that autophagyG may also contribute to reducing cellular death independently and through interactions with the apoptotic pathway in the myocardium[14]. IPC reduces necrosis at least partially by decreasing neutrophil accumulation within the area at risk [42]. The anti-inflammatory effects that reduce reperfusion injury occur by immune system cell suppression via adenosine A2A receptor activation [43]. Although a basic understanding of apoptosis and necrosis exist, further studies are needed to determine specifically how IPC contributes to alterations of necrosis, apoptosis and autophagy.
How the signal transduction occurs from the plasma membrane to the mitochondria is by multiple pathways either in series or parallel. Findings regarding the cellular components of IPC will be briefly summarized (Figure 1), in addition to the known members for each class, the common pharmacological activators or inhibitors, and the known interactions between signaling components (Table 2). All inhibitors listed have different degrees of target selectivity and efficacy, which need to be considered when interpreting results[15]. It is also difficult to discern in some studies whether the traditional recognition of proteins being upstream or downstream from other proteins in the signaling process is valid or whether protein inhibition blocks a protein-protein complex from forming. The contributions of the Na+/H+ exchangerG and heat shock proteinsG were previously reviewed[3] and are not discussed.
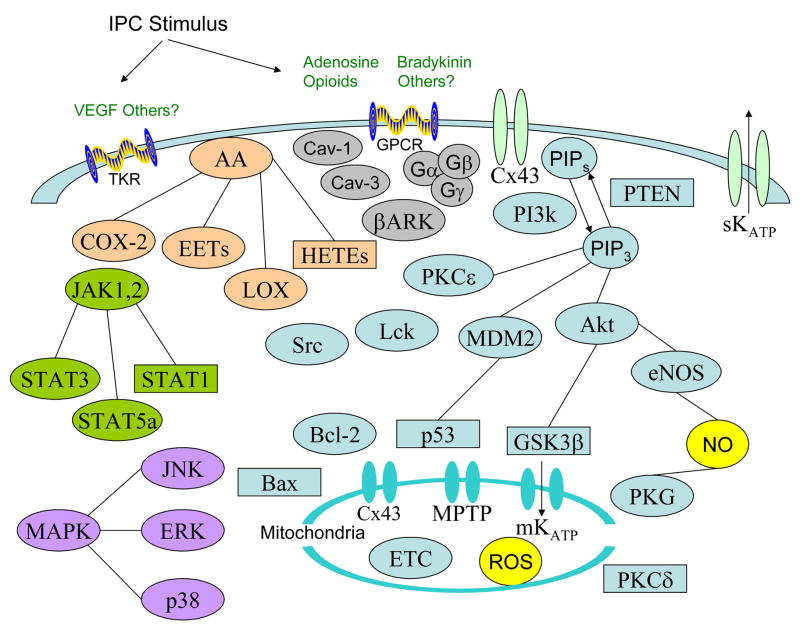
Figure 1. Signaling components activated during IPC.
Upon initiation of the IPC stimulus, endogenous triggers such as adenosine, opioids, and bradykinin are released. These endogenous agents bind to membrane receptors and when released in large enough quantities they achieve a threshold to activate intracellular signaling. Additional receptors, such as tyrosine kinase receptors (TKRs), may also be involved.
Receptor activation results in receptor internalization via caveolin and βARK. This leads to the activation of multiple cellular pathways, including arachidonic acid metabolites (AA), ion channels, such as sKATP and connexin 43 (Cx43), JAK/STAT, MAPK, tyrosine kinases such as Src and Lck, Bcl-2 family members such as Bcl-2 and Bax, and the PI3k pathway. The PI3k pathway is known to regulate PKCε, MDM2/p53, Akt, GSK3β, eNOS and the MPTP. Activation of these components leads to modulation of mitochondrial components such as Cx43, MPTP, mKATP and the electron transport chain (ETC), which modulate production of reactive oxygen species (ROS). Those components which are activated by IPC are represented by circles while the agents inactivated by IPC are in rectangles.
Table 2. Components of the IPC signal mechanism.
Protein classes and protein isoforms implicated in IPC
The proteins initiated by IPC involve different classes and isoforms, including those components reported to be present in cardiac myocytes. Furthermore, common pharmacological inhibitors and activators used in preclinical studies are listed, in addition to potential protein-protein interactions between signaling components.
| Component | Isoforms | Isoforms Present In Cardiomyocytes | Pharmacological Inhibitors | Pharmacological Activators | Component Interacts With |
|---|---|---|---|---|---|
| G proteinA | Gi | Gi | Pertussis Toxin | None | GPCR, βark, caveolin |
| Caveolin | Cav-1, Cav-2, Cav-3 | Cav-1, Cav-2, Cav-3 | NoneB | None | eNOS, GLUT4, GPCR, δOR |
| Src Tyrosine Kinase | Fyn, Yrk, Fgr, Yes, Src, Lyn, Hck, Lck, Blk | Fyn, Fgr, Yes, Src, Lyn, Lck, Blk | Lavedustin-A Genistein, MNS | None | PI3k, PKCε metalloproteinase |
| LOX | 5-LOX, 12-LOX, 15-LOX | 5-LOX, 12-LOX, 15-LOX | Baicalein, CDC, phenidone | None | PKCε |
| JAK | JAK1, JAK2, JAK3, TYK2 | JAK1, JAK2, JAK3 | AG-490, ZM39923 ZM-449829 | None | STAT, Bcl-2, PKCε, ERK |
| STAT | STAT1, STAT2,STAT3, STAT4, STAT5a, STAT5b, STAT6 | STAT1, STAT2,STAT3, STAT4, STAT5a, STAT5b, STAT6 | Stattic | None | JAK, Bcl-2, PKCε, ERK |
| PI3k | PI3kα, PI3kβ, PI3kδ/γ | PI3kα, PI3kβ, PI3kδ/γ | Wortmannin, Quercetin LY294002 | None | Akt, PKCε, GSK3β, p53, eNOS |
| PTENC | PTEN | PTEN | None | None | PI3k PIPs |
| PKC | classical: α, βI, β II, γ novel: δ, ε, η, θ, μ atypical: ζ, λ | classical: α, βI, βII, γnovel: δ, ε, η, θ, μ atypical: ζ, λ | Chelerythrine, Rottlerin,CGP53353, NPC15437 Ro-318220D, Go-6976, Go-6983, LY333531 | PMA, DAG | PI3k, ROS, mKATP, sKATP,p38, JNK, ERK, Src, Lck, MPTP, connexin 43 |
| Akt | Akt1, Akt2, Akt3 | Akt1, Akt2, Akt3 | API-2, 10-DEBC | None | PI3k, GSK3, NOS |
| ERK | ERK1, ERK2, ERK5 | ERK1, ERK2, ERK5 | PD98059E U0126E | None | PKCε |
| p38 | α, β, γ, δ | α, β, γ, δ | SB203580, JX401 | Anisomycin | HSP27, connexin 43 |
| JNK | JNK1, JNK2, JNK3 | JNK1, JNK2, JNK3 | SP600125 curcumin | Anisomycin | PKCε |
| Bcl-2 | Anti-apoptotic: Bcl-2, Bcl-XL Pro-apoptotic: Bax, Bak, Bnip3, Bnip3L, Bim, Bik, Bid, Bad, Puma, Noxa | Anti-apoptotic: Bcl-2, Bcl-XL Pro-apoptotic: Bax, Bak, Bnip3, Bnip3L, Bim, Bik, Bid, Bad, Puma, Noxa | HA14-1 | None | JAK, STAT, MPTP |
| p53 | p53, p53β, p53γ | p53F | Pifithrin-α,Pifithrin-μ | None | PI3k |
| NOS | eNOS, nNOS, iNOS | eNOS, iNOS | L-NAME, L-NIO, AMT,LNMMA, 1400W, L-NIL, 7-nitroindazole, L-canavanine | None | NO, PKG, Akt |
| PKG | PKG | PKG | KT5823 | None | mKATP |
| Connexin 43 | connexin 43 | connexin 43 | HeptanolG | None | p38, HSP90, TOM20 PKCε, GSK3β |
| GSK3 | GSK3α, GSK3β | GSK3α, GSK3β | SB216763, SB415286,kenpaullone, lithium, Indirubin3′-oxamine, NSC693868 | None | Connexin 43, MPTP mKATP, PI3k, Akt |
| MPTP | MPTP | MPTP | cyclosporin A, sanglifehrin A | atractyloside | PKC, Bcl-2, GSK3β |
| KATP | sKATP, mKATP | sKATP, mKATP | HMR-1098, HMR-1883,5-hydroxydecanoic acid, glyburide | P-1075 BMS-191095 diazoxide nicorandil | PKC,PKG, adenosine |
A number of different G proteins are present in cardiomyocytes, but this discussion is beyond the scope of this manuscript
Indirect inhibition of caveolin is achieved by pharmacological blockade of receptor endosomal internalization
PTEN is also named TEP1 and MMAC1
This inhibitor was found to also be a potent inhbiitor of GSK3[15]
These inhibitors indirectly inhibit ERK by inhibition of MEK
Whether all p53 isoforms are present in cardiac myocytes is unknown
This inhibitor is an indirect inhibitor of connexin 43 by dissolving it from membranes
1. G-protein/G-protein receptor complexes
Upon trigger-induced activation of a Gi-protein coupled receptor by endogenous agents, there is dissociation of the G-protein into Gα and Gβγ subunits. The Gβγ subunit then couples to β-arrestin, β-adrenergic receptor kinase (βARK), clathrin and the clathrin adaptor AP-2 for endosomal internalization of the receptor by caveolin. Activation of the Gβγ component of Gi proteins is a critical component of IPC, since both the pharmacological inhibition of the Gi protein by pertussis toxin and transgenic overexpression of the Gβγ sequestering peptide, βARK, reduced IPC-induced recovery of left ventricular developed pressure (LVDP) and blocked IPC-induced improvement of cardiac necrosis[16].
2. CaveolinG
Initial studies indicated that blockade of receptor endosomal internalization via bafilomycin A1 or methyl-β-cyclodextrin abolished the IPC-induced recovery of LVDP[16] and reduction of cell death[17], respectively. The IPC protective mechanism was linked to a reduction of caveolin-1 complexes with endothelial nitric oxide synthase (eNOS)[18] and increased caveolin-3 complexes with GLUT4[18] and δ-opioid receptors (δOR)[17]. The association of eNOS and GLUT4 with caveolin was also reported to be dependent upon generation of reactive oxygen species (ROS)[18].
3. Arachidonic Acid Metabolites([19]-for review)
Upon activation of membrane bound phospholipases, accumulated arachidonic acid is metabolized to products including lipoxygenases (LOX), epoxyeicosatrienoic (EET) acids, hydroxyeicosatetraenoic (HETE) acid and cyclooxygenases (COX). The accumulation of LOX or EET metabolites is beneficial and a role for 12-LOX in IPC has been established[19]. HETE accumulation is detrimental in myocardial salvage and its role in IPC is less clear. Pharmacological inhibition of 20-HETE reduces infarct size, at least partially by a parallel and perhaps via an alternative pathway to IPC[19]. Acute IPC-induced cardioprotection does not require COX-1, however, COX-2 appears to be involved[19].
4. JAK/STATG, ([20]-for review)
Traditionally, Janus activated kinases (JAKs) were identified to transduce a receptor signal via JAK activation by transphosphorylation upon receptor dimerization. The IPC stimulus acutely phosphorylates and activates JAK1 and JAK2[20] and pharmacological inhibition by the selective JAK inhibitor, AG490, abolished the acute cardioprotection achieved by IPC[20]. In turn, the JAK isoforms activate signal transducers and activators of transcription (STAT) isoforms which complex with Bcl-2 family members[20] and ERK[21]. Besides the role established for IPC-induced nuclear translocation of STATs to regulate cellular transcription[20], STATs also regulate apoptosis, with STAT3[20] and STAT5a[22] activation being anti-apoptotic while STAT1 activation is pro-apoptotic[20]. The STAT6 isoform does not appear to be involved in the IPC mechanism[22].
5. Src tyrosine kinase familyG
Seven Src tyrosine kinase family members are present in cardiomyocytes and the Lck and Src tyrosine kinases mediate IPC[23]. Administration of the Src tyrosine kinase family inhibitor lavendustin-A or the broad spectrum tyrosine kinase inhibitor genistein, prior to IPC also abrogates cardioprotection[3, 23]. IPC-induced activation of Src is perhaps mediated via a metalloproteinase dependant mechanism[24], and is dependent upon protein kinase C epsilon (PKCε)[23].
6. PI3k/PTENG
The IPC stimulus, via phosphatidylinositol-3 kinase (PI3k) activation, initiates an extensive signaling cascade, which is linked to IPC-induced PKCε translocation[25], activation of Akt (protein kinase B) and eNOS[26] pathways, and inhibition of p53[27] and glycogen synthase kinase 3β (GSK3β)[28] pathways. Pharmacological inhibition of PI3k or over-expression of a catalytically inactive mutant of PI3kγ abolish IPC-induced cardioprotection[16]. The phosphatidylinositol system is regulated by phosphatase and tensin homolog (PTEN)G, which has been proposed to antagonize PI3k signaling. PTEN is perhaps inactivated by phosphorylation via IPC[29], however, the IPC stimulus used in this study had a long ischemia and reperfusion period which suggests that a role for PTEN in IPC needs further evaluation.
7. PKCG, ([30]-for review)
Two of the more widely studied PKC isoforms, PKCε and PKCδ, appear to perform opposing functions in the myocardium, with the mechanism consisting of PKCε-induced MPTP inhibition, while PKCδ is involved in the deleterious generation of ROS[30]. The other PKC isoforms have not been extensively studied in IPC-induced cardioprotection.
The activation of PKC, specifically PKCε is PI3k dependent, since inhibition of PI3k reduced IPC-induced PKCε translocation to the myocyte particulate fraction[25].
Administration of the PKCε activator, epsilon receptor for C activated kinases (εRACK), when given during ischemia, did not improve recovery of LVDP or reduce cumulative CPK release. It appears that PKCε activation preserves the myocardium from injury only when activated prior to ischemia[30]. However, PKCδ inhibition during reperfusion was cardioprotective, suggestive that PKCδ inhibition contributes more to reperfusion salvage[30].
8. MAPKG, ([3]-for review)
The role for MAPK associated activation in cardioprotection is controversial with studies supporting and refuting a role for ERK, p38 and JNK in IPC-induced cardioprotection[3]. Once more specific isoform inhibitors of each MAPK become available, further studies will be needed for all the MAPK members.
9. Bcl-2 family membersG
The Bcl-2 family members are modified by IPC, increasing members that enhance myocardial salvage such as Bcl-2[31] and reducing members that initiate cellular death such as Bax[32]. The members of the Bcl-2 family are modulated by JAK[20] and reciprocally regulate the MPTP[5] and STAT[20] in IPC.
10. MDM2/p53
IPC-induced cardioprotection also occurs partially by the phosphorylation of MDM2 after the IPC stimulus via a PI3k-dependent means, which in turn causes p53 degradation during index ischemia and reperfusion[27]. Pifithrin α-induced inhibition of p53 reduced myocardial infarction, however, its effect was not equivalent to IPC-induced reduction of myocardial infarct size, which suggests that this pathway only partially mediates the effect of IPC[27].
11. NOSG, ([4,26]-for reviews)
Activation of Akt via PI3k leads to the production of nitric oxide (NO) synthesized by eNOS. Blockade of NO via L-NAME abolishes IPC-induced cardioprotection, with exogenous NO administration mimicking IPC-induced myocardial salvage. However, IPC-induced cardioprotection also occurs independently of endogenous NO generation, which indicates that eNOS generated NO production is not an essential requirement for the acute IPC stimulus[26]. Production of NO leads to cGMP generation by guanylyl cyclase and activation of PKG, which leads to the opening of mKATP[4]. Furthermore, IPC-induced cardioprotection in guinea-pig hearts increases the generation of NO and reduces O2- by a mKATP channel-dependent mechanism[33].
12. Connexin 43G, ([34]-for review)
Localization of connexin 43 is mainly at the sarcolemma, however, this protein also localizes to the inner mitochondrial membrane after IPC via heat shock protein 90 (HSP90) and translocase of the outer membrane 20 (TOM20)[34]. Connexin 43 knockout mice lacked IPC-induced cardioprotection. Pharmacological displacement from membranes of connexin 43 by heptanol also blocked IPC. IPC increases the duration of phosphorylation of connexin 43 compared to control, with this mechanism involving the association of p38 and PKC with connexin 43. Connexin 43 also associates with glycogen synthase kinase 3β (GSK3β) during IPC[34].
13. GSK3βG and MPTPG
Tong and colleagues previously found inhibition of GSK3β mimics the effects of IPC-induced cardioprotection, with GSK3β inactivation being PI3k dependent[35]. MPTP closure is dependent upon phosphorylation of GSK3β at Ser9 and MPTP closure is a common myocardial salvage mechanism among hypoxic preconditioning, insulin, KATP channel openers and Na+/H+ exchanger antagonists [28]. MPTP opening by atractyloside also abolishes IPC-induced cardioprotection[36]. It is unclear how GSK3β inhibition leads to MPTP closure, but it likely results by altering GSK3β complexes with MPTP components[28].
14. KATP channelsG, ([37]-for review)
Evidence suggests involvement of both the sKATP and mKATP channels in acute IPC-induced cardioprotection[37]. Pharmacological blockade of the KATP channel abolishes acute IPC-induced cardioprotection, while putative openers of the channel mimic the myocardial salvage produced by IPC[37]. The method by which IPC stimulates sKATP or mKATP channel opening is somewhat unclear, however, pre-clinical studies link sKATP channel activation to adenosine release[37] and PKC[37] with mKATP channel activation involving PKC[37] and PKG[4].
15. Reactive Oxygen Species (ROS)
ROS production in acute IPC is somewhat of a paradox, since the triggering of the IPC stimulus requires ROS, while ROS generation is deleterious when the myocardium is reperfused. Interestingly, the IPC stimulus may also reduce ROS generation at reperfusion to induce the protective stimulus, since ROS scavengers abrogate IPC-induced cardioprotection even when administered after the IPC stimulus during index ischemia[7]. The source of ROS production is perhaps mitochondrial and regulated by connexin-43, mKATP, MPTP and the electron transport chain[5].
16. Novel IPC targets
Three novel targets recently identified for IPC are listed with references, but will not be discussed (Box 1). These include TRPV-1, H11 kinase and GRP78. Additional possible novel targets for IPC have also been discovered via gene array studies (Box 1).
Disease State Abrogation of IPC-induced Salvage([38]-for review)
The IPC stimulus efficacy in disease models is regulated by the duration of the disease state for the model selected, in addition to the parameters selected for the IPC stimulus. Therefore, a decrease in IPC-induced myocardial salvage is reported in some, but not all, disease models of diabetes, hypercholesterolemia, hypertension and models of aging[38]. These areas of investigation will be important once the IPC signaling mechanism is more completely understood in naïve subjects. Baseline levels of protein expression and phosphorylation for components that regulate IPC-induced cardioprotection are reported to be altered in disease states[38].
In a diabetic model where the protection afforded by one five minute cycle of IPC is abrogated, pharmacological inhibition of GSK3 was able to still effectively salvage the myocardium[39]. Myocardial salvage by GSK3 inhibition occurs even though baseline levels of ERK, Akt and STAT3 are altered[39]. This study may suggest GSK3 or other intracellular components, rather than IPC triggers, may need to be targeted in order for myocardial salvage to be effective in disease models.
Conclusions
The strategy of IPC, which relies on mechanical intervention to protect the myocardium, may be limited clinically since accurately timed ischemia and reperfusion pulsations are required and are difficult to offer outside of a hospital procedure room or surgical suite. IPC also requires a prior knowledge of an ischemic event; hence, the technique is limited to a scheduled procedure such as non-emergent percutaneous coronary intervention or coronary artery bypass grafting.
It is unclear whether the contributions of the IPC signaling components which salvage myocardium are responsible for protection from ischemia, from reperfusion injury or from both. However, since at least part of the IPC mechanism salvages the myocardium at reperfusion, this requires further investigation in order identify therapeutic agents that may be beneficial when given both during ischemia and during reperfusion.
A subset of promising therapeutic agents designed from research associated with IPC mechanisms are presently being investigated as potential agents to improve myocardial salvage from a myocardial infarction. These agents were recently reviewed[40] and the agents are partially summarized here (Table 3). Many of the pharmacological agents identified as potential therapeutic agents need further pre-clinical establishment of the required dose, timing and side effect profiles to further establish their prerequisites for use.
Table 3. Targets and related therapies to mimic IPC-induced cardioprotection.
Targets and related therapies
| Target | Strategic Approach To Target | Expected Outcome of Intervention At Target | Who is Working On The Target | Therapies In Trial | Ref |
|---|---|---|---|---|---|
| MPTP | Pore Inhibition | Myocardial Salvage | None exclusively | Pre-clinical | [3,4,5,36] |
| GSK | GSK3β Inhibition | Myocardial Salvage | None exclusively | Pre-clinical | [25,28] |
| PKC | PKCδ Inhibition | Myocardial Salvage | KAI Pharmacauticals | Phase II Clinical Trial | [3,4,5,30] |
| HETEs | 20-HETE Inhibition | Myocardial Salvage | None exclusively | Pre-clinical | [19] |
| KATP channelA | Opening of channel | Myocardial Salvage, Angina reversal | None exclusively | Pre-clinical | [37,40] |
| Adenosine Receptor | Receptor activation | Myocardial Salvage | AMISTAD investigators | AMISTAD-1 and AMISTAD-2 trials completed | [40] |
| Opioid Receptor | Receptor activation | Myocardial Salvage | Enhance Biotech | Pre-clinical | [40] |
Clinically approved in European countries and Japan for chronic angina
Since other pharmacological agents have been investigated for stroke, tissue transplantation and reperfusion injury in other organs, it is important to pool data from all organs to determine if common mechanisms are involved in myocardial salvage. Thus, novel therapeutics with the greatest potential can be developed to reduce injury in many organs. In this regard, whether myocardial salvage has a unique mechanism or if all tissues undergo identical salvage signaling cascades is important to understand.
Although the general concepts of IPC are known, there are many questions regarding the IPC mechanism that still need to be addressed (Box 2). Studies have been limited regarding the effects of IPC in disease models. In addition it is unknown whether pharmacological agents commonly found in the clinical setting, such as anti-hypertensives, anti-diabetics and NSAIDs, when given acutely or chronically alter the efficacy of IPC-induced myocardial salvage. On the molecular level, an understanding of the protein-protein interactions which occur during IPC and index ischemia/reperfusion will be needed and the protein-protein interactions reported in non-myocardial cells will need to be investigated in the realm of cardiac IPC.
Over the last 21 years, since the discovery of cardiac IPC, the mechanism involved is progressively being elucidated. With persistent investigation into the basic science mechanisms regarding cardiac IPC and well-designed clinical trials, perhaps there will be in the not too distant future additional mechanical or pharmacological interventions to target reduction of cardiac injury during a myocardial infarction.
Box 1. Novel Targets of Interest
TRPV-1 – capsaicin sensitive channel
Zhong, B. and Wang, D.H. (2007) TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293 (3), H1791–H1798
H11 kinase – small heat shock protein
Danan, I.J. et al. (2007) Therapeutic potential of H11 kinase for the ischemic heart. Cardiovasc Drug Rev 25 (1), 14–29
GRP78 – member of the heat shock protein 70 family
Shintani-Ishida, K. et al. (2006) Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun 345 (4), 1600–1605
Gene array studies –
Onody, A. (2003) Effect of classical preconditioning on the gene expression pattern of rat hearts: a DNA microarray study. FEBS Letters 536, 35–40
Das, DK. (2006) Cardiac genomic response following preconditioning stimulus. Cardiovascular Research 70, 254–163
Box 2. Future Studies Needed
General Issues:
Can a cocktail of pharmacological inhibitors mimic the full effect of IPC?
What contribution does IPC have in salvaging myocardium at reperfusion compared to during ischemia?
What are the differences in IPC-induced cardioprotection in different genetic strains?
Why is the IPC stimulus abrogated in models of aging?
How do disease states such as hypercholesterolemia, hypertension and diabetes modify IPC-induced cardioprotection and the signaling cascade?
Does gender contribute to the efficacy of the IPC stimulus and furthermore, is the role of gender in cardioprotection dependent upon species and age?
Are there parallel or separate pathways activated in IPC and if separate pathways, do these pathways communicate with one another?
Are there additional endogenous biomolecules responsible for the IPC trigger?
Is the IPC stimulus modified in the presence of acute or chronic use of pharmacological agents such as statins, NSAIDs, anti-hypertensives and anti-diabetics?
Does ischemia/reperfusion in other organ systems have a similar mechanism of salvage?
Unresolved Questions Regarding Signaling:
Are there other sources of ROS besides mitochondrial ROS?
Is ERK activation needed for phosphorylation and inhibition of GSK3β at Thr43 during IPC?
Is there an interaction between p53 and GSK3β in IPC?
Do heat shock proteins control the balance of oxidant state by regulating O2- and NO production as shown with HSP90 regulating NO and O2- production via eNOS in non-myocardial cells?
Is JAK complexing with PI3k required to transduce the IPC signal?
Acknowledgments
This work was supported by NIH grant HL08311 and HL074314 (GJG).
Glossary of Terms
- Apoptosis
Process where cell death is programmed without the initiation of an inflammatory response
- Autophagy
A process in which macromolecules and organelles are processed and recycled by the lysosomal degradative pathway. This process is useful in maintaining cellular differentiation and homeostasis
- Bcl-2 family
Regulators of the intrinsic apoptotic pathway, this family contains both pro-apoptotic and anti-apoptotic members that all share a common homology domain, known as the Bcl-2 homology domain
- Caveolin
Localized in caveolae, which are invaginations of the sarcolemmal membrane, these biomolecules primarily function in membrane trafficking and internalization
- Connexin 43
Connexin 43 is the most abundant connexin in ventricular myocytes and is part of the structure forming cellular gap junctions and non-junctional hemichannels
- Delayed IPC
Brief timed sequences of ischemia and reperfusion that reduce the extent of myocardial injury when initiated 24–72 hours prior to index ischemia
- GSK3
GSK3 has two different isoforms and contributes to cellular events including transcription, metabolism, adhesion and apoptosis. The two isoforms appear to locoalize differently in the cell, with GSK3β, unlike GSK3α, localizing to the mitochondria
- Intra-cardiac acute IPC
Brief timed sequences of ischemia and reperfusion that reduce the extent of myocardial injury when initiated in a region prior to and separate from the area experiencing index ischemia. For example, IPC pulses administered to the left circumflex artery given prior to index ischemia and reperfusion of the left anterior descending coronary artery (LAD) improves myocardial salvage of the LAD
- IPC Protective Threshold
The existing hypothesis for the role of myocyte cell surface receptors in cardiac IPC. It is suggested that after the initiation by IPC the release of three classes of protective substances, adenosine, opioids and bradykinin, activate their respective receptors to initiate myocardial salvage. This threshold is raised by blockade of any of these three receptor classes and this blockade can be overcome by increasing the cycles of IPC to release more of the three classes of protective endogenous substances
- Ischemic preconditioning (IPC)
Brief timed sequences of ischemia and reperfusion that reduce the extent of myocardial injury when initiated just prior to index ischemia and reperfusion
- Ischemic postconditioning (POC)
Brief timed sequences of ischemia and reperfusion that reduce the extent of myocardial injury when initiated after index ischemia and prior to reperfusion
- JAK/STAT
There are 3 JAK protein members and 7 STAT member proteins in myocytes. When JAK proteins are activated, the tyrosine motifs in the cytoplasmic tail of the JAK protein are phosphorylated, which allows STAT protein binding to JAK. This activation leads to both acute cellular processes which control apoptosis and delayed cellular responses such as protein transcription
- KATP channels
KATP channels are both sarcolemmal and mitochondrial in origin, with the sarcolemmal KATP (sKATP) channel composed of the Kir6.2 and SUR2A subunits and the inner mitochondrial membrane KATP (mKATP) channel yet to be identified. The specificity of 5-HD as a mKATP channel antagonist has recently been challenged and cloning of the mKATP channel has yet to be achieved, which may question the existence of the mKATP channel
- MAPK
The family of MAPK consists of ERK, p38 and JNK. This family regulates numerous processes including proliferation, gene expression and apoptosis. Since these proteins are regulated by multiple signals and components of the signaling pathway, their specificity is partially regulated by the scaffolding protein member that they bind
- MPTP
A pore assembled at the mitochondrial membrane that is hypothesized to connect the inner mitochondrial membrane with the outer mitochondrial membrane to allow passage of biomolecules of less than 1500 daltons between the mitochondria and the cytosol. The composition of the MPTP is still under investigation, however, it is hypothesized to consist of the adenine nucleotide translocator (ANT) at the inner membrane and the voltage-dependent anion channel (VDAC) at the outer membrane
- Necrosis
Process where cellular integrity is lost, resulting in the release of cytosolic contents and an immunological/inflammatory response
- NOS
There are two known NOS isoforms which exist in cardiomyocytes, eNOS and iNOS, with the presence of nNOS in cardiomyocytes unknown, however, unlikely due to hypothesized localization in neuronal cells. The NOS isoform, eNOS, has been suggested to not be required for acute IPC to salvage myocardium, while iNOS has traditionally been found to regulate the delayed cardioprotective signal
- Pharmacological-induced Myocardial Salvage
Process where pharmacological agents given either prior to or during index ischemia and reperfusion reduce infarct size.
- PI3k/PTEN
Phosphatidylinositol-3 kinase (PI3k) generates phosphatidylinositols, phosphatidylinositols (3,4)P2 (PIP2) and phosphatidylinositols (3,4,5)P3 (PIP3) that are normally absent in unstimulated cells, however, they rapidly accumulate upon stimulation to activate downstream pathways by interacting with pleckstrin homology domains. Although most proteins only bind phosphatidylinositols weakly and non-specifically, a small subclass of proteins with pleckstrin homology domains, including PDK and Akt, show high affinity and specificity for PIP3. In turn, PTEN, a phosphatase, antagonizes PI3k signaling likely by the degradation of PIP3 and perhaps PIP2
- PKC
There are 11 different protein isoforms in this family, which regulate numerous signaling pathways. The PKC isoforms are attached to RACKs, which regulate both the localization of PKC isoforms and allow for selective signaling pathway activation
- Receptor Cross-Talk/Receptor Coupling
Receptors traditionally form complexes with other receptors in the myocardium. This coupling of receptors activates both the ligand bound receptor and the other receptor coupled to the ligand bound receptor. Due to receptor coupling, other receptors besides adenosine, bradykinin and opioid receptors may be involved in the IPC stimulus without requiring triggers for the receptor
- Remote IPC
Brief timed sequences of ischemia and reperfusion that reduce the extent of myocardial injury when initiated in a separate organ prior to myocardial index ischemia, such as the mesenteric artery of the small intestine or limb arteries supplying skeletal muscle
- Transferred Inter-cardiac IPC
A process where the coronary effluent transferred from an IPC-conditioned heart to a non-preconditioned heart causes infarct size reduction in the non-preconditioned heart when given prior to index ischemia and reperfusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Rosamond W, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, et al. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83(4):1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 4.Downey JM, et al. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007 doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 5.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 6.Gumina RJ, et al. Inhibition of the Na(+)/H(+) exchanger confers greater cardioprotection against 90 minutes of myocardial ischemia than ischemic preconditioning in dogs. Circulation. 1999;100(25):2519–2526. doi: 10.1161/01.cir.100.25.2519. discussion 2469–2572. [DOI] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, et al. Ischemic preconditioning targets the reperfusion phase. Basic Res Cardiol. 2007;102(5):445–452. doi: 10.1007/s00395-007-0656-1. [DOI] [PubMed] [Google Scholar]
- 8.Addya S, et al. Ischemic preconditioning-mediated cardioprotection is disrupted in heterozygous Flt-1 (VEGFR-1) knockout mice. J Mol Cell Cardiol. 2005;38(2):345–351. doi: 10.1016/j.yjmcc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70(2):212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Wang TL, et al. Somatostatin analogue mimics acute ischemic preconditioning in a rat model of myocardial infarction. J Cardiovasc Pharmacol. 2005;45(4):327–332. doi: 10.1097/01.fjc.0000156823.35210.21. [DOI] [PubMed] [Google Scholar]
- 11.Duda M, et al. Preconditioning protects endothelium by preventing ET-1-induced activation of NADPH oxidase and xanthine oxidase in post-ischemic heart. J Mol Cell Cardiol. 2007;42(2):400–410. doi: 10.1016/j.yjmcc.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Lazou A, et al. Dopamine mimics the cardioprotective effect of ischemic preconditioning via activation of alpha1-adrenoceptors in the isolated rat heart. Physiol Res. 2006;55(1):1–8. doi: 10.33549/physiolres.930694. [DOI] [PubMed] [Google Scholar]
- 13.Baines CP, et al. Myocardial protection by insulin is dependent on phospatidylinositol 3-kinase but not protein kinase C or KATP channels in the isolated rabbit heart. Basic Res Cardiol. 1999;94(3):188–198. doi: 10.1007/s003950050142. [DOI] [PubMed] [Google Scholar]
- 14.Hamacher-Brady A, et al. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281(40):29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 15.Davies SP, et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong H, et al. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res. 2004;94(8):1133–1141. doi: 10.1161/01.RES.0000126048.32383.6B. [DOI] [PubMed] [Google Scholar]
- 17.Patel HH, et al. Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol. 2006;291(1):H344–350. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 18.Koneru S, et al. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292(5):H2060–2072. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 19.Gross GJ, et al. Cytochrome P450 and arachidonic acid metabolites: Role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Research. 2005;68:18–25. doi: 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Bolli R, et al. Role of the JAK-STAT pathway in protection against myocardial ishcemia/reperfusion injury. Trends Cardiovasc Med. 2003;13:72–79. doi: 10.1016/s1050-1738(02)00230-x. [DOI] [PubMed] [Google Scholar]
- 21.Xuan YT, et al. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112(13):1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaura G, et al. STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H476–482. doi: 10.1152/ajpheart.00079.2003. [DOI] [PubMed] [Google Scholar]
- 23.Ping P, et al. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ Res. 1999;85(6):542–550. doi: 10.1161/01.res.85.6.542. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa Y, et al. The role of ADAM protease in the tyrosine kinase-mediated trigger mechanism of ischemic preconditioning. Cardiovasc Res. 2004;62(1):167–175. doi: 10.1016/j.cardiores.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 25.Tong H, et al. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87(4):309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 26.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40(1):16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Mocanu MM, Yellon DM. p53 down-regulation: a new molecular mechanism involved in ischaemic preconditioning. FEBS Lett. 2003;555(2):302–306. doi: 10.1016/s0014-5793(03)01260-2. [DOI] [PubMed] [Google Scholar]
- 28.Juhaszova M, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113(11):1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97(12):1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki K, et al. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70(2):222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Maulik N, et al. Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation. 1999;100(19 Suppl):II369–375. doi: 10.1161/01.cir.100.suppl_2.ii-369. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura M, et al. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000;45(3):661–670. doi: 10.1016/s0008-6363(99)00393-4. [DOI] [PubMed] [Google Scholar]
- 33.Beresewicz A, et al. Effect of classic preconditioning and diazoxide on endothelial function and O2- and NO generation in the post-ischemic guinea-pig heart. Cardiovasc Res. 2004;63(1):118–129. doi: 10.1016/j.cardiores.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Schulz R, et al. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007 doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- 35.Tong H, et al. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90(4):377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 36.Hausenloy DJ, et al. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55(3):534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 37.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003;285(3):H921–930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- 38.Pantos C, et al. Protection of the abnormal heart. Heart Fail Rev. 2007;12(34):345–362. doi: 10.1007/s10741-007-9036-z. [DOI] [PubMed] [Google Scholar]
- 39.Gross ER, et al. Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes. 2007;56(1):127–136. doi: 10.2337/db06-0907. [DOI] [PubMed] [Google Scholar]
- 40.Gross ER, Gross GJ. Pharmacological therapeutics for cardiac reperfusion injury. Expert Opin Emerging Drugs. 2007;12(3):367–388. doi: 10.1517/14728214.12.3.367. [DOI] [PubMed] [Google Scholar]
- 41.Lankford AR, et al. Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2006;290(4):H1469–H1473. doi: 10.1152/ajpheart.00181.2005. [DOI] [PubMed] [Google Scholar]
- 42.Wang NP, et al. Ischemic preconditioning reduces neutrophil accumulation and myocardial apoptosis. Ann Thorac Surg. 1999;67(6):1689–1695. doi: 10.1016/s0003-4975(99)00305-7. [DOI] [PubMed] [Google Scholar]
- 43.Yang Z, et al. Infarct-spearing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111(17):2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]