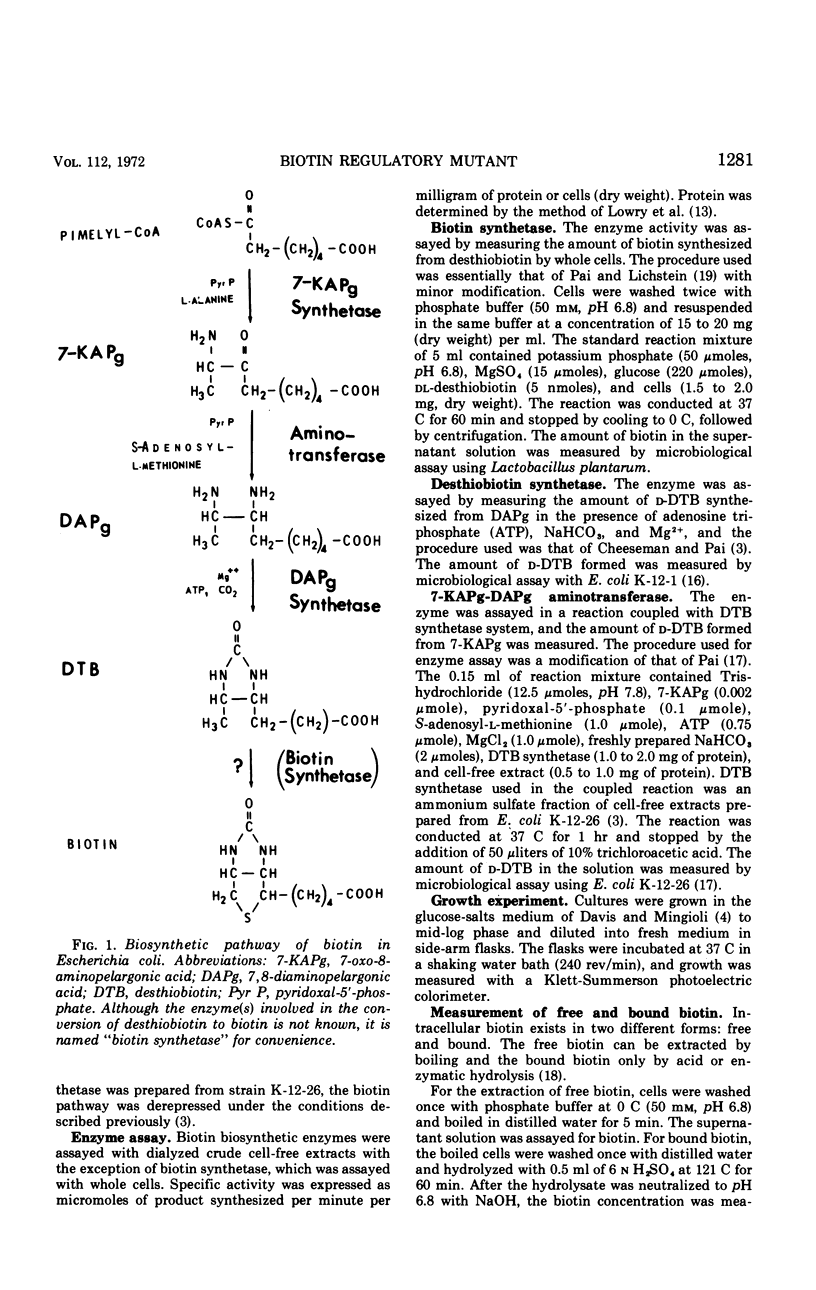
Abstract
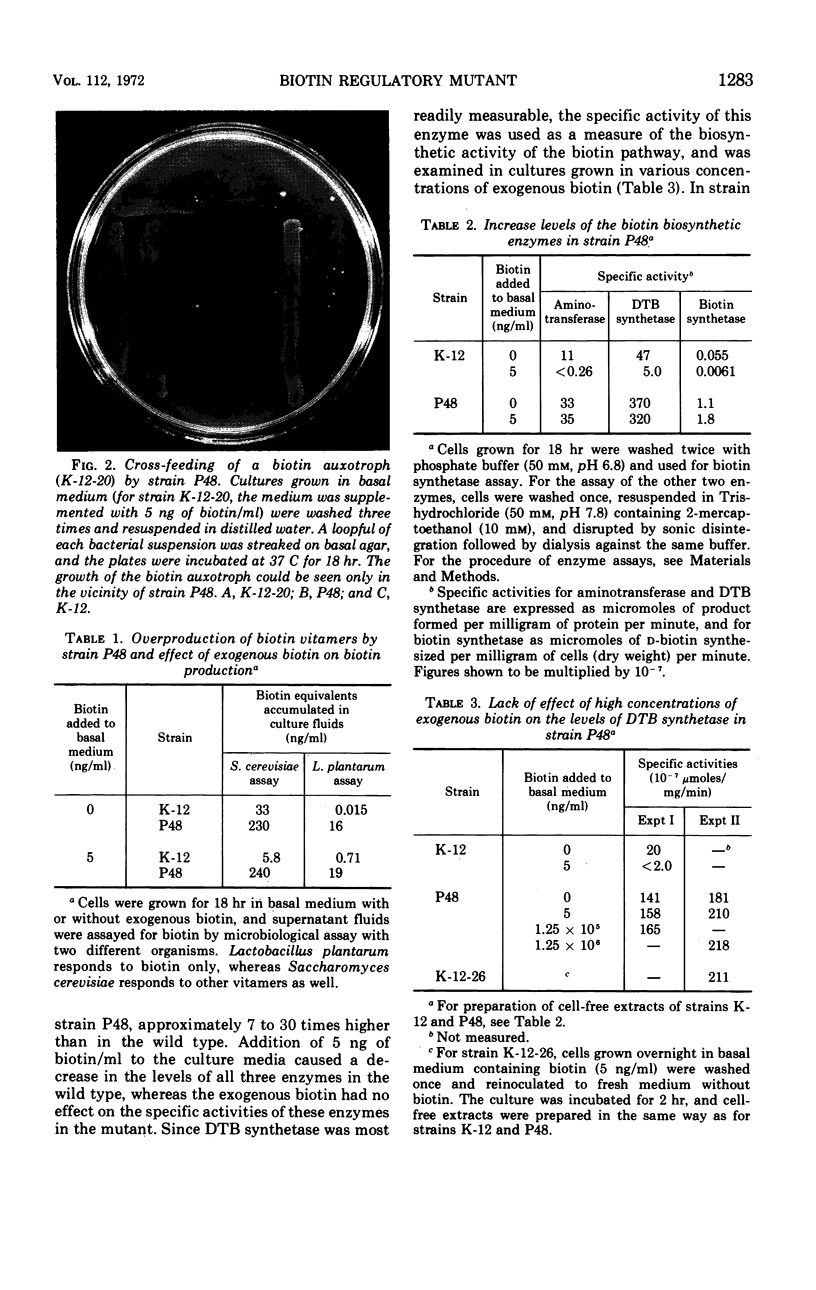
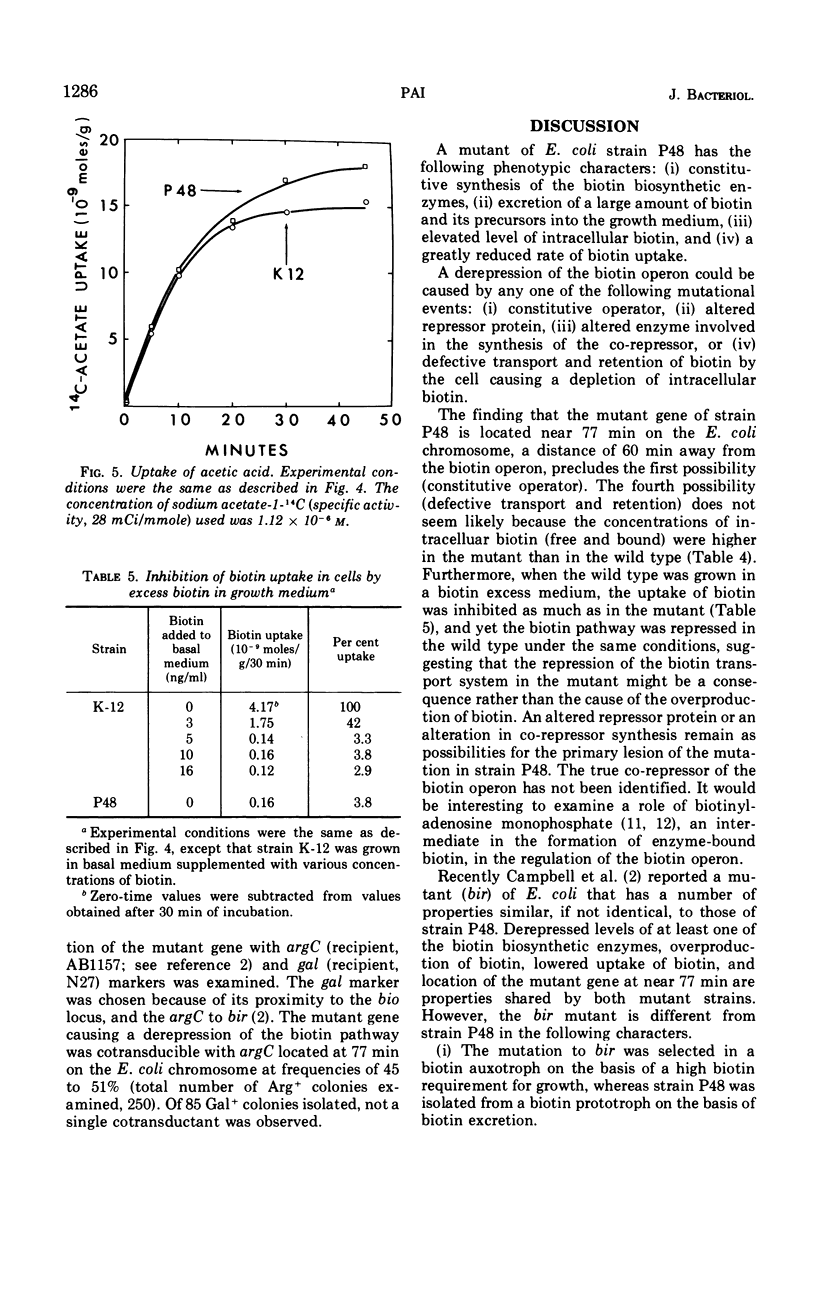
A cross-feeding technique was used to isolate a mutant of Escherichia coli K-12 that excretes 1,000 times more biotin into the growth medium than the parent strain. The mutant has high levels of the biotin biosynthetic enzymes even when grown in the presence of biotin. Desthiobiotin synthetase, the level of which was used as a measure of the biosynthetic activity of the biotin pathway, is not repressed by biotin at the concentration 250,000 times that sufficient to repress the enzyme in the wild type. The mutant gene is cotransducible with argC located at 77 min on the E. coli chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A., Del Campillo-Campbell A., Chang R. A mutant of Escherichia coli that requires high concentrations of biotin. Proc Natl Acad Sci U S A. 1972 Mar;69(3):676–680. doi: 10.1073/pnas.69.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman P., Pai C. H. Partial purification and properties of D-desthiobiotin synthetase from Escherichia coli. J Bacteriol. 1970 Nov;104(2):726–733. doi: 10.1128/jb.104.2.726-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campillo-Campbell A., Kayajanian G., Campbell A., Adhya S. Biotin-requiring mutants of Escherichia coli K-12. J Bacteriol. 1967 Dec;94(6):2065–2066. doi: 10.1128/jb.94.6.2065-2066.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. A., Star C. Synthesis of 7-oxo-8-aminopelargonic acid, a biotin vitamer, in cell-free extracts of Escherichia coli biotin auxotrophs. J Bacteriol. 1968 Oct;96(4):1291–1297. doi: 10.1128/jb.96.4.1291-1297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. A., Stoner G. L. Biosynthesis of 7,8-diaminopelargonic acid, a biotin intermediate, from 7-keto-8-aminopelargonic acid and S-adenosyl-L-methionine. J Bacteriol. 1971 Dec;108(3):1135–1140. doi: 10.1128/jb.108.3.1135-1140.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A. Divergent orientation of transcription from the biotin locus of Escherichia coli. J Mol Biol. 1971 Feb 28;56(1):53–62. doi: 10.1016/0022-2836(71)90083-0. [DOI] [PubMed] [Google Scholar]
- INUI Y., AKEDO H. AMINO ACID UPTAKE BY ESCHERICHIA COLI GROWN IN PRESENCE OF AMINO ACIDS. EVIDENCE FOR REPRESSIBILITY OF AMINO ACID UPTAKE. Biochim Biophys Acta. 1965 Jan 25;94:143–152. doi: 10.1016/0926-6585(65)90018-x. [DOI] [PubMed] [Google Scholar]
- Krell K., Eisenberg M. A. The purification and properties of dethiobiotin synthetase. J Biol Chem. 1970 Dec 25;245(24):6558–6566. [PubMed] [Google Scholar]
- LANE M. D., ROMINGER K. L., YOUNG D. L., LYNEN F. THE ENZYMATIC SYNTHESIS OF HOLOTRANSCARBOXYLASE FROM APOTRANSCARBOXYLASE AND (+)-BIOTIN. II. INVESTIGATION OF THE REACTION MECHANISM. J Biol Chem. 1964 Sep;239:2865–2871. [PubMed] [Google Scholar]
- LANE M. D., YOUNG D. L., LYNEN F. THE ENZYMATIC SYNTHESIS OF HOLOTRANSCARBOXYLASE FROM APOTRANSCARBOXYLASE AND (+)-BIOTIN. I. PURIFICATION OF THE APOENZYME AND SYNTHETASE; CHARACTERISTICS OF THE REACTION. J Biol Chem. 1964 Sep;239:2858–2864. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PAI C. H., LICHSTEIN H. C. THE BIOSYNTHESIS OF BIOTIN IN MICROORGANISMS. I. THE PHYSIOLOGY OF BIOTIN SYNTHESIS IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Apr 12;100:28–35. doi: 10.1016/0304-4165(65)90423-x. [DOI] [PubMed] [Google Scholar]
- Pai C. H. Biosynthesis of biotin: synthesis of 7,8-diaminopelargonic acid in cell-free extracts of Escherichia coli. J Bacteriol. 1971 Mar;105(3):793–800. doi: 10.1128/jb.105.3.793-800.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H. Biosynthesis of desthiobiotin in cell-free extracts of Escherichia coli. J Bacteriol. 1969 Sep;99(3):696–701. doi: 10.1128/jb.99.3.696-701.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Lichstein H. C. Biosynthesis of biotin in microorganisms. IV. Repression and derepression of (+ -)-biotin synthesis from (+)-desthiobiotin. Arch Biochem Biophys. 1966 Apr;114(1):138–144. doi: 10.1016/0003-9861(66)90314-6. [DOI] [PubMed] [Google Scholar]
- Pai C. H., Lichstein H. C. Biosynthesis of biotin in microorganisms. VI. Further evidence for desthiobiotin as a precursor in Eschericia coli. J Bacteriol. 1967 Dec;94(6):1930–1933. doi: 10.1128/jb.94.6.1930-1933.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. O., Lichstein H. C. Regulation of biotin transport in Saccharomyces cerevisiae. J Bacteriol. 1969 Nov;100(2):565–572. doi: 10.1128/jb.100.2.565-572.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe B., Eisenberg M. A. Genetic and biochemical analysis of the biotin loci of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):515–524. doi: 10.1128/jb.96.2.515-524.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe B. Lambda phage transduction of the bio A locus of Escherichia coli. Virology. 1970 Nov;42(3):643–661. doi: 10.1016/0042-6822(70)90310-7. [DOI] [PubMed] [Google Scholar]



