Abstract
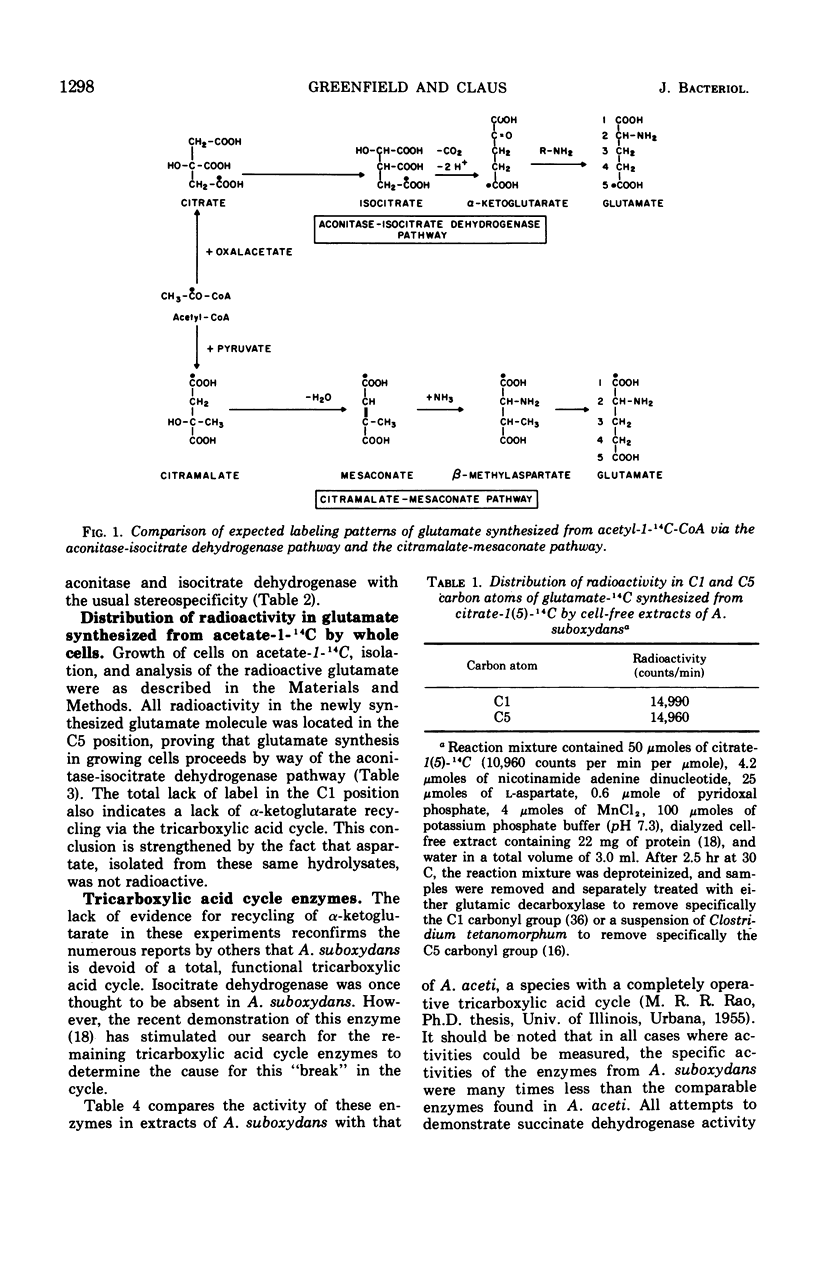
Acetobacter suboxydans does not contain an active tricarboxylic acid cycle, yet two pathways have been suggested for glutamate synthesis from acetate catalyzed by cell extracts: a partial tricarboxylic acid cycle following an initial condensation of oxalacetate and acetyl coenzyme A. and the citramalate-mesaconate pathway following an initial condensation of pyruvate and acetyl coenzyme A. To determine which pathway functions in growing cells, acetate-1-14C was added to a culture growing in minimal medium. After growth had ceased, cells were recovered and fractionated. Radioactive glutamate was isolated from the cellular protein fraction, and the position of the radioactive label was determined. Decarboxylation of the C5 carbon removed 100% of the radioactivity found in the purified glutamate fraction. These experiments establish that growing cells synthesize glutamate via a partial tricarboxylic acid cycle. Aspartate isolated from these hydrolysates was not radioactive, thus providing further evidence for the lack of a complete tricarboxylic acid cycle. When cell extracts were analyzed, activity of all tricarboxylic acid cycle enzymes, except succinate dehydrogenase, was demonstrated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSKY T., SHEMIN D. THE FORMATION OF ISOLEUCINE FROM BETA-METHYLASPARTIC ACID IN ESCHERICHIA COLI W. J Biol Chem. 1965 Jul;240:2971–2975. [PubMed] [Google Scholar]
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Belly R. T., Claus G. W. Effect of amino acids on the growth of Acetobacter suboxydans. Arch Mikrobiol. 1972;83(3):237–245. doi: 10.1007/BF00645124. [DOI] [PubMed] [Google Scholar]
- Claus G. W., Orcutt M. L., Belly R. T. Phosphoenolpyruvate carboxylation and aspartate synthesis in Acetobacter suboxydans. J Bacteriol. 1969 Feb;97(2):691–696. doi: 10.1128/jb.97.2.691-696.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELEY J., KERSTERS K. OXIDATION OF ALIPHATIC GLYCOLS BY ACETIC ACID BACTERIA. Bacteriol Rev. 1964 Jun;28:164–180. [PMC free article] [PubMed] [Google Scholar]
- ENGLARD S., COLOWICK S. P. On the mechanism of the aconitase and isocitric dehydrogenase reactions. J Biol Chem. 1957 Jun;226(2):1047–1058. [PubMed] [Google Scholar]
- FEWSTER J. A. Growth of acetobacter suboxydans and the oxidation of aldoses, related carboxylic acids, and aldehydes. Biochem J. 1958 Aug;69(4):582–595. doi: 10.1042/bj0690582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flipse R. J., Dietz R. W. Metabolism of bovine semen. XVII. Oxidative metabolism of glutamate. J Dairy Sci. 1969 Jan;52(1):113–116. doi: 10.3168/jds.S0022-0302(69)86511-2. [DOI] [PubMed] [Google Scholar]
- Gottschalk G., Barker H. A. Presence and stereospecificity of citrate synthase in anaerobic bacteria. Biochemistry. 1967 Apr;6(4):1027–1034. doi: 10.1021/bi00856a011. [DOI] [PubMed] [Google Scholar]
- Gottschalk G., Barker H. A. Synthesis of glutamate and citrate by Clostridium kluyveri. A new type of citrate synthase. Biochemistry. 1966 Apr;5(4):1125–1133. doi: 10.1021/bi00868a003. [DOI] [PubMed] [Google Scholar]
- Gottschalk G. The stereospecificity of the citrate synthase in sulfate-reducing and photosynthetic bacteria. Eur J Biochem. 1968 Aug;5(3):346–351. doi: 10.1111/j.1432-1033.1968.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Greenfield S., Claus G. W. Isocitrate dehydrogenase and glutamate synthesis in Acetobacter suboxydans. J Bacteriol. 1969 Dec;100(3):1264–1270. doi: 10.1128/jb.100.3.1264-1270.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON K. R., ROSE I. A. THE ABSOLUTE STEREOCHEMICAL COURSE OF CITRIC ACID BIOSYNTHESIS. Proc Natl Acad Sci U S A. 1963 Nov;50:981–988. doi: 10.1073/pnas.50.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E., CHELDELIN V. H. Oxidations in Acetobacter suboxydans. Biochim Biophys Acta. 1954 May;14(1):108–116. doi: 10.1016/0006-3002(54)90137-7. [DOI] [PubMed] [Google Scholar]
- KITOS P. A., WANG C. H., MOHLER B. A., KING T. E., CHELDELIN V. H. Glucose and gluconate dissimilation in Acetobacter suboxydans. J Biol Chem. 1958 Dec;233(6):1295–1298. [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Di- and triphosphopyridine nucleotide isocitric dehydrogenases in yeast. J Biol Chem. 1951 Mar;189(1):123–136. [PubMed] [Google Scholar]
- Maragoudakis M. E., Sekizawa Y., King T. E., Cheldelin V. H. Glutamate biosynthesis in Acetobacter suboxydans. VI. Formation from acetate plus pyruvate. Biochemistry. 1966 Aug;5(8):2646–2653. doi: 10.1021/bi00872a024. [DOI] [PubMed] [Google Scholar]
- Maragoudakis M. E., Strassman M. Biosynthesis of alpha-isopropylmalic and citric acids in Acetobacter suboxydans. J Bacteriol. 1967 Sep;94(3):512–516. doi: 10.1128/jb.94.3.512-516.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- RAO MRR Acetic acid bacteria. Annu Rev Microbiol. 1957;11:317–338. doi: 10.1146/annurev.mi.11.100157.001533. [DOI] [PubMed] [Google Scholar]
- STOUTHAMER A. H. Oxidative possibilities in the catalase-positive Acetobacter species. Antonie Van Leeuwenhoek. 1959;25:241–264. doi: 10.1007/BF02542850. [DOI] [PubMed] [Google Scholar]
- Sato K., Yamada Y., Aida K., Uemura T. Enzymatic studies on the oxidation of sugar and sugar alcohol. 8. Particle-bound L-sorbose dehydrogenase from Gluconobacter suboxydans. J Biochem. 1969 Oct;66(4):521–527. doi: 10.1093/oxfordjournals.jbchem.a129177. [DOI] [PubMed] [Google Scholar]
- Sekizawa Y., Maragoudakis M. E., King T. E., Cheldelin V. H. Glutamate biosynthesis in an organism lacking a Krebs tricarboxylic acid cycle. V. Isolation of alpha-hydroxy-gamma-ketoglutarate (HKG) in Acetobacter suboxydans. Biochemistry. 1966 Jul;5(7):2392–2398. doi: 10.1021/bi00871a032. [DOI] [PubMed] [Google Scholar]
- Underkofler L. A., Bantz A. C., Peterson W. H. Growth Factors for Bacteria: XIV. Growth Requirements of Acetobacter suboxydans. J Bacteriol. 1943 Feb;45(2):183–190. doi: 10.1128/jb.45.2.183-190.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS P. J., RAINBOW C. ENZYMES OF THE TRICARBOXYLIC ACID CYCLE IN ACETIC ACID BACTERIA. J Gen Microbiol. 1964 May;35:237–247. doi: 10.1099/00221287-35-2-237. [DOI] [PubMed] [Google Scholar]


