Abstract
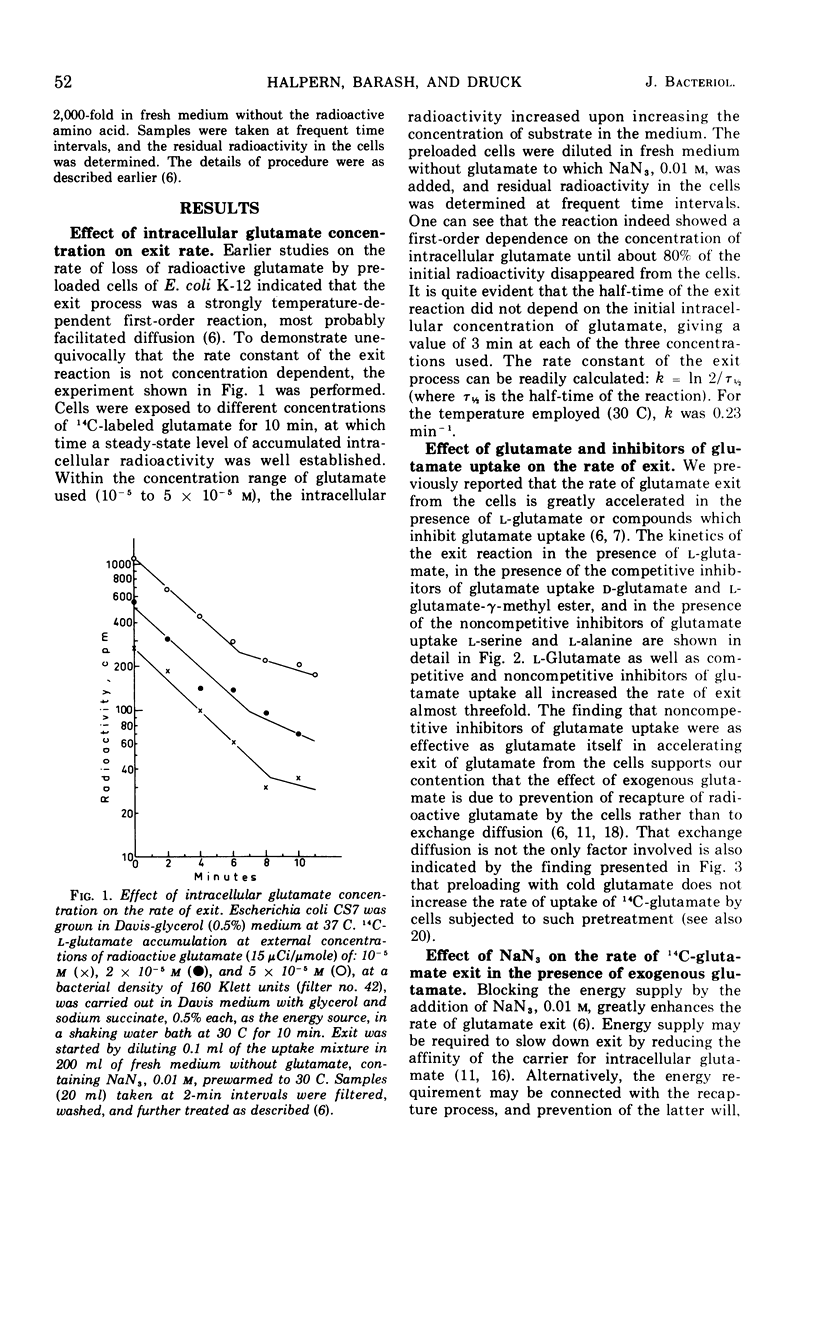
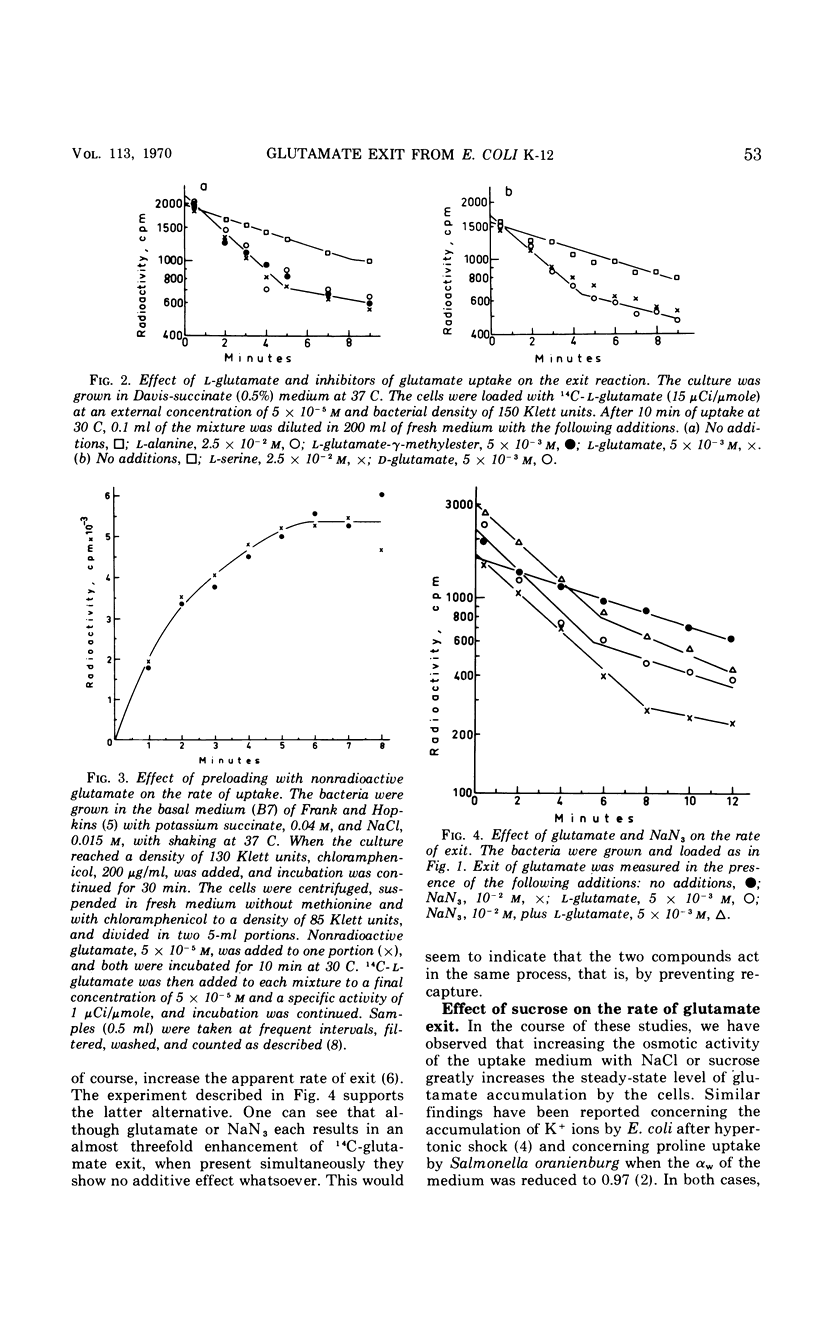
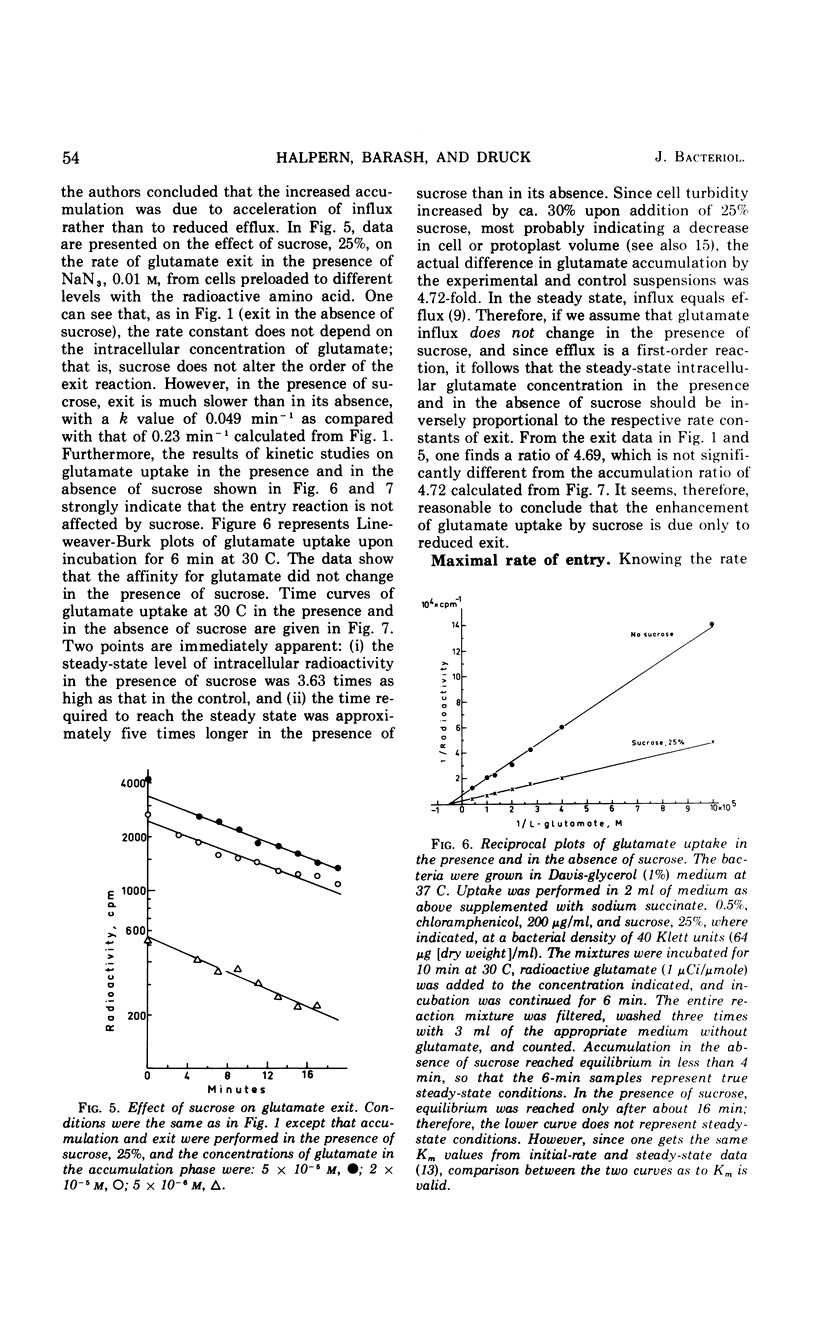
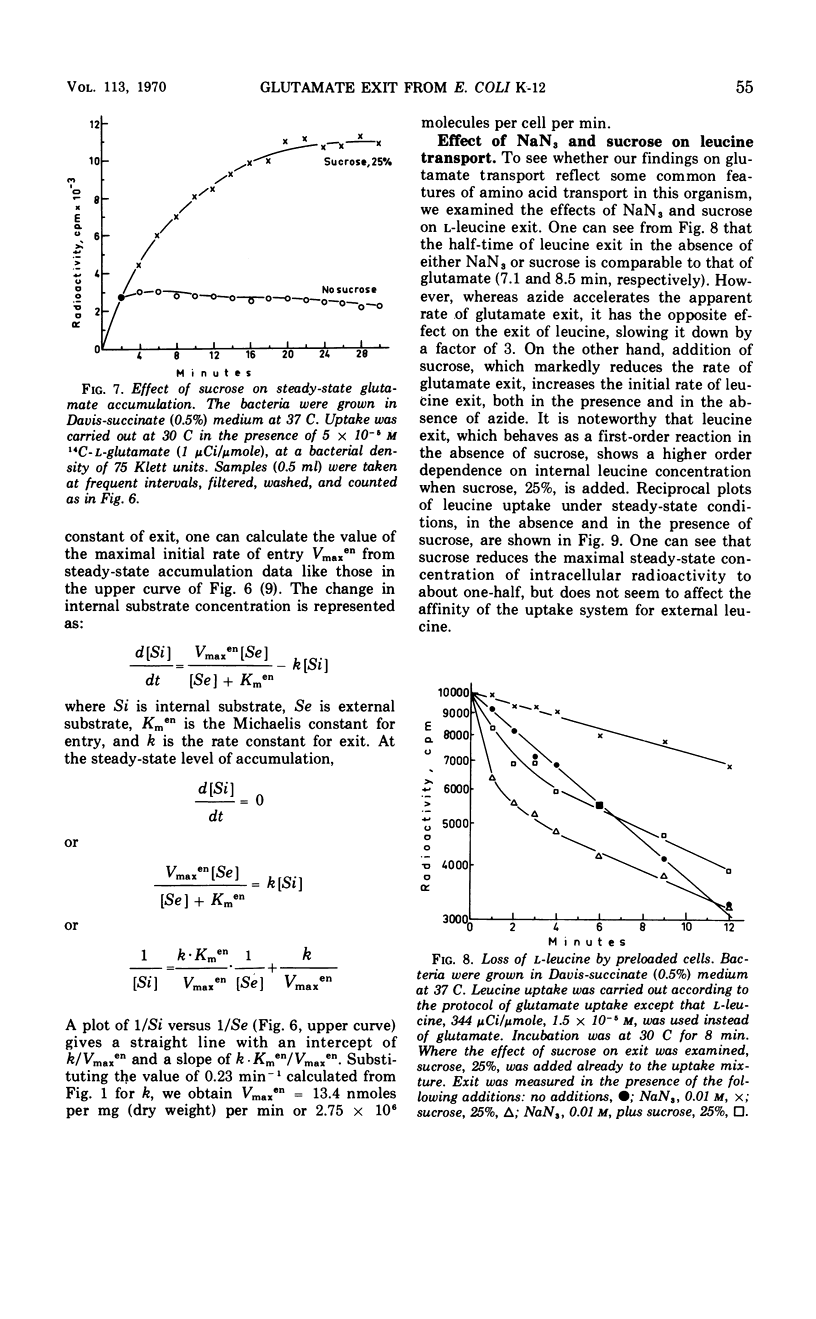
The exit of glutamate from Escherichia coli K-12 cells preloaded with the radioactive amino acid and its relation to the reaction of entry were studied. Experiments with cells preloaded to different intracellular concentrations of radioactive glutamate confirmed our earlier conclusion that glutamate exit was a first-order reaction. l-Glutamate, competitive inhibitors of glutamate uptake (d-glutamate and l-glutamate-γ-methyl ester), noncompetitive inhibitors of glutamate uptake (l-serine and l-alanine), and the energy poison NaN3 all accelerated glutamate exit 2.8-fold. No additive effect was observed in the presence of NaN3 together with l-glutamate. Preloading with cold l-glutamate did not increase the rate of uptake of radioactive glutamate. It is concluded that the acceleration of glutamate exit in the presence of l-glutamate in the medium is not due to exchange diffusion and that l-glutamate and azide affect exit indirectly by preventing recapture. Sucrose, 25%, slowed down glutamate exit by a factor of about 4.7 and increased the steady-state level of glutamate accumulation to about the same extent. Increasing the intracellular K+ concentration enhanced glutamate uptake but did not affect the half-time of exit. It is concluded that separate carriers are most probably involved in mediating the entry and exit reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barash H., Halpern Y. S. Glutamate-binding protein and its relation to glutamate transport in Escherichia coli K-12. Biochem Biophys Res Commun. 1971 Nov 5;45(3):681–688. doi: 10.1016/0006-291x(71)90470-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L., Hopkins I. Sodium-stimulated transport of glutamate in Escherichia coli. J Bacteriol. 1969 Oct;100(1):329–336. doi: 10.1128/jb.100.1.329-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. II. Characteristics of the exit process. J Biol Chem. 1960 Jun;235:1586–1590. [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquez J. A., Sherman J. H. The effect of metabolic inhibitors on transport and exchange of amino acids in Ehrlich ascites cells. Biochim Biophys Acta. 1965 Sep 27;109(1):128–141. doi: 10.1016/0926-6585(65)90097-x. [DOI] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. STABILITY OF ALPHA-HYDROGEN OF AMINO ACIDS DURING ACTIVE TRANSPORT. Biochemistry. 1965 Mar;4:561–565. doi: 10.1021/bi00879a029. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. THE ROLE OF PERMEASE IN TRANSPORT. Biochim Biophys Acta. 1964 Jan 27;79:177–200. doi: 10.1016/0926-6577(64)90050-6. [DOI] [PubMed] [Google Scholar]
- Knowles C. J., Smith L. Effect of osmotic pressure of the medium on the volume of intact cells of Azotobacter vinelandii and on the rate of respiration. Biochim Biophys Acta. 1971 Apr 6;234(1):144–152. doi: 10.1016/0005-2728(71)90139-3. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Energy expenditure is obligatory for the downhill transport of galactosides. J Mol Biol. 1971 Aug 14;59(3):447–459. doi: 10.1016/0022-2836(71)90309-3. [DOI] [PubMed] [Google Scholar]
- Levine M., Oxender D. L., Stein W. D. The substrate-facilitated transport of the glucose carrier across the human erythrocyte membrane. Biochim Biophys Acta. 1965 Sep 27;109(1):151–163. doi: 10.1016/0926-6585(65)90099-3. [DOI] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of glutamate transport and glutamate decarboxylase in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1409–1415. doi: 10.1128/jb.93.4.1409-1415.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbie J. P., Wilson T. H. Transmembrane effects of beta-galactosides on thiomethyl-beta-galactoside transport in Escherichia coli. Biochim Biophys Acta. 1969 Mar 11;173(2):234–244. doi: 10.1016/0005-2736(69)90107-2. [DOI] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]


