Abstract
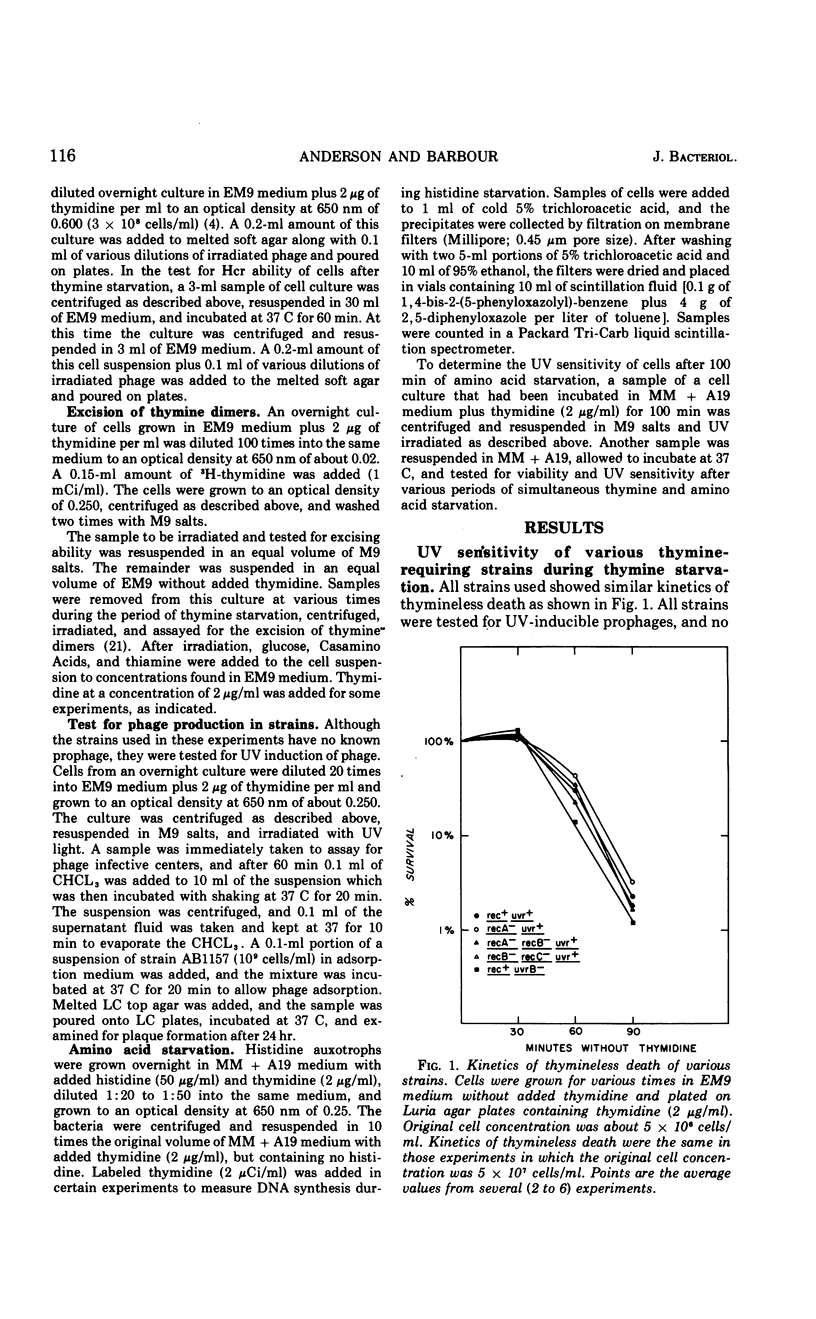
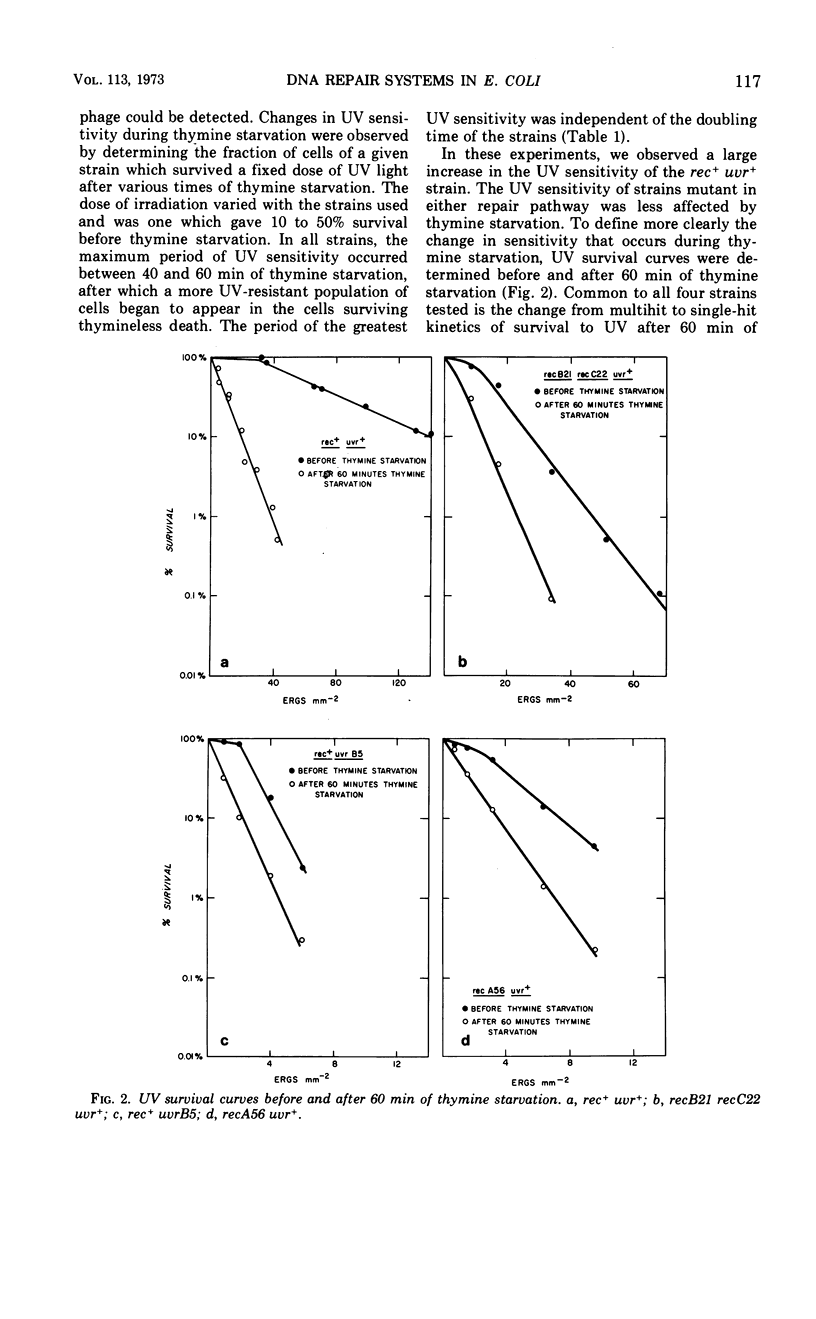
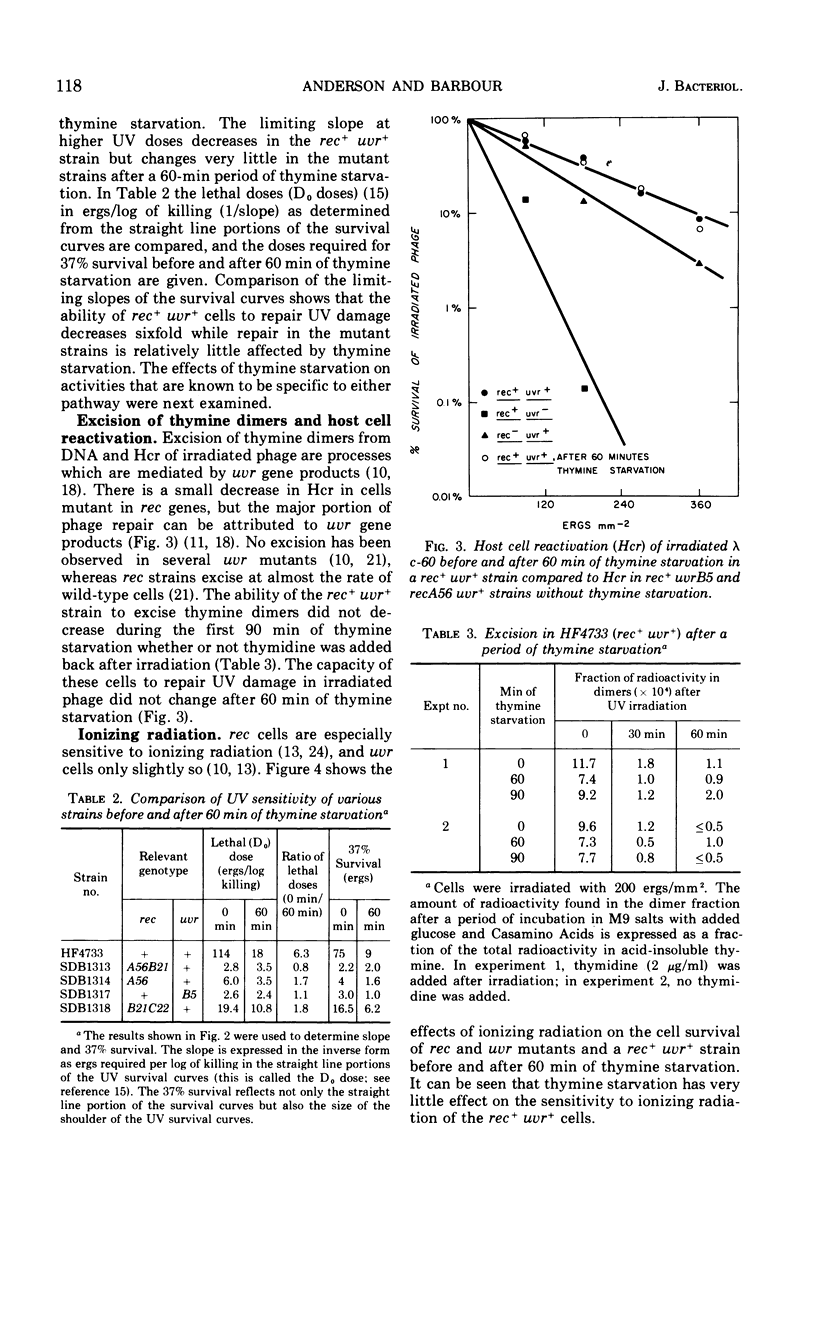
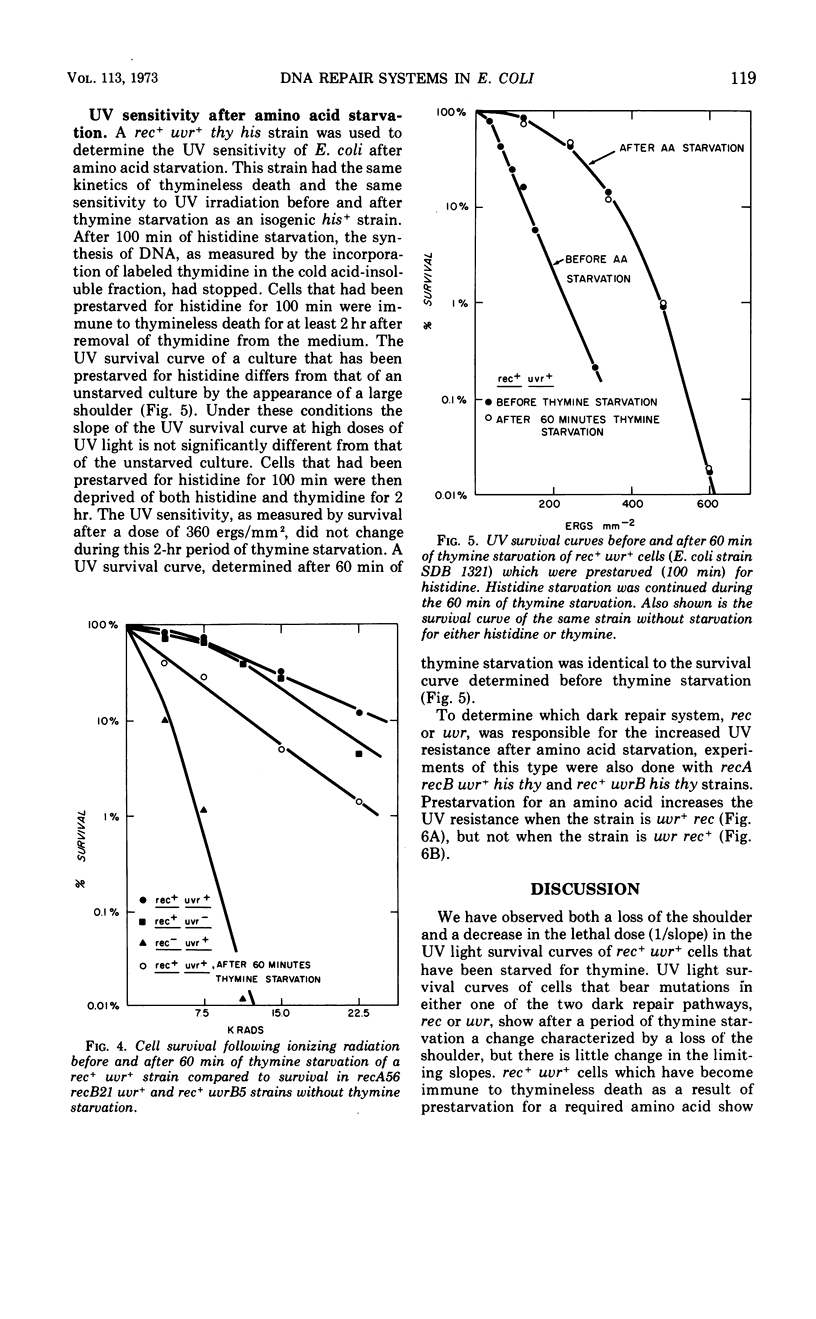
Thymine starvation of Escherichia coli K-12 results in greatly increased sensitivity to ultraviolet light (UV). Our studies, using isogenic strains carrying rec and uvr mutations, have shown the following. (i) Common to all strains tested is a change from multihit to single-hit kinetics of survival to UV after 60 min of thymine starvation. However, the limiting slope of UV survival curves decreases in the rec+uvr+ strain and changes very little in several rec mutant strains and one uvrB mutant strain. Thus, when either the rec or uvr system is functioning alone, the limiting slopes of the UV survival curves are relatively unaffected by thymine starvation. (ii) Thymine starvation does not significantly inhibit repair processes carried out by either repair system alone; i.e., host cell reactivation of irradiated phage (carried out by the uvr system), excision of thymine dimers (uvr), or X-ray repair (rec). (iii) In a rec+uvr+ strain, repair appears to be a synergistic rather than additive function of the two systems. However, after thymine starvation, repair capacity is reduced to about the sum of the repair capacities of the independent systems. (iv) The kinetics of thymineless death are not changed by rec and uvr mutations. This indicates that the lesions responsible for thymineless death are not repaired by rec or uvr systems. (v) Withholding thymine from thy rec+uvr+ bacteria not undergoing thymineless death has no effect on UV sensitivity. Under these conditions one sees higher than normal UV resistance in the presence or absence of thymine. This is due to increased repair carried out by the uvr system. To explain these results we postulate that thymine starvation does not inhibit either the rec or uvr repair pathway directly. Rather it appears that thymine starvation results in increased UV sensitivity in part by inhibiting a function which normally carries out efficient coordination of rec and uvr pathways.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Bruns L. Relationship between radiation response and the deoxyribonucleic acid replication cycle in bacteria: dependence on the excision-repair system. J Bacteriol. 1970 Aug;103(2):400–403. doi: 10.1128/jb.103.2.400-403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLANT J., SUSKIND S. R. Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J Bacteriol. 1961 Aug;82:187–194. doi: 10.1128/jb.82.2.187-194.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. The U.V. sensitivity of bacteria: its relation to the DNA replication cycle. Photochem Photobiol. 1966 Jan;5(1):1–12. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L., Stedeford J. B. Some properties of excision-defective recombination-deficient mutants of Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1134–1141. doi: 10.1128/jb.97.3.1134-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Peacey M., Gross J. D. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. doi: 10.1016/0022-2836(71)90376-7. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M., Cordone L., Krsmanovic-Simic D., Errera M. Complementary action of recombination and excision in the repair of ultraviolet irradiation damage to DNA. J Mol Biol. 1970 Apr 14;49(1):203–212. doi: 10.1016/0022-2836(70)90386-4. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Anderson J. A., Barbour S. D. Excision repair properties of isogenic rec mutants of Escherichia coli K-12. J Bacteriol. 1972 Sep;111(3):723–730. doi: 10.1128/jb.111.3.723-730.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


