Abstract
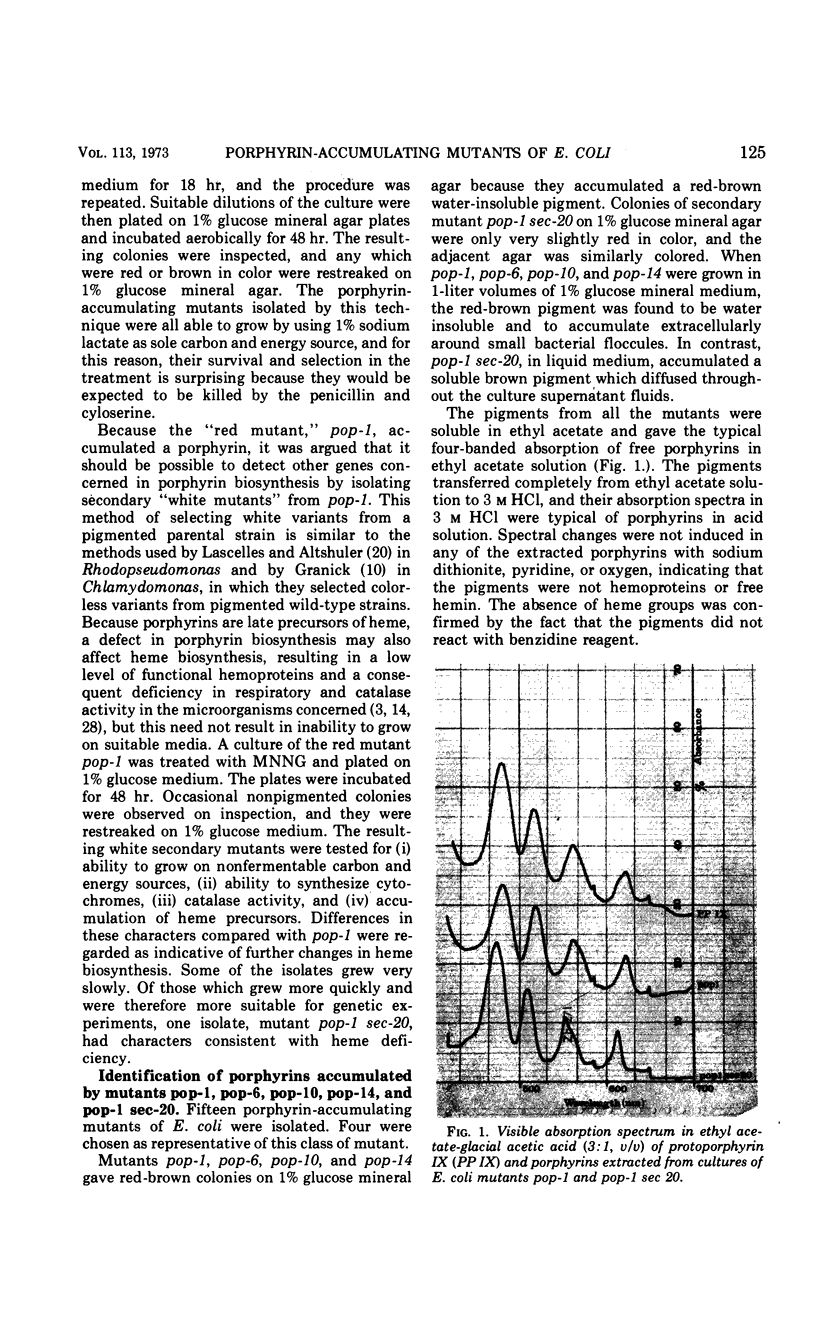
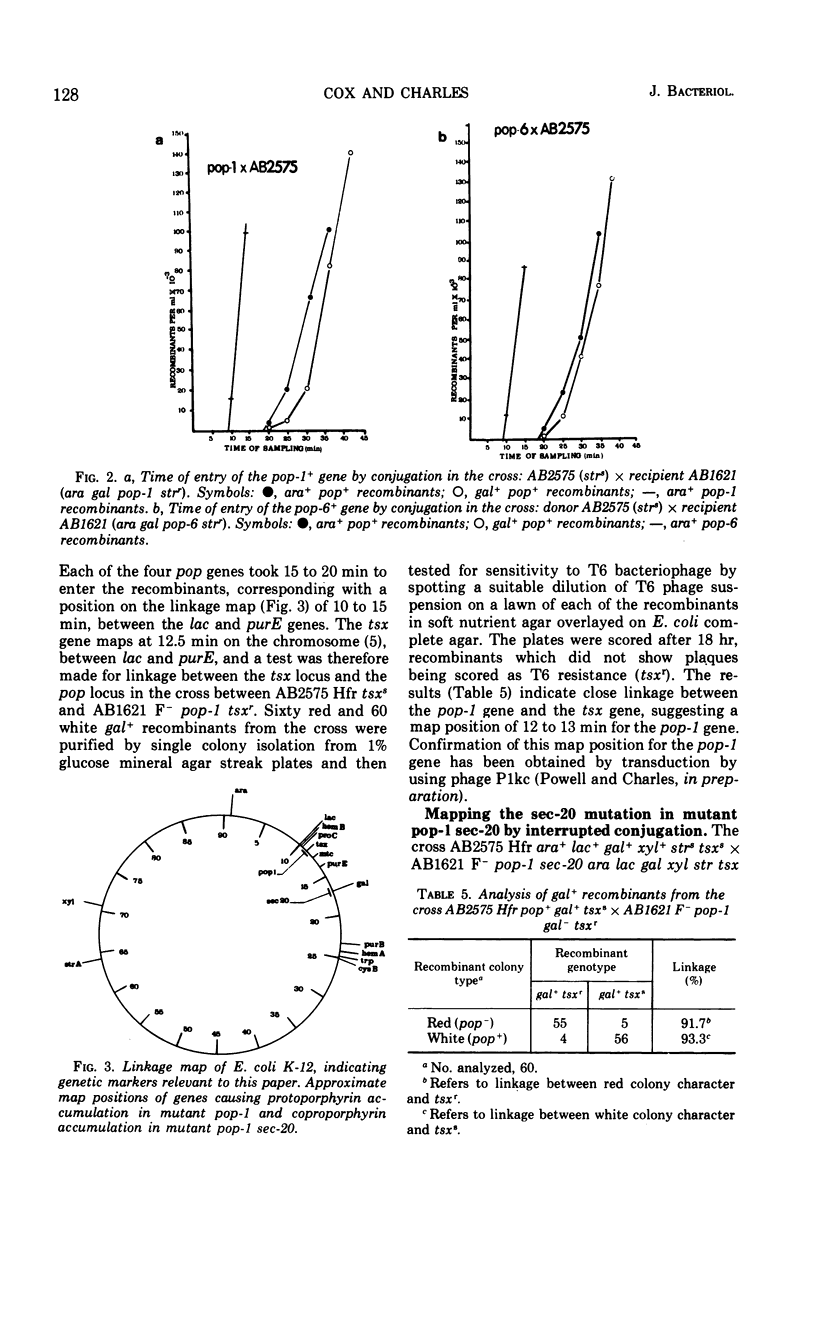
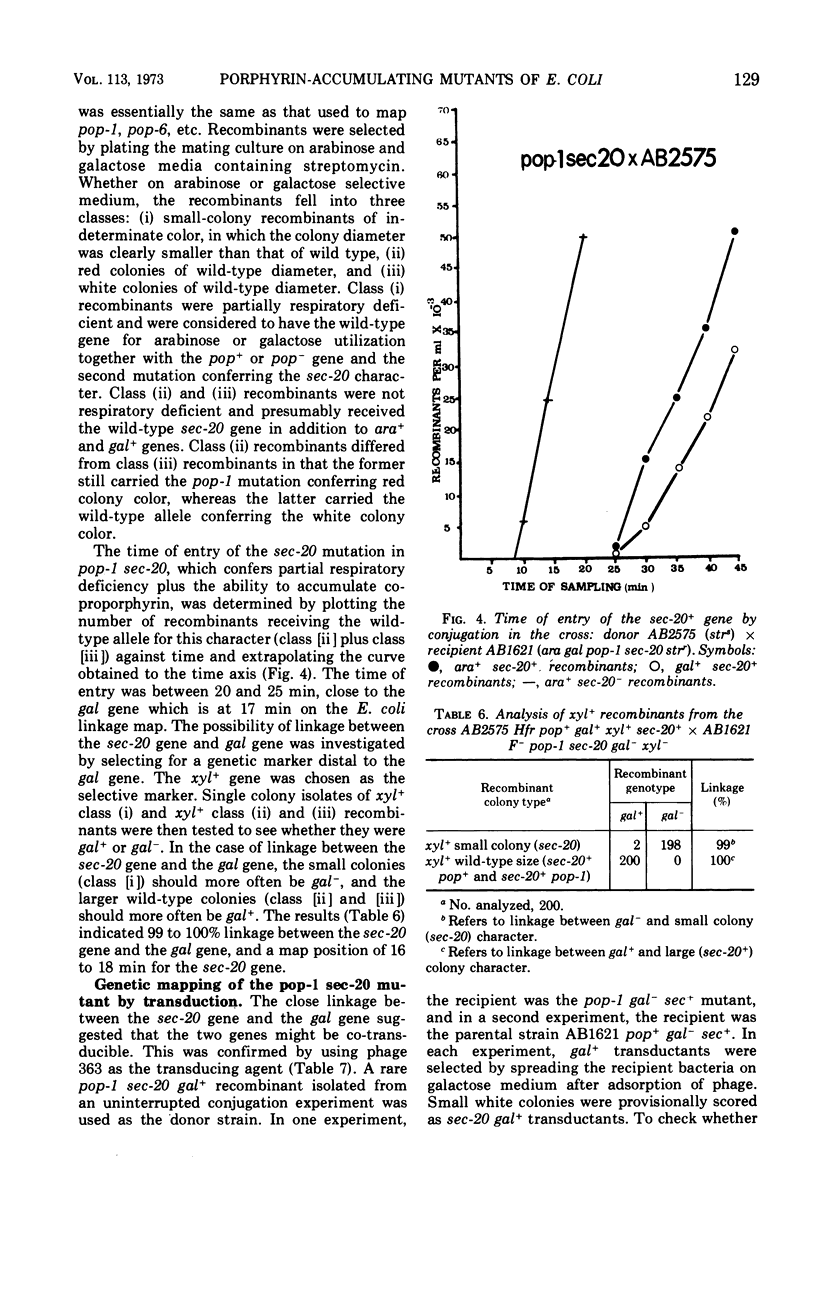
Four mutants (pop-1, pop-6, pop-10, and pop-14) which accumulate a red water-insoluble pigment were obtained in Escherichia coli K-12 AB1621. For each mutant, the red pigment was shown to be protoporphyrin IX, a late precursor of heme. Mutagenic treatment of mutant pop-1 yielded a secondary mutant, pop-1 sec-20, which accumulated a brown water-soluble pigment. The brown pigment was shown to be coproporphyrin III. Mutant pop-1 resembled the parental strain in its cytochrome absorption spectrum, catalase activity, and ability to grow on nonfermentable carbon and energy sources; therefore, its ability to produce and utilize heme was unimpaired. Judged on the same criteria, the secondary mutant, pop-1 sec-20, was partially heme and respiratory deficient. Growth in anaerobic conditions decreased by 25% the accumulation of protoporphyrin by pop-1; under the same conditions, pop-1 sec-20 did not accumulate coproporphyrin or coproporphyrinogen. The mutations causing protoporphyrin accumulation in all four pop mutants were found to map in the lac to purE (10–13 min) region of the E. coli chromosome. In the case of mutant pop-1, the mutation was shown to be strongly linked to the tsx locus (12 min). In mutant pop-1 sec-20, the second mutation causing coproporphyrin accumulation was co-transducible with the gal locus at a frequency of 88 to 96%. The mechanism of porphyrin accumulation by the mutants is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELJANSKI M. Sur la formation d'enzymes respiratoires chez un mutant d'Escherichia coli streptomycino-résistant et auxotrophe pour l'hémine. Ann Inst Pasteur (Paris) 1957 Mar;92(3):396–412. [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU T. C., SISTER A A GREEN, CHU E. J. Paper chromatography of methyl esters of porphyrins. J Biol Chem. 1951 Jun;190(2):643–646. [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles H. P., Roberts G. A. Carbon dioxide as a growth factor for mutants of Escherichia coli. J Gen Microbiol. 1968 Apr;51(2):211–224. doi: 10.1099/00221287-51-2-211. [DOI] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Sugimura T., Iwasaki M. Genetic analysis of a respiration-deficient mutant of Saccharomyces cerevisiae lacking all cytochromes and accumulating coproporphyrin. Genetics. 1967 Oct;57(2):213–226. doi: 10.1093/genetics/57.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Formation of protoporphyrin from coproporphyrinogen in extracts of various bacteria. J Bacteriol. 1970 May;102(2):398–403. doi: 10.1128/jb.102.2.398-403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J., Altschuler T. Mutant strains of Rhodopseudomonas spheroides lacking delta-aminolevulinate synthase: growth, heme, and bacteriochlorophyll synthesis. J Bacteriol. 1969 May;98(2):721–727. doi: 10.1128/jb.98.2.721-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J., Hatch T. P. Bacteriochlorophyll and heme synthesis in Rhodopseudomonas spheroides: possible role of heme in regulation of the branched biosynthetic pathway. J Bacteriol. 1969 May;98(2):712–720. doi: 10.1128/jb.98.2.712-720.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S., Sugimura T. Coproporphyrinogenase in a respiration-deficient mutant of yeast lacking all cytochromes and accumulating coproporphyrin. J Bacteriol. 1968 Dec;96(6):1997–2003. doi: 10.1128/jb.96.6.1997-2003.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K., Chou G. Isolation of spontaneous mutant strains of Pseudomonas putida. Biochem Biophys Res Commun. 1969 Jul 7;36(1):179–184. doi: 10.1016/0006-291x(69)90666-4. [DOI] [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes. Bacteriol Rev. 1954 Jun;18(2):106–130. doi: 10.1128/br.18.2.106-130.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Horodniceanu T. Locus determining normal colony formation on the chromosome of Escherichia coli K-12. J Bacteriol. 1967 Oct;94(4):1268–1269. doi: 10.1128/jb.94.4.1268-1269.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Sanderson K. E., Surdeanu M., Sonea S. Hemin-deficient mutants of Salmonella typhimurium. J Bacteriol. 1970 May;102(2):531–536. doi: 10.1128/jb.102.2.531-536.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff D. L. Delta-aminolevulinic acid-requiring mutant from Escherichia coli. J Bacteriol. 1967 Apr;93(4):1473–1474. doi: 10.1128/jb.93.4.1473-1474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]