Abstract
Recombinant immunotoxins are hybrid proteins composed of an Fv that binds to a tumor antigen fused to a bacterial or plant toxin. Immunotoxin BL22 targets CD22 positive malignancies and is composed of an anti-CD22 Fv fused to a 38-kDa fragment of Pseudomonas exotoxin A (PE38). BL22 has produced many complete remissions in drug-resistant Hairy cell leukemia, where many treatment cycles can be given, because neutralizing antibodies do not form. In marked contrast, only minor responses have been observed in trials with immunotoxins targeting solid tumors, because only a single treatment cycle can be given before antibodies develop. To allow more treatment cycles and increase efficacy, we have produced a less immunogenic immunotoxin by identifying and eliminating most of the B cell epitopes on PE38. This was accomplished by mutation of specific large hydrophilic amino acids (Arg, Gln, Glu, Lys) to Ala, Ser, or Gly. The new immunotoxin (HA22–8X) is significantly less immunogenic in three strains of mice, yet retains full cytotoxic and anti-tumor activities. Elimination of B-cell epitopes is a promising approach to the production of less immunogenic proteins for therapeutic purposes.
Keywords: antibody engineering, BL22, HA22, immunotherapy, Pseudomonas exotoxin A
Immunotoxins (ITs) are hybrid proteins that are composed of a cancer-specific antibody attached to a bacterial or plant toxin (1). Initially ITs were made by chemically coupling toxins to whole antibodies. Now they are made using a combination of antibody and protein engineering (2, 3). ITs kill cells by binding to a cell surface protein, being internalized by endocytosis and eventually reaching the cytosol, where they arrest protein synthesis by inactivating EF2 or ribosomes (4, 5). Our laboratory has developed recombinant immunotoxins (RITs) in which the Fv portion of an antibody is directly fused to a 38-kDa portion of the bacterial toxin Pseudomonas exotoxin A (PE). Three RITs are currently in clinical trials and all three have shown anti-tumor activity in phase 1 trials. LMB-2 [anti-Tac-(Fv)-PE38] targets CD25 expressed on many T cell malignancies and some B cell malignancies (6). BL22 [anti-CD22-(Fv)-PE38] targets CD22 expressed on most B cell malignancies (7), and SS1P anti-mesothelin-(Fv)-PE38 targets the mesothelin antigen expressed on mesotheliomas and on ovarian, lung, pancreatic, and gastric cancers (8). Because these ITs contain a portion of a bacterial protein, they can induce the formation of neutralizing antibodies, hindering their efficacy. In patients with B- and T-cell malignancies the formation of neutralizing antibodies is infrequent because of the immune-suppressed state of patients with these malignancies (6, 7). However, in patients with solid tumors treated with SS1P and other ITs, antibody formation was very frequently detected 21 days after the first treatment cycle, preventing readministration of the IT (9).
Previous studies have shown that the formation of antibodies to foreign proteins can be prevented by coupling the protein to high-molecular-weight polyethylene glycol (10). We have had limited success with this approach because of inactivation of the IT and only minor decreases in immunogenicity. Another approach is to treat patients with cyclophosphamide or fludarabine that damages the immune system (11, 12).
Alternative approaches are to identify and remove B-cell or T-cell epitopes (13–17). We have recently used a mouse model to identify the major B-cell epitopes in the PE38 portion of RITs made by our group (18). Our approach was to immunize mice, with PE38-containing ITs, isolate monoclonal antibodies (mAbs) reacting with conformational epitopes on PE38, and use these to determine the number of epitopes on PE38. We found that PE38 contains seven major conformational epitopes located in specific positions on the protein and not diffusely distributed over the entire surface of PE38. The finding that the epitopes are clustered enabled us to determine the precise location of most of the epitopes by mutating large hydrophilic amino acids on the surface of PE38 to alanine or glycine and showing that specific mAb binding to the selected epitope was abolished or greatly decreased (18). These results indicated that we might be able to reduce significantly the immunogenicity of PE38, if we combined in one IT several individual mutations that each by itself eliminated one epitope. We describe here the production of a PE38-containing IT that is significantly less immunogenic than its parental IT and does not contain new epitopes yet retains full cytotoxic activity in vitro and in mice with lymphomas.
Results
Mutant Proteins.
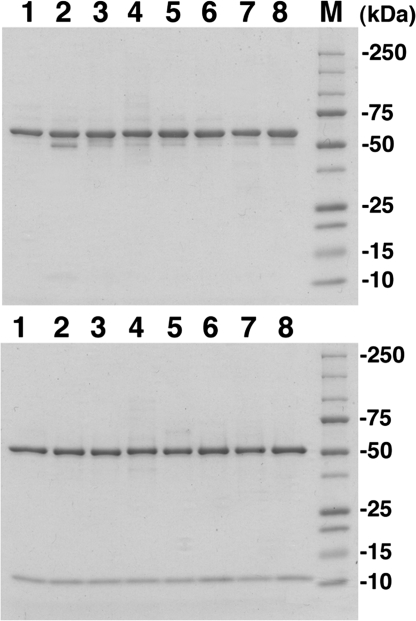
Our goal was to prepare a mutant IT in which as many B-cell epitopes as possible were removed. The locations of the seven major B-cell epitopes on the PE38 portion of the IT HA22 were established previously by mutating hydrophilic amino acids with large exposed areas to small amino acids (Ala, Gly, and Ser) and showing the binding of mAbs to the different epitopes was abolished or greatly diminished in the mutant protein (18). A list of the mutant ITs that we used in this study, the location of each mutation, and the epitope to which it is assigned are shown in Table 1. In the case of proteins containing more than one mutation, we indicate the location of all of the mutations and the epitope groups affected. The purified disulfide-bonded parental IT (HA22) migrates at 63 kDa on a nonreducing gel and is resolved into two bands of 52 and 12 kDa under reducing conditions. Fig. 1 shows the purity of HA22 (lane 1) and seven different IT mutants used in the current studies containing from one to eight mutations (lanes 2–8). When analyzed under reducing conditions, all of the ITs appear to be >95% pure, showing the expected light chain at ∼12 kDa and the heavy chain PE38 fusion protein at ∼52 kDa. Under nonreducing conditions, all eight proteins migrated at 63 kDa; only the IT with a Q332A mutation showed an extra weak band at ∼50 kDa. We conclude that the mutant ITs can be obtained in a highly purified form needed for additional experiments.
Table 1.
Cytotoxic activity on Daudi or Raji cells of mutant immunotoxins expressed relative to unmutated HA22
| Mutations | Epitope | Cytotoxic activity (%) |
|---|---|---|
| R313A | 3 | 118 ± 49 |
| Q332S | 1 | 130 ± 3 |
| R432G | 4a | 200 ± 37 |
| R467A | 2c | 98 ± 30 |
| R490A | 5 | 96 ± 34 |
| R513A | 6a | 94 ± 20 |
| E548A | 6a | 17 ± 7 |
| K590S | 7 | 200 ± 25 |
| Q332A + R467A + R490A + K590S | 1, 2c, 5, 7 | 132 ± 36 |
| R313A + Q332S + R467A + R490A + K590S | 1, 2c, 4a, 5, 7 | 206 ± 23 |
| R313A + Q332S + R432G + R467A + R490A + K590S | 1, 2c, 3, 4a, 5, 7 | 221 ± 6 |
| R313A + Q332S + R432G + R467A + R490A + R513A + K590S | 1, 2c, 3, 4a, 5, 6a, 7 | 121 ± 82 |
| R313A + Q332S + R432G + R467A + R490A + R513A + E548S + K590S | 1, 2c, 3, 4a, 5, 6a, 7 | 139 ± 60 |
Fig. 1.
Polyacrylamide electrophoresis gels of purified recombinant immunotoxins. The purified proteins (2 μg) were run on 4–20% gradient SDS polyacrylamide electrophoresis gels under nonreducing conditions (Upper) and under reducing conditions (Lower). The gels were stained with Coomasie Blue. Lane 1, HA22(dsFv)-PE38 wild type; lane 2, Q332S in PE38; lane3, R490A; lane 4, K590S; lane 5, R313A + Q332S + R467A + R490A + K590S; lane 6, R313A + Q332S + R432G + R467A + R490A + K590S; lane 7, R313A + Q332S + R432G + R467A + R490A + R513A + K590S; lane 8, R313A + Q332S + R432G + R467A + R490A + R513A + E548S + K590S (HA22–8X); M, molecular mass standards are (top to bottom) 250, 75, 50, 25, 15, and 10 kDa, respectively.
Cytotoxic Activities of Mutant ITs.
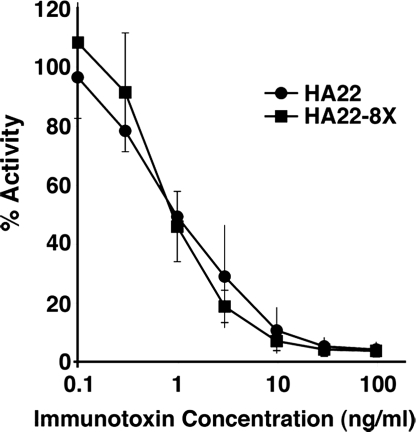
We measured the cytotoxic activities of the purified ITs to determine if the mutations produced any change in activity. The data in Table 1 show the average cytotoxic activities (IC50's) relative to HA22 of the 13 ITs used in the current studies. Each IT was assayed at least three times. We carried out both protein synthesis assays by measuring [3H]leucine incorporation and cytotoxicity assays with WST-8 (Dojindo). These assays were in close agreement (data not shown). The data in Table 1 show that all of the mutant ITs were as active as HA22, and in several cases the mutant proteins were even more active. We have not yet examined the basis of the increase in activity. Table 1 shows activities of eight ITs with single mutations and five other ITs in which five, six, seven, or eight mutations are combined. The molecule with eight mutations is designated HA22–8X and combines mutations in most of the major epitope groups previously identified. Fig. 2 shows data from a typical cytotoxicity experiment with HA22–8X. The mutations in HA22–8X are Q332S in epitope 1, R467A in epitope 2a, R313A in epitope 3, R432G in epitope 4a, R490A in epitope 5, R513A and E548S both in epitope 6a, and K590S in epitope 7.
Fig. 2.
Specific cytotoxic activity of HA22 and HA22–8X on Raji cells. The cytotoxicity assays were performed in triplicate at least twice. Data are expressed as the mean ± SD.
In some cases an epitope can be subdivided into two or three subgroups (e.g., 2a, b, c) and mutations that abolished binding to all of the subgroups could not be identified. For example, we were not able to determine the location of epitope 2b. In all cases we chose to mutate a residue that abolished binding to the largest number of members of the group (e.g., 2c, 4a, 6a). We had previously found that mutation Q332A eliminated binding of mAbs to epitope 1 (18), but when we combined this mutation with others, we found there was a significant loss of cytotoxic activity. To try to overcome this problem we made a Q332S mutation instead of a Q332A mutation and combined it with R313A, R467A, R490A, and K590S. Although the mutants with Q332S were fully active in a cytotoxicity assay, this mutation did not eliminate epitope 1, suggesting that loss of epitope 1 in the Q332A mutant is because of some conformational change not produced by a more hydrophilic serine residue. Nevertheless we decided to keep the Q332S mutation in the ITs containing several mutations, because it did eliminate a large hydrophilic amino acid, which could be immunogenic.
Residual Epitopes on the HA22–8X Mutant.
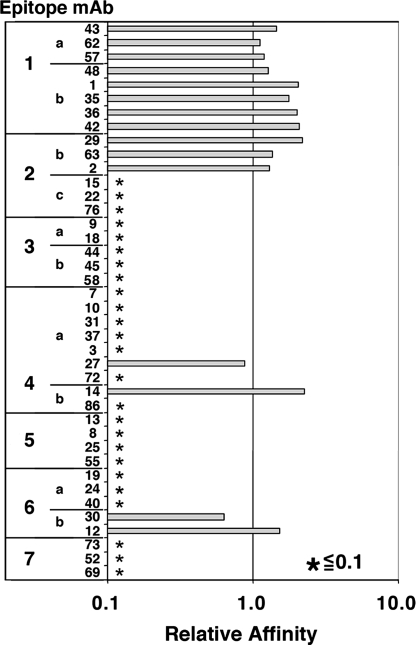
Previous data showed that individually each of the mutations eliminated binding to the corresponding mAbs (18). In the current study, we examined the binding properties of IT HA22–8X, in which eight mutations were combined in one molecule. For the assessment of binding, we used a previously established indirect ELISA termed an immune complex captured ELISA (ICC-ELISA). The ICC-ELISA detects Ag–Ab reactions that occur in solution, avoiding possible conformational changes in the epitope structure by adsorption onto a plastic surface. Binding was quantitatively evaluated as an affinity change compared to unmutated PE38 in a competition assay format as described previously (18). In this assay, the concentration of each mAb that is required for forming an immunocomplex with 50% of a small amount of PE38 was determined. Fig. 3 summarizes the affinity change for each mAb when bound to HA22–8X. We found that the binding of mAbs in epitopes 2c, 3a, 3b, 4a, 4b, 5a, 6a, and 7 was reduced to <10% of the binding affinity to an unmutated IT. However, the binding affinity of mAbs to epitopes 1a, 1b, 2b, and 6b was not reduced, indicating these epitopes are still present in the 8X mutant. In addition, there was residual binding of single mAbs to subepitopes in 4a and 4b. These studies show that there is a significant decrease in the number of B-cell epitopes present in HA22–8X, although not all have been removed.
Fig. 3.
Relative affinity of mAbs from different epitope groups with HA22–8X mutant. Numbers 1–7 and letters a–c indicate epitope groups, numbers in the second column indicate mAbs in each epitope group (18), bars represent relative affinity with 1 indicating similar reactivity of mAb with HA22–8X and HA22 and * indicating reactivity with mutant HA22–8X is <0.1 relative to HA22. Experimental procedures are described in Materials and Methods.
Anti-Tumor Activity.
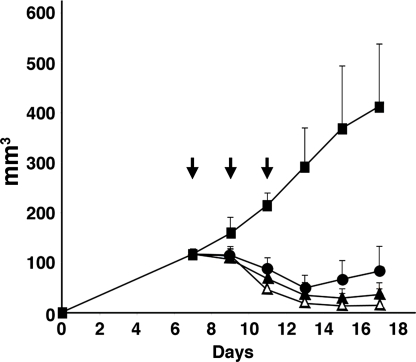
ITs against CD22 have been previously evaluated for anti-tumor activity in mice using CA46 cells growing as s.c. tumors (19). We compared the activity of HA22–8X with HA22 in this tumor model, using groups of eight mice as shown in Fig. 4. When mice were treated with 0.2 mg/kg of either of the ITs every other day times three, HA22–8X had greater anti-tumor activity on days 15 and 17, compared to HA22 (P < 0.05). In both cases the tumors decreased in size. Growth resumed on day 14 with HA22 and on day 17 with HA22–8X. Another group of mice were treated with 0.4 mg/kg of HA22–8X and much greater efficacy was observed with no tumor detected in four of eight mice on day 17 (P < 0.05). We conclude that the cytotoxic and anti-tumor activities of the native and mutant ITs are very similar.
Fig. 4.
Anti-tumor activity of HA22 and HA22–8X. Groups of eight SCID mice bearing CA46 tumors were treated every other day times three, as indicated by arrows, with PBS (filled squares), or with three doses of HA22 at 0.2 mg/kg (filled circles), or HA22–8X at 0.2 mg/kg (filled triangles) or at 0.4 mg/kg (open triangles). Tumor size was measured every other day and was calculated using the formula described in Materials and Methods. Data are expressed as the mean ± SD. There were significant differences of the anti-tumor activity between HA22 (0.2 mg/kg) and HA22–8X (0.2 mg/kg) on days 15 and 17 (P < 0.05).
HA22–8X is Less Immunogenic Than HA22.
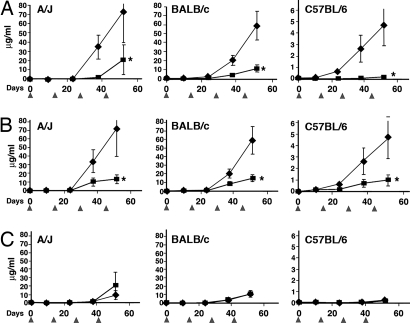
To investigate the immunogenicity of HA22 and HA22–8X, mice were dosed i.v. with 5 μg of IT every 14 days and serum was collected for analysis of antibody levels 10 days after each immunization (Fig. 5). The assays for antibody titers were designed to recognize conformational epitopes (18) and were carried out using the DELFIA fluoroimmunometric assay described in Materials and Methods. In this assay, antigen and antibody are allowed to react in solution before being captured and the amount of antibody bound to PE38 is then detected. The titers of antibody are expressed as micrograms per milliliter, using a standard curve generated using a mAb to PE38 (IP30 mAb). In the initial experiments we used BALB/c mice and observed that the antibody titers were significantly lower after both the third and the fourth ITs (Fig. 5). To be sure the MHC haplotype did not affect the results, we carried out immunization studies in two other strains of mice: A/J and C57BL/6. These mice have different MHC class II molecules that bind different groups of peptides to react with T-cell receptors: (A/J for haplotype a, C57BL/6 for b, and BALB/c for d) (20). The data in Fig. 5A show that the mutant IT was also significantly less immunogenic than the native IT in these mice with the most striking decreases observed with C57BL/6 mice. The overall titers varied among the three mouse strains, but in all cases the mutant protein was significantly less immunogenic.
Fig. 5.
Generation and cross-reactivity of the antibodies to HA22 and HA22–8X. Groups of mice (15 BALB/c, 7 A/J, and 9 C57BL/6) received 250 μg/kg of HA22 or HA22–8X i.v. every 14 days and were bled 10 days after each injection. Antibody levels were measured on respective immunogens using ICC-DELFIA as described in Materials and Methods and expressed relative to a mAb to PE38. (A) Immunogenicity of HA22 and HA22–8X: (filled diamonds) anti-HA22 level in the HA22 immunized serum and (filled squares) anti-HA22–8X level in the HA22–8X immunized serum. (B) Antigenicity, antibody level of the antisera to HA22 with HA22 (191) and HA22–8X (γ). (C) Antibody level of antisera to HA22–8X with HA22 (191) and HA22–8X (γ). Data are expressed as the mean ± SE. *, P < 0.05, compared with the respective control group.
HA22–8X Is Less Antigenic.
To assess the antigenicity of the 8X mutant protein we compared its reactivity and that of HA22 with antisera to HA22. Fig. 5B shows that HA22–8X is significantly less reactive with the anti-HA22 antisera than HA22 itself. Using the fourth bleed, there was 59 μg/ml of antibody reacting with HA22 and only 14.8 μg/ml reacting with HA22–8X, an 85% reduction that is highly significant (P < 0.05). With antisera from A/J mice, the titer was decreased from 73 to 13 μg/ml (P < 0.05) and with C57BL/6 mice from 4.7 to 0.97 μg/ml (P < 0.05). These differences in the three strains of mice are very large and highly significant, showing that HA22–8X is less antigenic as well as less immunogenic. Differences were also significant after the third immunization.
HA22–8X Does Not Have New Epitopes.
To examine the possibility that HA22–8X induced the formation of antibodies to epitopes not present in HA22, the antisera raised against HA22–8X were assayed on HA22 and HA22–8X. The results using antisera generated after the fourth immunization are shown in Fig. 5C. The reactivity of the sera from the mice immunized with HA22–8X had the same reactivity with HA22–8X or with HA22. With BALB/c mice the average antibody levels in the HA22–8X-treated group against HA22 were 9.6 ± 3.0 μg/ml and against HA22 were 11.3 ± 3.9 μg/ml. This difference is not statistically significant (P = 0.98). With A/J mice the antibody levels were 9.5 ± 5.0 μg/ml and 20.8 ± 16.2 μg/ml (P = 1.00) and with C57BL/6 mice the antibody levels were 0.19 ± 0.096 μg/ml and 0.118 ± 0.098 μg/ml, respectively, again not statistically significant (P = 0.114). These data clearly show that mutating HA22 to HA22–8X did not create new epitopes.
Discussion
We show here that we can produce a mutant IT that is less immunogenic and antigenic in mice yet maintains full cytotoxic and anti-tumor activities. Because human and mouse mAbs have been shown to recognize the same epitopes on foreign proteins, we believe our studies are relevant to humans treated with ITs (21–24). We produced the less immunogenic IT by mapping the location of the seven major B-cell epitope groups in PE38 and then mutating amino acids within these epitopes to amino acids with no side chain (glycine) or small side chains (alanine or serine). Arginine is the most frequently mutated amino acid (five of the eight). The other three are glutamine, glutamate, and lysine. All contain large bulky hydrophilic side chains, residues known to frequently provoke a strong immune response (25). We believe this is the first example of the successful reduction of immunogenicity and antigenicity without any loss of biological activity. In previous studies in which immunogenicity was decreased, it was accompanied by a significant loss of biological activity (17, 26).
Properties of HA22–8X.
To produce HA22–8X we needed to combine mutations in different locations in PE38. Our strategy to reduce immunogenicity is based on the well-accepted observation that immunization of animals with peptides and proteins containing bulky hydrophilic residues often induces a strong immunogenic response. In our previous epitope-mapping studies we chose these residues for replacement. Alanine is the most common residue used in most mutational replacement studies, but we were concerned that the replacement of all eight surface residues with alanine might alter protein folding or cause instability. Therefore we used alanine for four mutations, serine for three others, and glycine for one.
T-Cell Epitopes.
To address the possibility that we were inadvertently mutating T-cell epitopes, we carried out immunogenicity studies in three different strains of mice: BALB/c, A/J, and C57BL/6. These mice have different MHC class II molecules that bind to different groups of peptides that react with T-cell receptors (A/J for haplotype a, C57BL/6 for b, BALB/c for d) (20). In all cases immunogenicity was decreased significantly, but the magnitude of the immune response and the percentage of decrease in antibody titer varied among the three groups. HA22 induced the lowest immune response in C57BL/6 mice and even after the fourth immunization the mice injected with HA22–8X still had very low titers to PE38. In the other two strains of mice the immune responses to HA22 were similar, with very low responses to the mutant IT evident after the third immunization and a two- to fivefold decrease after the fourth immunization.
In patients with hematological malignancies who have received chemotherapy consisting of drugs that selectively kill B cells, the frequency of formation of neutralizing antibodies to ITs containing PE38 is very low (6, 27). However, in patients with epithelial tumors and mesotheliomas, where the chemotherapy used to treat the malignancy does not damage immune cells, the frequency of formation of antibodies that neutralize the IT is high and limits the amount of IT that can be given (2, 9). In the current study we have shown that we can produce a less immunogenic IT by mutating residues containing bulky hydrophilic residues. We have shown previously that human sera from patients treated with ITs contain antibodies that bind to some of the epitopes defined by our mouse studies. We have not yet analyzed if any of these are missing in the HA22–8X mutant and this will be the focus of future studies. We also do not know if the 8X mutant will be sufficiently less immunogenic in humans to be useful and allow more treatment cycles. Nevertheless we are encouraged by our ability to make a less immunogenic protein that could allow more treatment cycles and are pursuing studies to identify and remove the remaining B-cell epitopes, particularly epitope 1. An additional approach to identify human B-cell epitopes has been described by Chester and colleagues (28), who used a phage display approach to identify a major B-cell epitope present on a bacterial enzyme used to activate a pro-drug for human cancer therapy.
Summary.
We have used site-directed mutagenesis to replace bulky hydrophilic residues in the toxin portion of an IT with small amino acids and produced an IT that is fully active but significantly less immunogenic in mice. We believe that this approach can produce an IT, which is less immunogenic in humans and will allow more treatment cycles to be given, resulting in increased efficacy in patients with cancer.
Materials and Methods
Construction, Expression, and Purification of ITs.
The expression plasmids of HA22(dsFv)-PE38 IT were derived from the plasmids pEM15 that encoded RFB4(VH)(R44C)-PE38 and pEM16 that encoded RFB4(VL)(G100C) (29). Mutations were introduced into the parent expression plasmid using PCR overlap extension (30). Beginning with the expression plasmid for HA22VH-PE38 (pEM15), coding and noncoding oligonucleotides were designed to introduce individual mutations at codons Q332S, R490A, and K590S. As our study progressed, individual mutations were successfully introduced into the previous construct. Finally, R313A + Q332S + R432G + R467A + R490A + R513A + K590S was used as a template to introduce E5485 resulting in HA22–8X.
All of the ITs were made by a standard protocol established in our laboratory (31). The final step in the purification scheme is size exclusion chromatography, which assures that the IT we isolate is a monomer and not aggregated (31). Protein purity was assessed by SDS-PAGE performed under reducing and nonreducing conditions. HA22 is a disulfide-linked molecule composed of the heavy chain Fv fused to PE38 and the light chain Fv. The VH-PE38 and the VL components of the protein are expressed on two different plasmids. The proteins were expressed in Escherichia coli BL21(λDE3) in a T7-based expression system.
Testing Cytotoxic Activities of the Mutant Proteins.
Cytotoxic activity measured on Raji or Daudi cells is as previously described (31, 32). The cytotoxicity of each IT was assessed by protein synthesis inhibition assays (inhibition of incorporation of tritium-labeled leucine into cellular protein) and by WST-8 assays in 96-well plates, as previously described (29, 33). The activity of the molecule is defined by the IC50, the toxin concentration that reduced incorporation of radioactivity or cell growth by 50% compared with cells with that were not treated with IT.
Anti-Tumor Activity of Mutant IT HA22–8X.
The anti-tumor activity of ITs was determined in SCID mice bearing human lymphoma CA46 cells that express CD22 (19). All procedures were conducted in accordance with National Institutes of Health guidelines as approved by the Animal Care and Use Committee of the National Cancer Institute. A total of 200 μl cells (1 × 107) with matrigel (4 mg/ml; BD Biosciences) were injected s.c. into nude mice on day 0. Tumors >100 mm3 in size developed in animals by day 7. Starting on day 7, animals were treated with i.v. injections of HA22 or HA22–8X IT diluted in 0.2 ml of PBS/0.2% HSA or with diluent only in the control group. IT was given every other day (on days 7, 9, and 11), and each treatment group consisted of eight animals. Tumors were measured with a caliper every 2 or 3 days. Tumor volume was calculated using the formula tumor volume (mm3) = length × (width)2 × 0.4. Tumor sizes are expressed as mean ± SD.
Generation of Human CD22-Rabbit Fc Fusion Protein by Mammalian Cells.
The extracellular domain of the human CD22 was expressed as a fusion protein with rabbit IgG Fc in HEK 293T cells (34). The DNA fragment encoding rabbit IgG Fc was amplified by PCR as previously described (34) and inserted between BamHI and NotI sites of pcDNA3 (Invitrogen). The cDNA for the extracellular domain of CD22 was inserted between EcoRI and SacII to obtain the plasmid pOND-22Fc. Primers used were as follows: forward, 5′-AGT GTG CTG GAA TTC ACC ATG CAT CTC CTC GGC CCC TGG CTC-3′; reverse, 5′-CGA CGA CCA CCG CGG TCC GCT TCG CCT GCC GAT GGT CTC CGG-3′. The CD22-rFc protein was harvested from the culture supernatant and purified with Hi-trap protein A column (Amersham Biosciences). The purified proteins were quantitated by Coomasie Blue (Pierce) and checked on an SDS-PAGE gel.
ICC Assay by DELFIA.
To preserve the native antigen structure, we used an ICC-ELISA procedure (18) and measured the anti-PE38 antibody concentrations using a commercial fluoroimmunometric assay (DELFIA, Perkin–Elmer Life Science). ICC-DELFIA detects antigen–antibody reactions that occur in solution. Microtiter plates (MaxiSorp, Nalge Nunc) were coated with 100 ng/50 μl/well of CD22-rFc in PBS overnight at 4°C. In separate tubes, the anti-PE38 mAb diluted in blocking buffer (25% DMEM, 5% FBS, 25 mM Hepes, 0.5% BSA, 0.1% sodium azide in PBS) was mixed with 2 μg/ml of an anti-CD22 immunotoxin (HA22 or 8X) and the diluted mouse serum to be tested. Serum was diluted 250-fold. HA22 was used for HA22-ICC DELFIA and HA22–8X used for 8X-ICC-DELFIA. After washing the plates with PBS containing 0.05% Tween 20, the immunotoxin–serum mixtures were transferred to individual wells (50 μl/well). The amount of immune complex captured by the CD 22-rFc fusion proteins was detected by the mixture of biotinylated rabbit anti-mouse IgG (315–065-046, Jackson Immuno Research Laboratories) and Eu-labeled streptavidin (1244–360, Perkin–Elmer Life Science), followed by DELFIA inducer (4013–0010, Perkin–Elmer Life Science). DELFIA Eu-fluorescence was measured with a 1420 VICTOR3 multilabel counter (Perkin–Elmer Life Science). Each plate contained serial dilutions of anti-PE38 mAb IP30 as a concentration control (18). The antibody concentrations were expressed as mAb IP30 concentrations. A standard curve of the IP30 reactivity was created with a four-parameter logistic curve model by SoftMaxPro 4.0 (Molecular Devices). mAb IP30 reacts with HA22 and HA22–8X similarly in ICC-DELFIA (data not shown).
Immunization Studies.
For the immunogenicity study, we used three strains of mice with different haplotypes. Groups of 15 BALB/c mice, groups of 7 A/J mice, and groups of 9 C57BL/6 mice were immunized. Mice received four doses 250 μg/kg IT (HA22 or 8X) every 2 weeks (35). Ten days after each injection, mice were bled and serum was stored at −20°C until assayed. These studies were performed at the National Cancer Institute under an approved protocol.
Competition Assay to Measure the Reactivity of mAbs to HA22–8X Mutant.
HA22 and HA22–8X were tested for reactivity with each mAb in solution to keep both antigen and antibody in the native form during the reaction. We used 40 representative mAbs in the competition assay that cover all 7 major epitope groups and 12 subgroups. These mAbs were extensively characterized in our previous report (18). To standardize the different affinity of individual mAbs assigned to each epitope, the result was evaluated as relative affinity, which was defined as the ratio of IC50 concentrations each of HA22–8X and of HA22 that were required for the binding to the same amount of each mAb. These values are close to the affinity ratio determined by BIAcore Biosensor as previously described (18). In brief, a series of fourfold dilutions of HA22–8X or HA22 IT (0.04–10,000 ng/ml) were mixed with an appropriate concentration of each mAb in blocking buffer at 4°C overnight. On the next day, the IT-mAb mixtures were transferred to the microtiter plates that had been coated with mesothelin-rFc, followed by a 2-h incubation with 2 μg/ml SS1P. After 1 h, the uncomplexed mAb in the mixture was captured by SS1P without waiting for reequilibrium. The captured mAbs were finally detected by HRP-conjugated goat anti-mouse IgG (H + L), followed by TMB substrate. The concentrations of HA22–8X mutant that reduced the signal by 50% (IC50) were calculated by fitting to a four-parameter logistic curve. The relative affinity of HA22–8X to a mAb was calculated according to the formula relative affinity of a mutant to a mAb (IC50 of wild-type PE38 to the mAb binding/IC50 of the HA22–8X to the mAb binding) as previously described (18).
Statistics.
For comparison between the two experimental groups in the anti-tumor study and the immunogenicity study, the Mann–Whitney nonparametric method was used; P < 0.05 was considered statistically significant.
Acknowledgments.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
References
- 1.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 2.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 3.Reiter Y, Brinkmann U, Lee BK, Pastan I. Engineering antibody Fv fragments for cancer detection and therapy: Disulfide-stabilized Fv fragments. Nat Biotechnol. 1996;14:1239–1245. doi: 10.1038/nbt1096-1239. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann U, Pastan I. Immunotoxins against cancer. Biochim Biophys Acta. 1994;1198:27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 5.Reiter Y, Pastan I. Recombinant Fv immunotoxins and Fv fragments as novel agents for cancer therapy and diagnosis. Trends Biotechnol. 1998;16:513–520. doi: 10.1016/s0167-7799(98)01226-8. [DOI] [PubMed] [Google Scholar]
- 6.Kreitman RJ, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 7.Kreitman RJ, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 8.Hassan R, et al. Antitumor activity of SS(dsFv)-PE38 and SS1(dsFv)-PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin Cancer Res. 2002;8:3520–3526. [PubMed] [Google Scholar]
- 9.Hassan R, et al. Phase I study of SS1P a recombinant anti-mesothelin immunotoxin given as a bolus IV infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 10.Molineux G. Pegylation: Engineering improved biopharmaceuticals for oncology. Pharmacotherapy. 2003;23:3S–8S. doi: 10.1592/phco.23.9.3s.32886. [DOI] [PubMed] [Google Scholar]
- 11.Kushner BH, Cheung IY, Kramer K, Modak S, Cheung NKV. High-dose cyclophosphamide inhibition of humoral immune response to murine monoclonal antibody 3F8 in neuroblastoma patients: Broad implications for immunotherapy. Pediatr Blood Cancer. 2007;48:430–434. doi: 10.1002/pbc.20765. [DOI] [PubMed] [Google Scholar]
- 12.Leonard JP, et al. Abbreviated chemotherapy with fludarabine followed by tositumomab and iodine I 131 tositumomab for untreated follicular lymphoma. J Clin Oncol. 2005;23:5696–5704. doi: 10.1200/JCO.2005.14.803. [DOI] [PubMed] [Google Scholar]
- 13.De Groot AS, Knopp PM, Martin W. De-immunization of therapeutic proteins by T-cell epitope modification. Dev Biol. 2005;122:171–194. [PubMed] [Google Scholar]
- 14.Tangri S, et al. Rationally engineered therapeutic proteins with reduced immunogenicity. J Immunol. 2005;174:3187–3196. doi: 10.4049/jimmunol.174.6.3187. [DOI] [PubMed] [Google Scholar]
- 15.Laroche Y, et al. Recombinant staphylokinase variants with reduced antigenicity due to elimination of B-lymphocyte epitopes. Blood. 2000;96:1425–1432. [PubMed] [Google Scholar]
- 16.Bresson D, et al. Directed mutagenesis in region 713–720 of human thyroperoxidase assigns 713KFPED717 residues as being involved in the B domain of the discontinuous immunodominant region recognized by human autoantibodies. J Biol Chem. 2004;279:39058–39067. doi: 10.1074/jbc.M403897200. [DOI] [PubMed] [Google Scholar]
- 17.Mayer A, et al. Modifying an immunogenic epitope on a therapeutic protein: A step towards an improved system for antibody-directed enzyme prodrug therapy (ADEPT) Br J Cancer. 2004;90:2402–2410. doi: 10.1038/sj.bjc.6601888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onda M, et al. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol. 2006;177:8822–8834. doi: 10.4049/jimmunol.177.12.8822. [DOI] [PubMed] [Google Scholar]
- 19.Kreitman RJ, Wang Q, FitzGerald DJ, Pastan I. Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by cynomolgus monkeys. Int J Cancer. 1999;81:148–155. doi: 10.1002/(sici)1097-0215(19990331)81:1<148::aid-ijc24>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Kruisbeek AM. Commonly used mouse strains. Curr Protoc Immunol. 2001;17(Suppl) doi: 10.1002/0471142735.ima01cs17. A. 1C1. [DOI] [PubMed] [Google Scholar]
- 21.Atassi MZ, et al. Mapping of the antibody-binding regions on botulinum neurotoxin H-chain domain 855–1296 with antitoxin antibodies from three host species. J Protein Chem. 1996;15:691–700. doi: 10.1007/BF01886751. [DOI] [PubMed] [Google Scholar]
- 22.Goding JW. In: Monoclonal Antibodies: Principles and Practice. Goding JW, editor. San Diego: Academic; 1996. pp. 50–71. [Google Scholar]
- 23.Bugelski PJ, Treacy G. Predictive power of preclinical studies in animals for the immunogenicity of recombinant therapeutic proteins in humans. Curr Opin Mol Ther. 2004;6:10–16. [PubMed] [Google Scholar]
- 24.Atassi MZ, Dolimbek BZ. Mapping of the antibody-binding regions on the HN-domain (residues 449–859) of botulinum neurotoxin A with antitoxin antibodies from four host species: Full profile of the continuous antigenic regions of the H-chain of botulinum neurotoxin A. Protein J. 2004;23:39–52. doi: 10.1023/b:jopc.0000016257.91979.06. [DOI] [PubMed] [Google Scholar]
- 25.Price MR, Petrakou E, Sekowski M, Murray A. Immunogenicity of the hydrophilic region of the MUC1 mucin protein core. Oncol Rep. 1997;4:337–339. doi: 10.3892/or.4.2.337. [DOI] [PubMed] [Google Scholar]
- 26.Collen D, et al. Recombinant staphylokinase variants with altered immunoreactivity. I: Construction and characterization. Circulation. 1996;94:197–206. doi: 10.1161/01.cir.94.2.197. [DOI] [PubMed] [Google Scholar]
- 27.Kreitman RJ, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 28.Spencer DIR, et al. A strategy for mapping and neutralizing conformational immunogenic sites on protein therapeutics. Proteomics. 2002;2:271–279. doi: 10.1002/1615-9861(200203)2:3<271::aid-prot271>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Kreitman RJ, et al. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) toward fresh malignant cells from patients with B-cell leukemia. Clin Cancer Res. 2000;6:1476–1487. [PubMed] [Google Scholar]
- 30.Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: Tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 31.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–518. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 32.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 33.Brinkmann U, Pai LH, FitzGerald DJ, Willingham M, Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci USA. 1991;88:8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onda M, et al. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting, western blotting, and ELISA. Clin Cancer Res. 2005;11:5840–5846. doi: 10.1158/1078-0432.CCR-05-0578. [DOI] [PubMed] [Google Scholar]
- 35.Tsutsumi Y, et al. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc Natl Acad Sci USA. 2000;97:8548–8553. doi: 10.1073/pnas.140210597. [DOI] [PMC free article] [PubMed] [Google Scholar]