Abstract
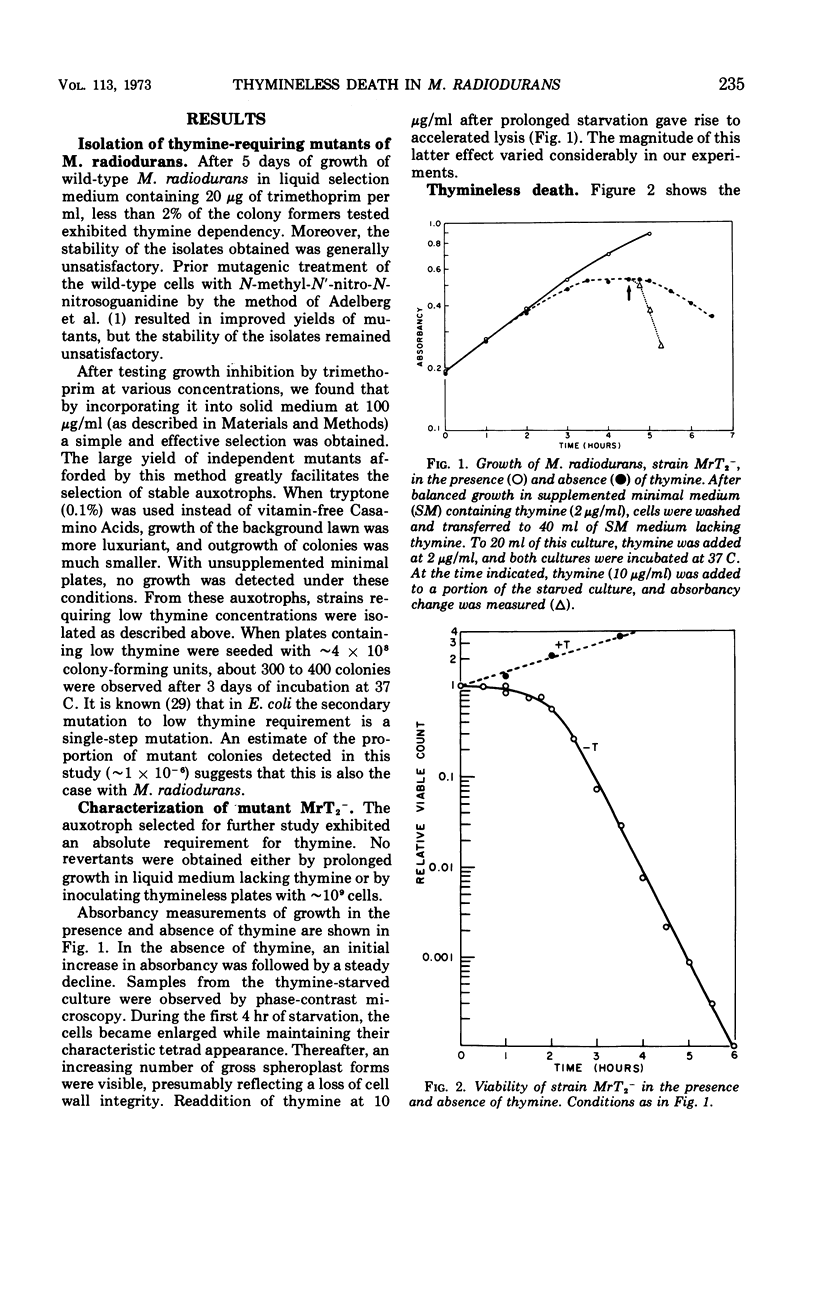
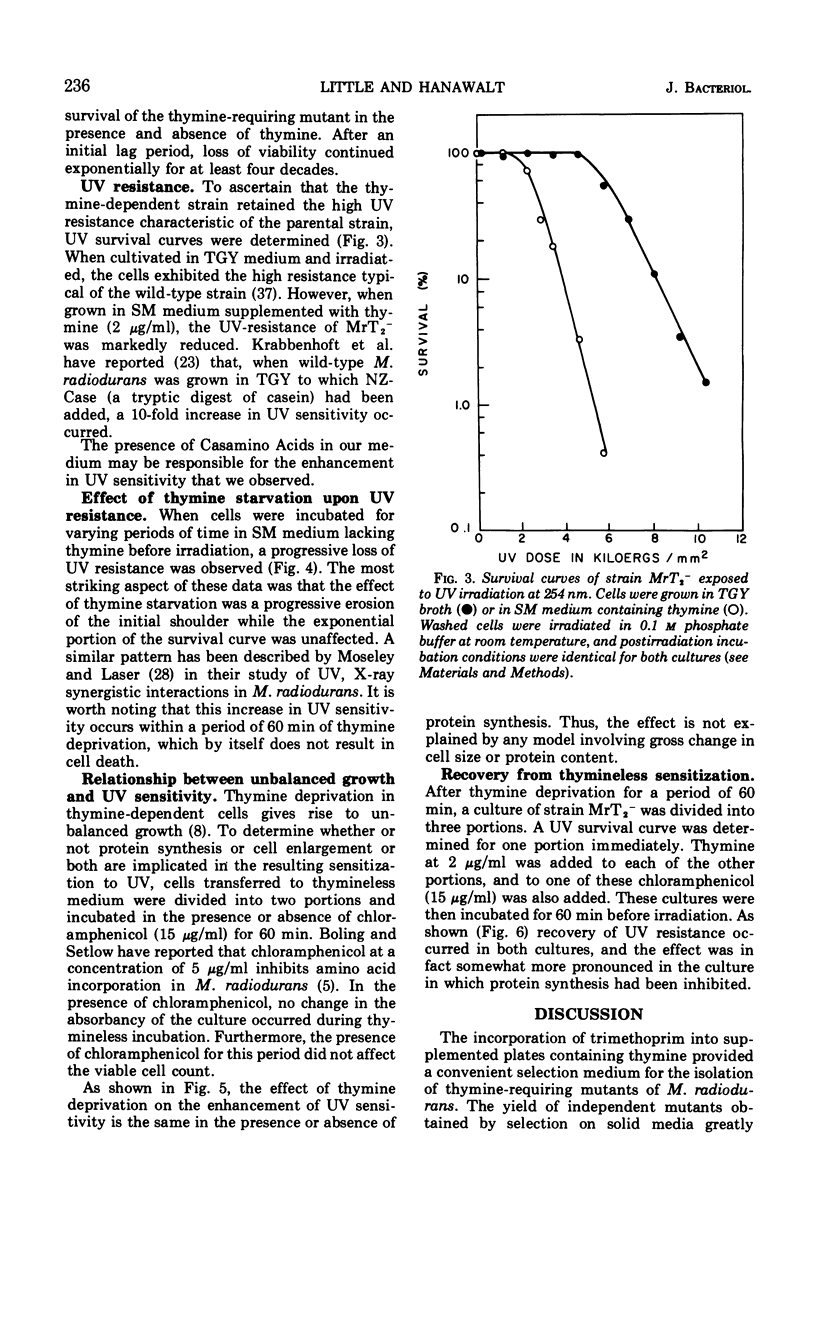
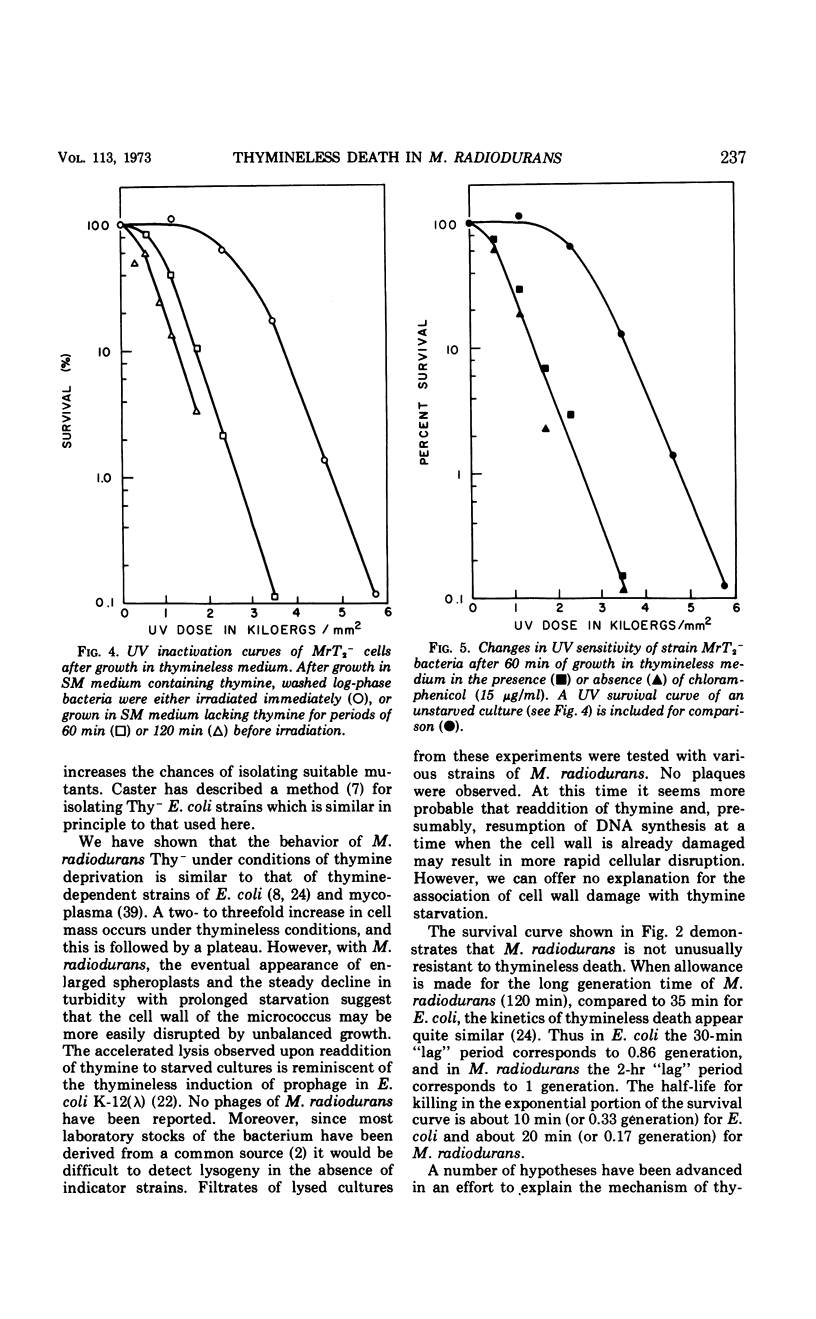
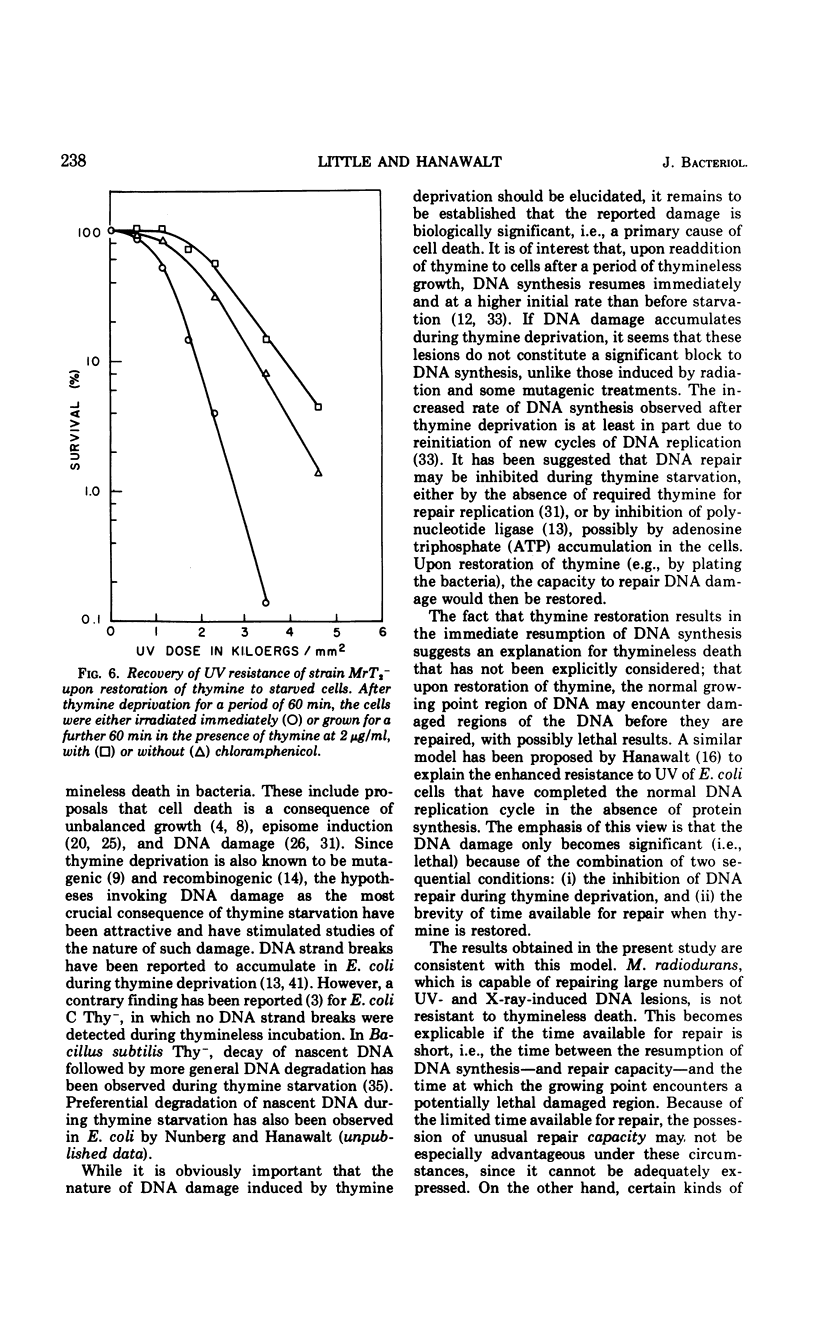
Thymine-requiring mutants of Micrococcus radiodurans have been isolated by selection on solid medium containing trimethoprim. Strains requiring either high concentrations of thymine (50 μg/ml) or low concentrations (2 μg/ml) for normal growth were obtained. The Thy− mutant requiring low thymine concentrations has been characterized. It was shown to retain the high ultraviolet light (UV) resistance typical of wild-type M. radiodurans, but it was not resistant to thymineless death. Preliminary exposure of the cells to thymineless conditions resulted in enhanced UV sensitivity, and this interaction occurred under conditions where “unbalanced growth” was inhibited by the addition of chloramphenicol. Upon addition of thymine to deprived cells, UV resistance was gradually restored, and this recovery took place in the absence of protein synthesis. A model is proposed to account for the similarity of thymineless death in bacteria whose deoxyribonucleic acid repair efficiencies differ widely.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., COUGHLIN C. A. Bacterial mutation induced by thymine starvation. Nature. 1956 Sep 8;178(4532):531–532. doi: 10.1038/178531a0. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. L., Hewitt R. R. Influence of thymine starvation on the integrity of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1971 Mar;105(3):733–738. doi: 10.1128/jb.105.3.733-738.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazill G. W. Lethal unbalanced growth in bacteria. Nature. 1967 Oct 28;216(5113):346–349. doi: 10.1038/216346a0. [DOI] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- Caster J. H. Selection of thymine-requiring strains from Escherichia coli on solid medium. J Bacteriol. 1967 Nov;94(5):1804–1804. doi: 10.1128/jb.94.5.1804-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. J., Feldschreiber P., Lett J. T. Repair of x-ray damage to the deoxyribonucleic acid in Micrococcus radiodurans. Nature. 1966 Jan 1;209(5018):49–52. doi: 10.1038/209049a0. [DOI] [PubMed] [Google Scholar]
- Dean C. J., Little J. G., Serianni R. W. The control of post irradiation DNA breakdown in Micrococcus radiodurans. Biochem Biophys Res Commun. 1970 Apr 8;39(1):126–134. doi: 10.1016/0006-291x(70)90767-9. [DOI] [PubMed] [Google Scholar]
- Donachie W. D. Control of cell division in Escherichia coli: experiments with thymine starvation. J Bacteriol. 1969 Oct;100(1):260–268. doi: 10.1128/jb.100.1.260-268.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Single-strand breaks in bacterial DNA associated with thymine starvation. J Mol Biol. 1969 Oct 14;45(1):1–7. doi: 10.1016/0022-2836(69)90205-8. [DOI] [PubMed] [Google Scholar]
- GALLANT J., SPOTTSWOOD T. MEASUREMENT OF THE STABILITY OF THE REPRESSOR OF ALKALINE PHOSPHATASE SYNTHESIS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1591–1598. doi: 10.1073/pnas.52.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLANT J., SUSKIND S. R. Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J Bacteriol. 1961 Aug;82:187–194. doi: 10.1128/jb.82.2.187-194.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C. The U.V. sensitivity of bacteria: its relation to the DNA replication cycle. Photochem Photobiol. 1966 Jan;5(1):1–12. [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Hirota Y. Hybridization between Escherichia coli K-12 and 15T- and thymineless death of their derivatives. J Bacteriol. 1965 Nov;90(5):1496–1497. doi: 10.1128/jb.90.5.1496-1497.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Efficiency of utilization of thymine and 5-bromouracil for normal and repair DNA synthesis in bacteria. Biochim Biophys Acta. 1968 May 21;157(3):532–545. doi: 10.1016/0005-2787(68)90151-2. [DOI] [PubMed] [Google Scholar]
- Krabbenhoft K. L., Anderson A. W., Elliker P. R. Influence of culture media on the radiation resistance of Micrococcus radiodurans. Appl Microbiol. 1967 Jan;15(1):178–185. doi: 10.1128/am.15.1.178-185.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MENNIGMANN H. D., SZYBALSKI W. Molecular mechanism of thymine-less death. Biochem Biophys Res Commun. 1962 Nov 27;9:398–404. doi: 10.1016/0006-291x(62)90023-2. [DOI] [PubMed] [Google Scholar]
- Mennigmann H. D. Induction in Escherichia coli 15 of the colicinogenic factor by thymine-less death. Biochem Biophys Res Commun. 1964 Jul 1;16(4):373–378. doi: 10.1016/0006-291x(64)90043-9. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Laser H. Similarity of repair of ionizing and ultra-violet radiation damage in Micrococcus radiodurans. Nature. 1965 Apr 24;206(982):373–375. doi: 10.1038/206373a0. [DOI] [PubMed] [Google Scholar]
- Moseley B. E. Repair of ultraviolet radiation damage in sensitive mutants of Micrococcus radiodurans. J Bacteriol. 1969 Feb;97(2):647–652. doi: 10.1128/jb.97.2.647-652.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T. Mutational Site of the Gene Controlling Quantitative Thymine Requirement in ESCHERICHIA COLI K-12. Genetics. 1966 Dec;54(6):1329–1336. doi: 10.1093/genetics/54.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hanawalt P. Nonconservative DNA replication in bacteria after thymine starvation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1728–1735. doi: 10.1073/pnas.54.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJ H. D., DURYEE F. L., DEENEY A. M., WANG C. H., ANDERSON A. W., ELLIKER P. R. Utilization of carbohydrates and amino acids by Micrococcus radiodurans. Can J Microbiol. 1960 Jun;6:289–298. doi: 10.1139/m60-033. [DOI] [PubMed] [Google Scholar]
- Reiter H., Ramareddy G. Loss of DNA behind the growing point of thymine-starved Bacillus subtilis 168. J Mol Biol. 1970 Jun 14;50(2):533–548. doi: 10.1016/0022-2836(70)90210-x. [DOI] [PubMed] [Google Scholar]
- SETLOW J. K., DUGGAN D. E. THE RESISTANCE OF MICROCOCCUS RADIODURANS TO ULTRAVIOLET RADIATION. I. ULTRAVIOLET-INDUCED LESIONS IN THE CELL'S DNA. Biochim Biophys Acta. 1964 Aug 12;87:664–668. doi: 10.1016/0926-6550(64)90284-1. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serianni R. W., Bruce A. K. Radioresistance of Micrococcus radiodurans during the growth cycle. Radiat Res. 1968 Nov;36(2):193–207. [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Macromolecular synthesis and thymineless death in Mycoplasma laidlawii B. J Bacteriol. 1968 Dec;96(6):2066–2076. doi: 10.1128/jb.96.6.2066-2076.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R. Thymine Starvation and Single-Strand Breaks in Chromosomal Deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1391–1392. doi: 10.1128/jb.104.3.1391-1392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


