Abstract
South Africa has high rates of tuberculosis (TB), including multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains. Expanding access to culture and drug susceptibility testing (DST) for TB diagnosis may help control this epidemic, but the potential impact of existing and novel TB diagnostics is uncertain. By fitting to World Health Organization epidemiological estimates, we developed a compartmental difference-equation model of the TB/HIV epidemic among South African adults. Performing culture and DST in 37% of new cases and 85% of previously treated cases was projected to save 47,955 lives (17.2% reduction in TB mortality, 95% simulation interval (S.I.) 8.9−24.4%), avert 7,721 MDR-TB cases (14.1% reduction, 95% S.I. 5.3–23.8%), and prevent 46.6% of MDR-TB deaths (95% S.I. 32.6–56.0%) in South Africa over 10 years. Used alone, expanded culture and DST did not reduce XDR-TB incidence, but they enhanced the impact of transmission-reduction strategies, such as respiratory isolation. In South Africa, expanding TB culture and DST could substantially reduce TB, and particularly MDR-TB, mortality. Control of XDR-TB will require additional interventions, the impact of which may be enhanced by improved TB diagnosis.
Keywords: clinical laboratory techniques, drug resistance, theoretical models
Accurate diagnosis remains an important obstacle to tuberculosis (TB) control in countries with a high TB burden (1). Sputum smear microscopy—the backbone of TB diagnosis worldwide—is <50% sensitive for detecting active TB in most laboratories (2), and its performance is particularly poor in human immunodeficiency (HIV)-infected patients, who experience more rapid progression and higher mortality from TB (3, 4). Other diagnostic modalities are hampered by inconsistent performance characteristics [e.g., chest X-rays (5) and antibiotic trials (6)], inability to distinguish active from latent infection [e.g., tuberculin skin testing and IFN-gamma release assays (7)], and resource requirements [e.g., nucleic acid amplification (8)]. Currently, the closest “gold standard” of TB diagnosis is culture of Mycobacterium tuberculosis from clinical specimens (1). Presently, culture is also required to perform drug susceptibility testing (DST) for most TB drugs. With multidrug-resistant (MDR) TB now accounting for ≈5% of all TB cases (9), and extensively drug-resistant (XDR) TB confirmed in at least 45 countries (10), appropriate diagnosis of drug-resistant strains has become increasingly urgent (11). By detecting cases of sputum-smear negative or drug-resistant TB earlier in their disease course, more widespread use of TB culture and DST might enhance TB control efforts in high-burden countries, particularly those with a high TB/HIV prevalence. However, TB culture and DST also entail substantial cost, human resource investment, and diagnostic delay (12, 13). Estimating the potential impact of expanded culture and DST could therefore inform the appropriate role of existing TB diagnostic tools in high-burden countries, while also serving as a realistic benchmark for development of novel TB diagnostics.
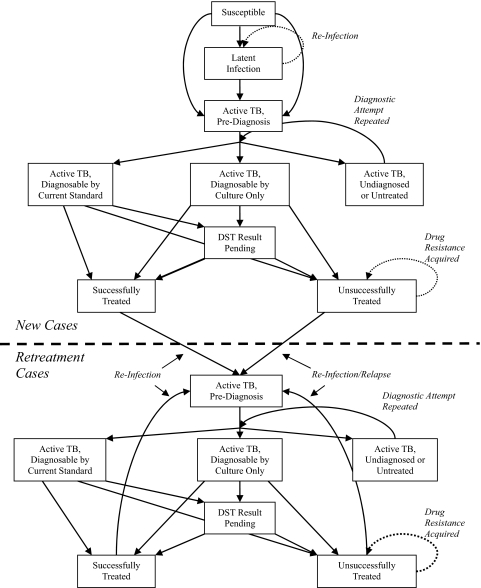
South Africa has a high TB burden, the world's largest TB-HIV coinfected population (2), and recent experience with XDR-TB outbreaks (14). However, unlike most African countries, South Africa also has existing country-wide laboratory capacity for TB culture. Thus, to assess the potential impact of improved TB diagnosis, we developed a mathematical model of TB in the South African adult population (age 15–49) under expanded access to culture and DST in South Africa (Fig. 1).
Fig. 1.
Mathematical model of South African tuberculosis epidemic. Each box represents a TB subpopulation and is subdivided by HIV status (positive or negative), drug resistance (if TB-infected: nonresistant, multidrug-resistant, or extensively drug-resistant), and infectivity (if active TB: highly or less infectious).
Model Description.
The model population is divided into compartments defined by four dimensions: TB disease state (susceptible, latent infection, diseased, or cured), HIV status (positive or negative), TB drug susceptibility (nonresistant, MDR, or XDR), and TB infectivity (highly or less infectious). Complete details are in supporting information (SI) Text and SI Appendix. Briefly, key model assumptions include: All TB-infected individuals harbor a single TB strain with a specified drug resistance pattern at any given time.
Drug-resistant TB is acquired by infection (or reinfection) with resistant strains or by de novo mutation while on noncurative therapy.
Active TB is classified as either “highly infectious” (sputum smear-positive under ideal laboratory conditions) or “less infectious” (all other active TB).
Of TB patients who are diagnosed by culture, 70% (80%, minus 10% loss to follow-up) receive a treatment regimen. TB treatment regimens (defined as receipt of ≥1 month of medication) are either inactive or active.
Inactive regimens (e.g., isoniazid, rifampin, pyrazinamide and ethambutol for patients with MDR-TB) have no effect on infectiousness or mortality.
Active regimens rapidly render the patient noninfectious and return the patient's mortality risk to that of individuals without TB disease.
Of patients receiving active regimens, 70% have their bacillary burden reduced to a level from which relapse cannot occur, assuming that this percentage is similar to South Africa's estimated treatment success rate (cure plus treatment completion) in 2005 (2). The remaining 30% remain at risk for relapse, which may occur with a strain that acquired drug resistance through mutation.
South African guidelines recommend culture for all new TB suspects with a negative sputum smear, and culture plus DST for all TB suspects who have been previously treated or who remain smear-positive after receiving two months of TB therapy (15). Because the nationwide utilization of culture and DST is not known, we assumed culture utilization as in one representative province (Free State) in 2004 and 2005: 4–8% of all new registered adult TB cases (1,423/29,651) and 36–37% of previously treated cases (3,003/8,186) (Table S1). We assumed that cultures included DST for previously treated TB suspects but not new patients, performing sensitivity analysis for receipt of DST results in only 75% of previously treated suspects, similar to the rate (79%) seen in a clinic serving a large township of Cape Town (M. Goniwe and V. Azevedo, unpublished results).
We estimated the potential impact of improved TB diagnosis in South Africa under eight independent scenarios. For scenarios involving expanded use of TB culture, we assumed that policy changes could effectively expand culture use in new TB cases to levels currently achieved in retreatment cases (37%), and in retreatment TB cases to include all patients currently receiving sputum smear (85%; see Table S1). The primary outcomes were the projected 10-year cumulative number of TB deaths, MDR-TB cases, and XDR-TB cases under each scenario. The eight scenarios (see also Table 1) were as below, with scenario 5 representing the primary intervention:
Baseline: Culture performed without DST in 5% of new TB suspects and with DST in 37% of previously treated suspects.
Expanded culture, new cases (no DST): Culture performed in 37% of all TB suspects, including DST if previously treated.
Expanded culture, new cases (with DST): Culture performed as in scenario 2, including DST with all cultures.
Expanded culture, retreatment cases: Culture performed without DST in 5% of new TB suspects, and with DST in 85% of previously treated suspects (approximating culture with every sputum smear in these patients).
Expanded culture, all TB suspects: Culture and DST performed in 37% of new TB suspects, and in 85% of previously treated TB suspects.
Expanded culture, gradual roll-out: Culture and DST utilization rates increased by 5% per year until reaching the levels in scenario 5.
New diagnostic test: Culture and DST performed at baseline levels for five years, then replaced by a hypothetical new test with 100% sensitivity for TB, no diagnostic delay, and ability to detect drug resistance (after 1-month delay), at utilization levels described in scenario 5.
Expanded culture plus XDR isolation: Culture used as in scenario 5, plus 50% of XDR-TB cases isolated and rendered noninfectious, only after receipt of DST results.
Table 1.
Projected impact of expanded tuberculosis culture and drug susceptibility testing (DST) in South Africa
| Scenario | Percentage of TB suspects receiving culture* | Cumulative 10-year projected TB outcomes |
|||||
|---|---|---|---|---|---|---|---|
| Total TB mortality |
MDR-TB† incidence |
XDR-TB incidence |
|||||
| Cases (×1,000) | Percent averted (95% SI) | Cases (×1,000) | Percent averted (95% SI) | Cases (×1,000) | Percent averted (95% SI) | ||
| 1: Baseline | Never treated, 5%, no DST; previously treated, 37% | 278 | 0% | 54.8 | 0% | 9.0 | 0% |
| 2: Expanded culture, new cases (no DST) | Never treated, 37%, no DST; previously treated, 37% | 243 | 12.6% (5.2, 19.5) | 55.0 | −0.4% (−1.9, 0.0) | 9.0 | −0.7% (−2.0, −0.2) |
| 3: Expanded Culture, New Cases (+ DST) | Never treated, 37%; previously treated: 37% | 240 | 13.6% (6.4, 20.4) | 51.8 | 5.5% (1.1, 11.0) | 9.1 | −1.2% (−2.4, −0.4) |
| 4: Expanded culture, retreatment cases | Never treated, 5%, no DST; previously treated, 85% | 267 | 4.1% (2.2, 6.1) | 48.9 | 10.6% (4.6, 17.7) | 9.0 | −1.0% (−1.5, −0.2) |
| 5: Expanded culture, all TB suspects | Never treated, 37%; previously treated, 85% | 230 | 17.2% (8.9, 24.4) | 47.0 | 14.1% (5.3, 23.8) | 9.1 | −1.9% (−3.3, −0.7) |
| 6: Expanded culture, gradual roll-out | Never treated, 10% (year 1), increasing by 5% per year to 37%; previously treated, 40% (year 1), increasing by 5% per year to 85% | 245 | 11.8% (5.8, 17.4) | 50.6 | 7.7% (2.5, 14.2) | 9.1 | −1.1% (−1.9, −0.3) |
| 7: New diagnostic test‡ | As in Scenario 1 (years 1–5). New test, with access as in Scenario 5 (years 6–10) | 231 | 16.8% (10.0, 25.3) | 51.6 | 5.8% (1.9, 10.9) | 9.1 | −1.1% (−2.8, −0.3) |
| 8: Expanded culture plus XDR isolation | As in Scenario 5; 50% of XDR-TB cases isolated upon receiving DST results | 228 | 18.0% (10.3, 25.9) | 47.1 | 14.1% (5.3, 23.8) | 5.1 | 43.5% (36.5, 65.6) |
*Culture includes drug susceptibility testing unless otherwise indicated.
†MDR-TB incidence excludes XDR-TB.
‡In this scenario, culture is replaced after 5 years with a test having 100% sensitivity for TB, no diagnostic delay, and DST capability similar to that of culture.
Results
According to the assumptions of this mathematical model, South African adults (ages 15–49) will suffer 278,154 TB deaths and 54,765 incident MDR-TB cases between the years of 2008 and 2017, if current control measures are continued. Table 1 lists cumulative 10-year TB outcomes under each of the 8 scenarios described above; Fig. 2 presents TB and MDR-TB mortality on a year-by-year basis. Performing TB culture and DST in 37% of all new TB suspects and 85% of previously treated suspects was projected to save 47,955 lives, or 17.2% [95% simulation interval (S.I.) 8.9–24.4%] of all TB deaths that would occur during this time (scenario 5, Table 1). Culture and DST as used in scenario 5 have a more modest impact on TB incidence, averting 60,193 (3.0%, 95% S.I. 1.1–5.9%) of 2,010,095 TB cases, but have a relatively greater effect on MDR strains, averting 7,721 incident MDR-TB cases (14.1%, 95% S.I. 5.3–23.8%) and 7,069 MDR-TB deaths (46.6%, 95% S.I. 32.6–56.0%). For comparison, increasing the TB cure rate from 70% to 87.5% among patients treated with active regimens, without any change in diagnostic strategy, was projected to avert 165,138 TB cases (8.2%) and 10,966 MDR-TB cases (20.0%), but only 14,758 TB deaths (5.3%) and 1,775 MDR-TB deaths (11.7%). Simultaneously increasing culture/DST utilization and cure rates would substantially reduce TB mortality (21.7%), TB incidence (11.3%), and MDR-TB incidence (34.1%).
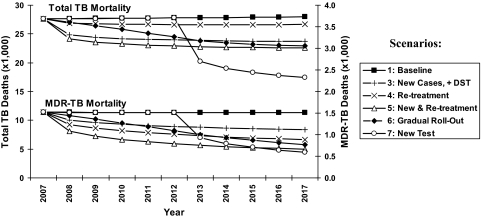
Fig. 2.
Annual TB and MDR-TB mortality with expanded culture and DST. Projected annual deaths under six scenarios of culture/DST access (see Materials and Methods and Table 1). Total TB mortality, per year, is shown by the upper set of lines corresponding to the left vertical axis, and MDR-TB mortality is shown by the lower set of lines corresponding to the right vertical axis. Cumulative 10-year impact corresponds to the area between the baseline curve (squares) and the curve corresponding to a given scenario. Scenario 2 is not shown because of overlap with scenario 3 (total TB mortality) and scenario 1 (MDR-TB mortality), and scenario 8 is not shown because of overlap with scenario 5. MDR-TB excludes XDR-TB.
Scenarios 2–4 (Table 1) reveal the relative impact of expanding utilization of culture and DST in new and previously treated TB suspects. Specifically, expanding culture and DST utilization from 5% to 37% in new TB suspects only was projected to avert 13.6% (95% S.I. 6.4–20.4%) of TB mortality and 5.5% (95% S.I. 1.1–11.0%) of MDR-TB incidence, versus 4.1% (95% S.I. 2.2–6.1%) and 10.6% (95% S.I. 4.6–17.7%) if culture and DST are expanded from 37% to 85% in previously treated suspects only. Performing culture without (versus with) DST in new TB cases had a similar effect on TB mortality averted (12.6%, 95% S.I. 5.2–19.5%) but increased projected MDR-TB incidence (0.4% increase, 95% S.I. 0.0%–1.9% increase).
Gradual expansion of culture and DST utilization rates (scenario 6, Table 1) was projected to avert 32,082 (11.8%, 95% S.I. 5.8–17.4%) TB deaths and 4,213 (7.7%, 95% S.I. 2.5–14.2%) MDR-TB cases over 10 years, or 15,153 (5.4%, 95% S.I. 3.0–7.3%) more TB deaths and 3,507 (6.4%, 95% S.I. 2.8–9.8%) more MDR-TB cases than if utilization were increased immediately (scenario 5). A new point-of-care diagnostic test with 100% sensitivity and no diagnostic delay, introduced five years into the future (scenario 7), could effect similar reductions in TB mortality (16.8%, 95% S.I. 10.0–25.3%) as immediate expansion of culture and DST, but the impact on MDR-TB incidence would be less (5.8% reduction, 95% S.I. 1.9–10.9%).
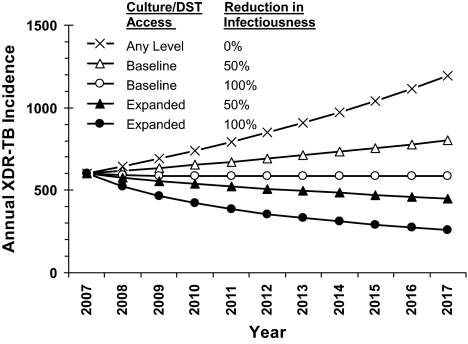
This model was not designed to predict the future course of the XDR-TB epidemic in South Africa. However, assuming XDR-TB to have the same rate of emergence, infectivity, and mortality as untreated MDR-TB resulted in an approximately linear rise of XDR-TB incidence, from 602 cases in 2007 to 1,191 in 2017 (Fig. 3). Expansion of culture and DST alone did not reduce XDR-TB incidence (0.7–1.9% increase). However, if expanded culture/DST was combined with eliminating infectivity (e.g., respiratory isolation) among 50% of XDR-TB cases upon receipt of DST results (scenario 8, Table 1), overall XDR-TB incidence was projected to decline to 448 cases in 2017 (Fig. 3, filled triangles), a 43.5% (95% S.I. 36.5–65.6%) reduction in cumulative 10-year XDR-TB incidence from baseline. If all XDR-TB cases were isolated upon DST result, XDR-TB incidence declined further to 259 projected cases at 10 years (Fig. 3, filled circles). However, if baseline levels of culture utilization were maintained, XDR-TB incidence remained stable even when all diagnosed XDR-TB cases were isolated (Fig. 3, open circles).
Fig. 3.
Annual XDR-TB incidence by culture utilization and isolation rates for diagnosed XDR-TB cases. Projected XDR-TB cases per year, assuming 0% (squares), 50% (triangles), or 100% (circles) of diagnosed XDR-TB cases are isolated. Culture/DST access refers to scenario 1 (Baseline) or scenario 5 (Expanded) (Table 1). Without isolation, projected XDR-TB incidence is similar (±2%) for baseline vs. expanded access.
The projected impact of expanded culture and DST was relatively robust to variation in parameter estimates; no univariate sensitivity analysis, and only 5.4% of probabilistic multivariate simulations, produced estimates of <10% of TB deaths averted. The estimate of deaths averted by expanded culture and DST was most sensitive to variation in the following four parameters: diagnostic delay of culture, proportion of diagnosed patients receiving treatment, TB case-detection rate in the absence of culture, and sensitivity of culture (Fig. S1).
Discussion
This mathematical model suggests that expanding the use of TB culture and drug susceptibility testing could have substantial impact on rates of TB and MDR-TB in South Africa. Specifically, our model projects that >47,000 lives could be saved, and >7,000 MDR-TB cases averted, by increasing utilization of TB culture and DST to levels achievable with current laboratory infrastructure. Gradual expansion of culture/DST utilization over 10 years could save 32,000 lives, resulting in 15,000 more TB deaths than achieved by immediate expansion. Expanded culture and DST alone may not affect XDR-TB rates, but they may enhance the impact of complementary strategies, such as isolation of diagnosed cases. The projected impact of culture and DST depends most strongly on the speed and sensitivity of culture, treatment rates in diagnosed TB patients, and TB case-detection rates in the absence of culture.
These findings have several key implications for TB control in South Africa and other countries experiencing similar TB/HIV epidemics. First, the impact of improving TB diagnosis is greatest when strategies can be implemented rapidly. Immediately scaling up an existing technology (TB culture) has a greater projected 10-year impact than implementing a superior diagnostic assay developed five years into the future. Thus, waiting for a better test results in more TB cases and deaths than rapid expansion of existing methods. In countries with capacity to perform TB culture and DST, maximal utilization of this infrastructure should be an urgent priority. At the same time, testing of rapid molecular tests for TB drug resistance—which have higher sensitivity than sputum smear and been successfully piloted in South Africa (16)—should continue at maximum speed.
Second, resource-allocation decisions should consider that the long-term gains of enhanced TB diagnosis will likely exceed short-term predictions. Models that ignore compounded effects over time may underestimate the long-term impact and cost-effectiveness of TB diagnostics, particularly for MDR-TB [which accounts for nearly 50% of South Africa's TB budget (2)]. For example, a recent static model (17) estimated that 44–66% access to a test with 85% sensitivity could reduce TB mortality by 11.2%. By comparison, our model estimates that expanded culture and DST (scenario 5) would reduce TB mortality and MDR-TB incidence, respectively, by 12.8% and 4.2% in the first year, but by 21.6% and 19.4% in year 10. During this time, program costs would likely decline because of reductions in TB incidence. Thus, static models would underestimate the long-term effectiveness of TB diagnostics while overestimating their cost.
Third, expanding use of culture and DST alone may not have a measurable impact on XDR-TB but could play an important role if combined with other control strategies. Similar to a recent model of XDR-TB control in Tugela Ferry, South Africa (18), the present model suggests that efforts to reduce XDR-TB transmission (e.g., by respiratory isolation) are likely to have greater impact than improved diagnostics alone (Fig. 3). However, our model further demonstrates that, if such measures are implemented, expansion of TB culture and DST on a country-wide basis could add value by increasing the number of XDR-TB patients identified. As with the Tugela Ferry model, our model suggests that a combination of control strategies will likely be required to contain the emerging XDR-TB epidemic.
Finally, our sensitivity analyses suggest that efforts to expand access to TB culture and DST should focus on areas where diagnostic sensitivity for smear-negative TB is low. Strengthening coordination between resource-poor peripheral sites and central labs may have greater yield than expanding culture utilization in clinics with existing lab access.
Our model, as with any mathematical representation, has certain limitations. The simplified model structure cannot fully capture the heterogeneity of TB epidemics. For example, our model does not account for concentrated outbreaks (e.g., nosocomial outbreaks of XDR-TB) (14), colocalization of HIV and TB (e.g., by geography or socioeconomic status), or differential infectivity of TB strains (19). Such heterogeneities are particularly likely to play an important role when the numbers of patients in specified subpopulations are small, as with XDR-TB.
Moreover, the values of many model parameters, particularly those not derived from present-day subSaharan Africa, are uncertain. Although we performed sensitivity analyses and fit key parameters to World Health Organization (WHO) epidemiological estimates, we cannot exclude the possibility of simultaneous or large errors in parameter values, which could result in projections outside the variability ranges provided. Additionally, our estimates may be biased by underlying errors in model assumptions. For example, we assume that all active TB is either highly infectious or less-infectious, and thus that extrapulmonary TB has a pulmonary (infectious) component in most patients. Similarly, to develop an equilibrium model (thus optimizing validity of model assumptions), we must assume that MDR-TB is inherently less infectious than drug-sensitive TB, as the assumption of equal infectivity results in steady-state MDR-TB rates that are unrealistically high (20). Laboratory studies have suggested that drug resistance might not incur a fitness cost (21); if true, our model underestimates the impact of TB culture on MDR-TB incidence (Fig. S2). Furthermore, because we cannot accurately project future uptake rates, we implicitly assume that antiretroviral use remains constant at 2005 levels, although variation of HIV and HIV/TB mortality rates by 25% did not substantially affect our outcome estimates.
Our model cannot evaluate certain relevant outcomes, including the future trajectory of XDR-TB under baseline conditions, the cost-effectiveness of TB diagnosis, or the impact of improving TB laboratory services in other high-burden countries where TB culture is not routinely available. A static model estimated that country-wide implementation of TB culture in South Africa would cost $327 per disability-adjusted life year (DALY) averted, or <5% of national gross domestic product (a common benchmark for very cost-effective interventions) (22). Thus, mathematical models suggest that expanded access to culture and DST could have substantial impact on TB rates with a favorable cost-effectiveness profile. Nevertheless, such models cannot fully inform the policy decision of whether and how to expand culture utilization in South Africa.
In conclusion, this mathematical model projects that rapid expansion of TB culture and drug sensitivity testing among South African adults could save >47,000 lives and prevent >7,000 MDR-TB cases during the 10-year period from 2008 to 2017. This corresponds to a reduction of 17% in total TB mortality, 14% in MDR-TB incidence, and 47% in MDR-TB mortality. Used alone, expanded culture and DST may not reduce XDR-TB incidence, but they enhance the impact of strategies to reduce transmission. The impact of TB culture and DST will likely be greatest where smear-negative TB is under-diagnosed. In South Africa and other countries with high burdens of TB and HIV, rapid improvement of TB diagnosis, using existing tools, represents an urgent public health priority.
Materials and Methods
Values for model input parameters were generated via a four-step process. First, we reviewed the literature for initial parameter estimates. Second, we selected 10 model parameters and paired them one-to-one with 10 key epidemiological estimates of the South African TB epidemic (year 2005) from the WHO. These estimates include TB incidence, prevalence, and mortality by HIV status (column 3 in Table S2) (2, 23). We used an iterative process to generate an equilibrium model, defined as one whose output matched each of the 10 WHO estimates (column 4 in Table S2) within 1% for 10 consecutive years, in the absence of XDR-TB. Third, we allowed XDR-TB to emerge from MDR strains, assuming MDR- and XDR-TB to have equal infectivity and mutation rates. The time point at which annual incidence of new XDR-TB cases approximated 600, or 10% of the total estimated MDR-TB burden, was taken as July 1, 2007. Fourth, interventions to improve TB diagnosis, as described below, were initiated at this time. The model therefore incorporates two dynamic processes (XDR-TB emergence and implementation of diagnostic interventions) on the background of a stable TB/HIV epidemic from 2005. Such stability is consistent with the estimated −0.1% change in TB incidence between 2004 and 2005 (2) and the 0.2% change in HIV prevalence between 2004 and 2006 (24). This approach implicitly assumes South African patterns of TB and HIV treatment in 2005. Future changes in clinical practice (e.g., increased access to antiretroviral therapy) were not explicitly modeled but are partially described by sensitivity analyses of specific model parameters (e.g., TB/HIV mortality rates).
We performed univariate sensitivity analysis on model parameters over the ranges given in Table S2, using the percentage of total TB deaths averted in scenario 5 as the outcome. We varied parameters over a range from 75% to 125% of their initial value except in cases of greater uncertainty, or in which the specified range was illogical (e.g., >1.0 for a probability estimate). We report all parameters for which such variation changed the outcome by more than ±5%. We performed multivariate sensitivity analysis by simultaneously setting all parameters in four predefined groups (TB transmission and mortality, existing diagnostic ability, culture/DST characteristics, and TB treatment) to the boundary value that was most favorable or least favorable to culture, respectively. To estimate variability associated with simultaneous changes in all parameters, we sampled randomly from a triangular probability distribution of each parameter, >10,000 independent iterations, thus generating 95% simulation intervals, defined as the intervals bounded by the 2.5 and 97.5 percentiles of all simulations. Complete details and results of univariate and best/worst-case sensitivity analyses appear in Tables S1–S5, and SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. Larry Moulton for useful insight into the model structure and Dr. Peter Small for helpful comments on an earlier draft. This work was supported by a Bill and Melinda Gates Foundation Grant to the Consortium to Respond Effectively to the AIDS/TB Epidemic (CREATE) and National Institutes of Health Grants T32 GMO7309 (to D.W.D.), K24 AI01637 (to R.E.C.), and K23 AI51528 (to S.E.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800965105/DCSupplemental.
References
- 1.Getahun H, Harrington M, O'Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: Informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva: WHO; 2007. [Google Scholar]
- 3.Burgess AL, et al. Integration of tuberculosis screening at an HIV voluntary counselling and testing centre in Haiti. AIDS. 2001;15:1875–1879. doi: 10.1097/00002030-200109280-00018. [DOI] [PubMed] [Google Scholar]
- 4.Hargreaves NJ, et al. “Smear-negative” pulmonary tuberculosis in a DOTS programme: Poor outcomes in an area of high HIV seroprevalence. Int J Tuberc Lung Dis. 2001;5:847–854. [PubMed] [Google Scholar]
- 5.Harries AD, et al. Management of pulmonary tuberculosis suspects with negative sputum smears and normal or minimally abnormal chest radiographs in resource-poor settings. Int J Tuberc Lung Dis. 1998;2:999–1004. [PubMed] [Google Scholar]
- 6.O'Brien RJ, Talbot EA. The utility of an antibiotic trial for diagnosis of AFB-negative tuberculosis. Int J Tuberc Lung Dis. 2003;7:198. [PubMed] [Google Scholar]
- 7.Menzies D, Pai M, Comstock G. Meta-analysis: New tests for the diagnosis of latent tuberculosis infection: Areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–354. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 8.Githui WA. Laboratory methods for diagnosis and detection of drug resistant Mycobacterium tuberculosis complex with reference to developing countries: A review. East Afr Med J. 2002;79:242–248. doi: 10.4314/eamj.v79i5.8861. [DOI] [PubMed] [Google Scholar]
- 9.Aziz MA, Wright A, Laszlo A, De Muynck A, et al. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): An updated analysis. Lancet. 2006;368:2142–2154. doi: 10.1016/S0140-6736(06)69863-2. [DOI] [PubMed] [Google Scholar]
- 10.WHO/IATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-Tuberculosis Drug Resistance in the World. Geneva: WHO; 2008. Report No. 4. [Google Scholar]
- 11.Dorman SE, Chaisson RE. From magic bullets back to the magic mountain: The rise of extensively drug-resistant tuberculosis. Nat Med. 2007;13:295–298. doi: 10.1038/nm0307-295. [DOI] [PubMed] [Google Scholar]
- 12.Hudson CP, Wood R, Maartens G. Diagnosing HIV-associated tuberculosis: Reducing costs and diagnostic delay. Int J Tuberc Lung Dis. 2000;4:240–245. [PubMed] [Google Scholar]
- 13.Apers L, et al. A comparison of direct microscopy, the concentration method and the Mycobacteria Growth Indicator Tube for the examination of sputum for acid-fast bacilli. Int J Tuberc Lung Dis. 2003;7:376–381. [PubMed] [Google Scholar]
- 14.Gandhi NR, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 15.South Africa Department of Health. The Management of Multidrug Resistant Tuberculosis in South Africa. Cape Town: Department of Health; 1999. [Google Scholar]
- 16.Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177(7):787–792. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 17.Keeler E, et al. Reducing the global burden of tuberculosis: The contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 18.Basu S, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: An epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porco TC, Small PM, Blower SM. Amplification dynamics: Predicting the effect of HIV on tuberculosis outbreaks. J Acquir Immune Defic Syndr. 2001;28:437–444. doi: 10.1097/00042560-200112150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Dye C, Williams BG, Espinal MA, Raviglione MC. Erasing the world's slow stain: Strategies to beat multidrug-resistant tuberculosis. Science. 2002;295:2042–2046. doi: 10.1126/science.1063814. [DOI] [PubMed] [Google Scholar]
- 21.Gagneux S, et al. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–1946. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 22.Dowdy DW, O'Brien MA, Bishai D. Cost-Effectiveness of novel diagnostic tools for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008 in press. [PubMed] [Google Scholar]
- 23.World Health Organization Global TB Database. [Accessed April 5, 2007]; Available at www.who.int/tb/country/global_tb_database/en/index.html.
- 24.Joint United Nations Programme on HIV/AIDS. 2006 Report on the global AIDS epidemic. Geneva: UNAIDS; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.