Abstract
Key cellular functions and developmental processes rely on cascades of GTPases. GTPases of the Rab family provide a molecular ID code to the generation, maintenance and transport of intracellular compartments. Here, we addressed the molecular design principles of endocytosis by focusing on the conversion of early endosomes into late endosomes, which entails replacement of Rab5 by Rab7. We modelled this process as a cascade of functional modules of interacting Rab GTPases. We demonstrate that intermodule interactions share similarities with the toggle switch described for the cell cycle. However, Rab5-to-Rab7 conversion is rather based on a newly characterized ‘cut-out switch' analogous to an electrical safety-breaker. Both designs require cooperativity of auto-activation loops when coupled to a large pool of cytoplasmic proteins. Live cell imaging and endosome tracking provide experimental support to the cut-out switch in cargo progression and conversion of endosome identity along the degradative pathway. We propose that, by reconciling module performance with progression of activity, the cut-out switch design could underlie the integration of modules in regulatory cascades from a broad range of biological processes.
Keywords: cut-out switch, endocytosis, GTPase, mathematical model, toggle switch
Introduction
The GTPase switch is a widespread molecular device regulating a variety of cellular processes such as signal transduction, cytoskeleton assembly, protein translation, organelle biogenesis and intracellular transport (Vetter and Wittinghofer, 2001). In the GTP-bound form, these proteins transduce their activity by interacting with specific effector proteins. Multiple processes regulated by GTPases need to be coordinated and, thus, the functional cycle of one GTPase is often coupled to another. For example, functional coordination between small GTPases of the Rab family regulates the vectorial transport along biosynthetic and endocytic pathways (Horiuchi et al, 1997; Christoforidis et al, 1999; McBride et al, 1999; Zerial and McBride, 2001; Pfeffer and Aivazian, 2004). Rab proteins constitute the largest family, comprising over 60 members in humans that confer structural and functional identity to intracellular membrane compartments (McBride et al, 1999; Pfeffer and Aivazian, 2004). In the endocytic pathway, different Rab proteins organize in a mosaic pattern, occupying different membrane domains on endosomes, termed Rab domains (Zerial and McBride, 2001; Miaczynska and Zerial, 2002; Pfeffer and Aivazian, 2004). Cargo is sequentially transported from Rab5 to Rab4 and Rab11 domains along the recycling route (Sonnichsen et al, 2000) and from Rab5 to Rab7 domains along the degradative route (Rink et al, 2005; Vonderheit and Helenius, 2005; Lakadamyali et al, 2006).
How Rab domains are assembled and functionally connected with each other is therefore critical for understanding the mechanisms underlying organelle biogenesis and cargo transport. One of the best-characterized family members is Rab5, a master endocytic regulator (Zerial and McBride, 2001). Similar to other Rab proteins, Rab5 behaves as a membrane protein but also dynamically shuttles between the membrane and the cytosol, chaperoned by Rab GDP dissociation inhibitor (GDI) (Pfeffer and Aivazian, 2004). On the endosome membrane, Rab5 undergoes cycles of GTP binding and hydrolysis (Rybin et al, 1996). The GTP-bound state is tightly regulated and depends on the balance between GDP/GTP exchange (denoted here as activation) and GTP hydrolysis, catalysed by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), respectively. Active Rab5 orchestrates the recruitment of a multi-effector protein machinery that performs various functions in endosome membrane tethering and fusion as well as microtubule- and actin-dependent motility (Christoforidis et al, 1999; Hoepfner et al, 2005; Pal et al, 2006). An important hallmark of this machinery is the cooperativity between Rab5 and its effectors. Cooperativity is due to effector dimerization, as in the case of EEA1 (Dumas et al, 2001), which enhances membrane binding. Furthermore, cooperativity is also through the formation of complexes between GEFs and effectors, such as the Rabaptin-5/Rabex-5 complexes (Horiuchi et al, 1997; Lippe et al, 2001a), as well as assembly of high-molecular-weight oligomers on the early endosome membrane (McBride et al, 1999).
Besides regulation of the nucleotide cycle and coordinated activity within Rab domains, GEFs, GAPs and effectors can bind to multiple Rab GTPases, allowing the progression of cargo between Rab domains along the endocytic pathways (Zerial and McBride, 2001; Markgraf et al, 2007). For example, the divalent effector Rabenosyn-5 interacts with both Rab5 and Rab4, coordinating cargo entry into early endosomes with sorting to the recycling route (de Renzis et al, 2002). In the degradative pathway, Rab5 interacts with the Class C VPS/HOPS complex, which is also a GEF and effector of the late endosomal regulator Rab7 (Rieder and Emr, 1997; Seals et al, 2000; Wurmser et al, 2000; Rink et al, 2005).
A key feature relevant for cargo transport is that the Rab5 domain assembly on endosomes is a dynamic and reversible process (Rink et al, 2005). The level of Rab5 on the early endosome membrane is not constant but fluctuates dynamically. Early endosomes grow in size due to frequent homotypic fusion events regulated by Rab5, thereby progressively accumulating cargo destined for degradation. Concomitantly, the surface density of Rab5 increases until it reaches a maximum. At this point, the Rab5 domain can undergo disassembly along with Rab7 domain assembly, through Rab conversion (Rink et al, 2005; Lakadamyali et al, 2006). Therefore, an early endosome is converted to a late endosome by a precipitous decline in Rab5 and a simultaneous and corresponding increase in Rab7. Rab5 thus displays a paradoxical behaviour: on the one hand, it must maintain and increase its activity to sustain the growth phase of early endosomes. On the other hand, decreasing its activity is necessary for the transfer of cargo to the late endocytic pathway (Rink et al, 2005). In addition to Rab conversion, evidence for a fission event of separate Rab5 and Rab7 domains on the same endosome has also been presented (Vonderheit and Helenius, 2005). In all cases, the data so far obtained support the shared view that assembly of distinct Rab domains is mutually exclusive, either through physical separation (of distinct membrane domains) or conversion processes (Rab7 replacing Rab5 around the entire perimeter of the endosome).
Resolving the paradox of growth and disassembly of Rab5 during conversion requires not only the identification of molecular components involved, such as Rab5 and Rab7 effectors and cofactors, but also the molecular design principles whereby this molecular network operates. To this end, the integration of mathematical modelling with biological measurements is essential, as demonstrated for other complex processes such as the cell cycle. In the cell cycle, the paradigmatic design principle for the generation of mutually exclusive identities is based on toggle switches interlinked by mutual inhibition (Ferrell, 2002; Tyson et al, 2003). Two components mutually inhibit each other if each one either represses the generation/activation or enhances the degradation/inactivation of the other component.
Here, to address the design principles of mutually exclusive Rab5 and Rab7 domains, we applied a combination of theoretical and experimental approaches. Out of two competing models, only one best fits the biological observations and predicts the behaviour of the system in relation to cargo transport, thereby reconciling module performance with progression of activity.
Results
Rab domains as a modular system
The endosomal system can be viewed as an interconnected and dynamic network of Rab domains, each with a characteristic biochemical composition, dynamically interacting with each other (Zerial and McBride, 2001). Replacement of Rab5 by Rab7 constitutes a key regulatory system for transport of cargo from early to late endosomes (termed Rab conversion in Rink et al, 2005). To develop a mathematical model that can describe the molecular design principles underlying the conversion of an early endosome into a late endosome by Rab5-to-Rab7 replacement, we considered a model of endocytic transport based on discrete but functionally interconnected modular blocks or modules, defined as a subset of molecules that closely interact to jointly perform a specific function (Hartwell et al, 1999; Hofmann et al, 2006). Each Rab module includes the factors that regulate the GTPase cycle as well as the effectors binding to Rab:GTP mediating its activity. The two modules are then connected with positive and negative feedback loops based on established or proposed molecular interactions.
Within each module, the activities of GEFs and GAPs have been modelled as Rab GDP/GTP exchange and GTP hydrolysis rates. We combined the densities of Rab:GTP and Rab:GTP:effector complexes into a single variable, named R5 and R7 for Rab5 and Rab7 respectively, as binding and unbinding processes should occur on a faster timescale than exchange and hydrolysis of guanine nucleotides. Owing to this simplification, the GDP/GTP exchange rate depends on the density variables R5 and R7 of the Rab that specifically recruits the corresponding GEF–effector complex (i.e. Rabaptin-5/Rabex-5 complex for Rab5, Class C VPS/HOPS complex for Rab7). A similar approach was applied to the GTP hydrolysis rate by the GAP.
The guanine nucleotide cycle and the corresponding interactions (Figure 1) were translated into a system of ordinary differential equations (see also Supplementary information):
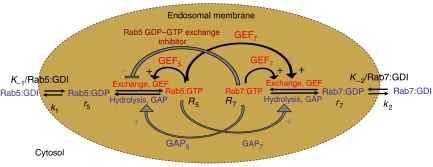
Figure 1.
Reaction scheme of membrane recruitment and GDP/GTP cycle of two Rab GTPases, Rab5 and Rab7. Early endosomes progressively accumulate cargo destined for degradation in the Rab5 domain while recycling cargo to the cell surface through fission of Rab4 and Rab11 domains (Zerial and McBride, 2001; Rink et al, 2005). Large Rab5-positive early endosomes at the peak of degradable cargo accumulation eventually undergo conversion into Rab7-positive late endosomes (Rink et al, 2005). In this case, the entire Rab5 domain is replaced by the Rab7 domain. This is in contrast to the alternative mechanism of fission between two coexisting Rab domains on the same endosome (Vonderheit and Helenius, 2005). The scheme shows that, during the conversion process, the two Rab GTPases form, together with GEFs, GAPs and effectors, two interconnected modules on the same endosome membrane. Initially, the endosome membrane is enriched in Rab5 and depleted of Rab7. Upon conversion, the membrane composition is reversed, that is, Rab7 prevails over Rab5. Such conversion results from programmed changes in the nucleotide cycle of the two Rab GTPases, which shuttle between inactive GDP-bound (blue) and active GTP-bound (red) conformations. GEFs catalyse the exchange of GDP with GTP, and GAPs catalyse the hydrolysis of GTP into GDP (Vetter and Wittinghofer, 2001), acting upon the respective Rab proteins (GEF5 for Rab5, GEF7 for Rab7, GAP5 for Rab5, GAP7 for Rab7). GEFs and GAPs acting on one Rab protein may also be part of effector complexes (e.g. Rabaptin-5/Rabex-5 for Rab5, Class C VPS/HOPS complex for Rab7), binding the active conformation of the same or the other Rab species. For example, GEF5 (Rabaptin-5/Rabex-5 complex) is both recruited by and acts upon Rab5, thus causing a positive feedback on Rab5 activation. However, the Class C VPS/HOPS complex is a GEF7 and effector of Rab7 but also an effector of Rab5 (Rink et al, 2005), thereby coupling the activity of Rab5 to activation of Rab7. Black curved arrows denote known (biochemically demonstrated) feedback mechanisms on Rab activation whereas grey arrows represent potential, but yet hypothetical, negative feedback mechanisms. The − and + signs denote a decrease or increase in the reactions.
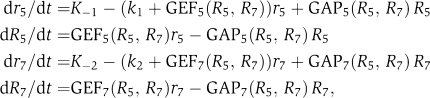 |
where the concentrations of GDP-bound Rab5 and Rab7 are r5 and r7, respectively. Owing to the high number (from a few hundreds up to 104) of Rab molecules on the endosomal membrane (Rink et al, 2005), all variables were considered as continuous. The mathematical equations take into account established activating (GEF) interactions (black, solid arrows in Figure 1), positive feedback loops (GEF–effector complexes), inhibitory (GAP) as well as hypothetical negative feedback loops (grey arrows). Suppose an effector oligomer (Christoforidis et al, 1999; McBride et al, 1999) provides n binding sites for Rab molecules and is quickly released if less than n molecules are bound, then the probability of a sustained recruitment increases with the nth power of the density of Rab molecules, that is, Hill kinetics. Moreover, if an effector is complexed to a GEF, for example, Rabaptin-5 with Rabex-5 (Horiuchi et al, 1997; Rieder and Emr, 1997; Seals et al, 2000; Wurmser et al, 2000; Lippe et al, 2001b; Rink et al, 2005), then the activation of the Rab proteins is also expected to follow Hill kinetics.
We have considered hyperbolic (Michaelis–Menten) as well as sigmoidal (Hill) kinetic rate laws (see Supplementary information), as the kinetic dependencies of GEF or GAP activities on the density of the recruiting Rab GTPase are not known in detail. As shown previously (Tyson et al, 2003; Angeli et al, 2004), it is not necessary to consider more complex rate laws if the two kinetic laws are sufficient to render the system bistable. By a combinatorial use of the two kinetic rate laws for each term in the equations, we obtained a total of 54 different models. Each model was algebraically solved for the quasi-steady state of the Rab7 module's activity, R7, as a function of the other module's activity yielding the system R7*=f1(R5*); R7*=f2(R5*). The graphical representations of the two functions f1 and f2 in the phase plane spanned by R5 and R7 are called nullclines. Qualitative model screening was then performed by combinatorially intersecting pairs of nullclines, that is, simultaneously solving for both modules' quasi-steady state (R7*=f1(R5*)=f2(R5*) yields quasi-steady states of R5 and R7 for the coupled modules), and classifying the number and location of intersection points (see Supplementary information).
The experimentally observed transition between two quasi-steady states of Rab domains requires bistability and a parameter change across one of the hysteresis thresholds. At the threshold value, the first Rab (Rab5) shall switch from the high- to the low-density state, and the second Rab (Rab7) from the low- to the high-density state. Correspondingly, three intersection points of the nullclines (two intersections representing the stable states that are separated by an unstable state represented by the third intersection) are the hallmark of bistability (Ferrell, 2002; Tyson et al, 2003). The main result of our analysis can be summarized as follows. We tested 54 possible models and, out of these, only two model subclasses provide the required functionality of the Rab5–Rab7 switch of modules (Figure 2):
Rab5 displays cooperative auto-activation and suppresses Rab7.
Rab5 activates Rab7; Rab7 displays cooperative auto-activation and suppresses Rab5.
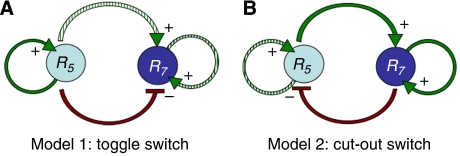
Figure 2.
Two competing models for Rab domain identity generation and endosome conversion. Green arrows denote reported interactions and red arrows denote proposed interactions that each may be essential (solid arrow) or just tolerated (dashed arrow). (A) Model 1 corresponds to a toggle switch that can switch off Rab5 as a result of weakened activation of Rab5 by its GEF (solid green arrow). Switching on of Rab7 follows if the repression (red arrow) by Rab5 becomes inferior to the activation (green dashed arrows). (B) Model 2 represents a cut-out switch that can switch off Rab5 as a result of enhanced (e.g. cargo-dependent, pH-dependent) activation of Rab5, as Rab5 fosters Rab7 activity (upper green arrow) to pass a threshold above which Rab7 self-sustains (right green arrow) its own activation. Rab7 activation at supra-threshold values strongly suppresses Rab5 (red arrow).
Models 1 and 2 only require a single inhibition instead of two as in the classical mutual inhibition model (Ferrell, 2002).
Experimental tests of model predictions
Either of the aforementioned models may theoretically correspond to different Rab modules operating in intracellular transport. To discriminate between the two models, we performed a mathematical analysis of either model (see Supplementary information) yielding a number of predictions that are listed in Table I. We next tested these predictions for the specific case of Rab5 and Rab7 modules by verifying cell-biological and biochemical findings reported previously and by explicitly monitoring module activities by means of single-endosome tracking in live cell images.
Table 1.
Experimental tests (numbers, see text for details), models and model predictions
| Experimental observations | References | Predictions of model 1 (toggle switch) | Predictions of model 2 (cut-out switch) |
|---|---|---|---|
| (1) Inhibition of GTP hydrolysis by Rab5 yields persistence of Rab5 and induction of Rab7, hence higher colocalization | Rink et al (2005) | Lower colocalization | Higher colocalization |
| (2) Inhibition of GDP/GTP exchange on Rab5 yields less degradation | Papini et al (1997) and Vitelli et al (1997) | More degradation | Less degradation |
| (3) Density/radius diagram of Rab5 on endosomes shows increasing Rab5 density | Rink et al (2005) | Decrease | Increase |
| (4) [Rab5](time) is increasing towards the conversion event | This work, Figure 3 | Decrease | Increase |
By comparing the model predictions with four independent experimental results, model 2, the cut-out switch, is corroborated by the present data. Figure 4C and D shows the numerical simulations for models 1 and 2, respectively. Figure 3 shows the statistical analysis of the time courses of 23 endosomes that undergo conversion: [Rab5](time) is increasing towards the conversion event.
First, the two models predict distinctive consequences on the conversion process upon inhibition of GTP hydrolysis by Rab5. Model 1 predicts that conversion should fail to initiate and, thus, no or little Rab7 should colocalize with Rab5 on the endosome membrane. In contrast, model 2 predicts that conversion should be initiated through the recruitment of Rab7, but fail to complete through the blocked removal of Rab5. The latter is precisely what is observed in cells expressing the hydrolysis-deficient mutant Rab5Q79L, which exhibits pronounced colocalization with Rab7 on endosomes (Rink et al, 2005).
Second, models 1 and 2 predict opposite effects on the conversion process upon inhibition of GDP/GTP exchange by Rab5. Model 1 predicts an increase in the activation of Rab7 and, thereby, faster conversion leading to enhanced cargo degradation. Conversely, model 2 predicts a delayed activation of Rab7 and delayed conversion. Again, only the predictions of model 2 are consistent with the experimental data. Expression of the dominant-negative Rab5S34N mutant defective in GDP/GTP exchange leads to a degradation-defective phenotype (Papini et al, 1997). Similarly, a degradation defect has also been observed for the Rab5N133I mutant displaying lower affinity for guanine nucleotides (Vitelli et al, 1997).
Third, the analysis of covariation of size and intensity (i.e. apparent density) of GFP-Rab5 in A431 cells showed that the increase in endosome size is paralleled by an increase in Rab5 density, up to a certain critical size and density value. At the same time, the analysis of early endosome dynamics by live cell imaging (by splitting long endosome tracks into sequences of 5 min in length) indicated a general trend of endosomes growing in size and intensity up to the conversion point (Rink et al, 2005). In other words, early endosomes progress along the size/intensity diagram from small and dim (low Rab5 density) to large and bright (high Rab5 density) as a function of time, consistent with model 2 but not model 1, as the simulations below demonstrate.
Fourth, we compared detailed experimental data to simulated model behaviour (Figures 3 and 4 and Supplementary information) and studied the corresponding quasi-steady–state behaviour by means of a bifurcation analysis. For the simulation, we assume that at least one rate constant changes during the lifetime of a Rab5 domain, possibly due to factors such as cargo accumulation, changes of intralumen pH, endosome size and intracellular position (all experimentally observed). As a control parameter, we selected the hydrolysis rate kh,5 for model 1 and the maximum guanine nucleotide exchange rate ke,5 for model 2. The specific choice purely serves the purpose of demonstration. Other parameters were kept constant, and different values of these parameters influence quantitatively but not qualitatively the behaviour of the models. As a result of the mathematical property of timescale separation, the fast model kinetics keep the Rab5 density close to the instantaneous quasi-steady-state solution, which itself slowly changes in response to variations of the control parameter. In the simulations, model 1 yields the standard hysteresis behaviour with the Rab5 density decreasing towards the limit point of the solution branch, that is, conversion. However, model 2 yields a counter-intuitive increase of Rab5 density towards conversion. Figure 4 presents the dependence of the quasi-steady state on the selected parameter by means of a bifurcation diagram.
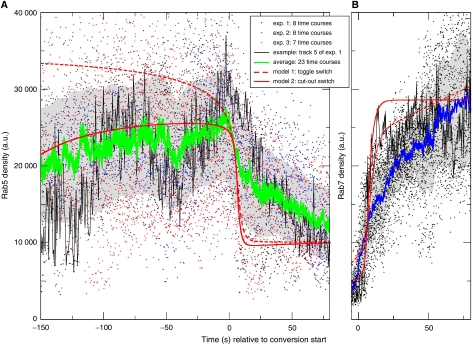
Figure 3.
Rab5 membrane density increases towards the conversion event. (A) Time-course data of Rab5 density on 23 individual endosomes tracked in 3 independent experiments (each value per time point shown by a dot) and a representative time course (black) are shown as a function of time. The average over 23 data points at each time point is given by the green curve together with the 70% confidence interval in grey. For each tracked endosome, the time point of conversion was determined by visual inspection and the recorded time courses were shifted by the conversion time point such that all 23 conversion events were synchronized at the chosen time point 0. Rab5 density has been computed by dividing the integral fluorescence by twice the cross-sectional area (Rink et al, 2005). Red curves denote model simulations for model 1 (dashed) and model 2 (solid curve), which were rescaled with respect to Figure 4C and D by (t−825)/4 → t, (R5−0.18)13 000+10 000 → R5 for model 1 and (t−850)/4−t, (R5−0.6)20 000+10 000 → R5 for model 2. (B) Analogously obtained and analysed data for Rab7 density on 15 converting endosomes from an independent experiment.
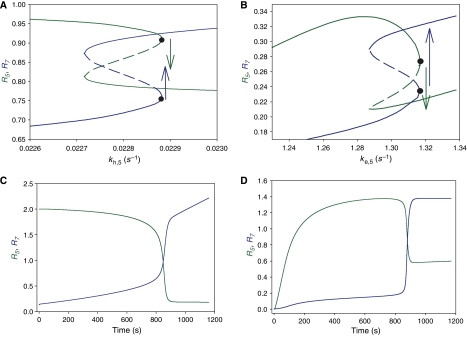
Figure 4.
Bifurcation diagrams showing quasi-steady-state solutions and simulation of models. (A) Bifurcation diagram for model 1 (toggle switch). When a parameter of the kinetic terms of Rab5 (density of Rab5:GTP, green line) varies as a function of time (here the hydrolysis rate kh,5 increases and other parameters are fixed), then the membrane density of Rab5 decreases until it suddenly drops during the conversion event (green arrow). Opposite to Rab5 switching off, Rab7 (density of Rab7:GTP, blue line) switches on at the same critical parameter value (blue arrow). (B) Bifurcation diagram for model 2 (cut-out switch; line colours as in (A); the exchange rate ke,5 increases and other parameters are fixed). Here the membrane density of Rab5 increases before dropping during the conversion event (green arrow). For graphical reasons, the parameter values chosen for both bifurcations differ from those of the simulations (see Supplementary information). (C) Numerical simulation of model 1 (solutions plotted in (A)). (D) Numerical simulation of model 2 (solutions plotted in (B)).
Finally, we performed live-cell video microscopy analysis of GFP-Rab5-expressing A431 cells as previously described (Rink et al, 2005) to compare the model predictions with the Rab5 dynamics in vivo before, during and after conversion from early to late endosomes. Different from the previous analysis, which considered endosomes at different stages of progression (Rink et al, 2005), here we analysed specifically tracks terminating with conversion (defined as Rab5 dissociation from the membrane). A total of 23 tracks of endosomes were isolated from 3 independent sequences and the corresponding Rab5 density over time was analysed. Figure 3A shows an increasing Rab5 density towards the conversion event (green curve). In demonstrating that the density of Rab5 on the membrane tends to increase during the lifetime of an early endosome before conversion, these experimental results support the predictions of model 2 (solid red curve in Figure 3A).
The complementary part of the Rab5/Rab7 conversion switch was also examined by conducting a similar analysis on cells expressing GFP-Rab7 (Figure 3B). Data for Rab7 density on 15 endosomes were obtained from an independent experiment. The results show a rapid increase in GFP-Rab7 density on endosomes that occurs with similar kinetics to those of the GFP-Rab5 decay (Figure 3A), thus reflecting in all likelihood conversion (Rink et al, 2005).
From the comparison of experimental data and model predictions in Table I we conclude that, although both models 1 and 2 can describe mutually exclusive Rab domains, model 2, but not model 1, is supported by the experimental evidence.
Discussion
The transition from early to late endosomes is regulated by the loss of the small GTPase Rab5 and the concomitant acquisition of Rab7 in a mechanism termed Rab conversion (Rink et al, 2005). The behaviour of the two master GTPases creates a paradox: on the one hand, early endosomes are required to maintain Rab5 and increase Rab5 activity as they (1) receive incoming cargo from the plasma membrane, (2) grow in size through homotypic early endosome fusion and (3) package cargo destined for degradation while sorting recycling cargo to the cell surface. On the other hand, when cargo has sufficiently accumulated in fewer and larger endosomes and the surface density of Rab5 reaches its peak (t1/2=10–15 min), Rab5 needs to be switched off and substituted by Rab7 to irreversibly commit cargo for degradation. To resolve this paradox, we considered these two master GTPases and corresponding regulators of nucleotide cycle and effectors as modules and applied a combination of theoretical and experimental approaches to unravel the yet unknown design principles of the switch system.
Here, we identified two design principles (corresponding to two classes of kinetic models) that can explain the maintenance and dynamic transition between successive Rab domains, each requiring only a single inhibitory interaction. The balance between the two conflicting interests (activate to grow faster versus deactivate to allow next Rab to take over) has to be shifted from high to low Rab5 activity in response to a threshold parameter value. As only model 2 is so far supported by the experimental data, we propose that Rab conversion is operated by a cut-out switch controlled by Rab7. According to this model, Rab5 activates Rab7 until Rab7 reaches a threshold upon which it inactivates Rab5 through a negative feedback loop. To our knowledge, this is the first example of a cut-out switch used in a biological system.
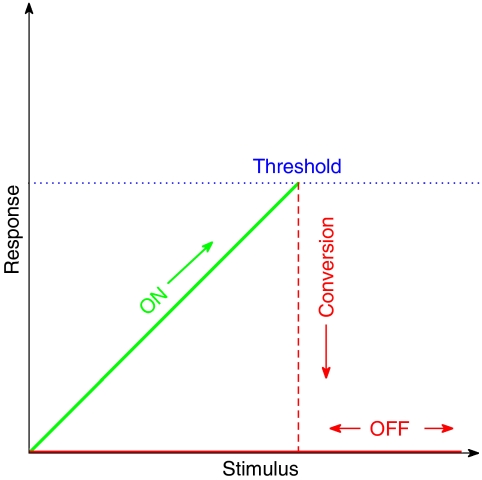
The cut-out switch is known from engineering applications (Morecoft and Hehre, 1933; Oliver, 1990). An idealized stimulus–response diagram is sketched in Figure 5. The key feature of this design is that the stimulus–response characteristic shows an increasing response of the ON state until it drops into the OFF state. By contrast, the standard toggle switch displays decreasing stimulus–response characteristics towards the transition to the OFF state. What could then be the advantage of a cut-out switch over a toggle switch for dynamic changes in membrane identity? We envisage two possible advantages. First, this design principle reconciles the two opposing levels of Rab activity during endosome growth, namely high Rab5 activity before, and required for, conversion, and low activity after conversion. Second, directionality of cargo transport is guaranteed by the asymmetry of the molecular design. The ‘interlinked toggle switches' design in Drosophila segmentation on the other hand requires external (maternal) gradients. An analogous design for the endocytic pathway could sequentially order endocytic compartments by coupling interlinked Rab toggle switches to a chemical gradient that increases with the distance from the plasma membrane towards the nucleus. In contrast, for a cut-out switch design, such a gradient is not required for ordering the Rab system. Moreover, a mutual inhibition model for the Rab system would also require cooperativity as the system is coupled to a large and, therefore, constant reservoir of Rab GTPases complexed to Rab GDI in the cytosol (see Supplementary information). Most other applications of toggle switches of the mutual inhibition type are found in (quasi-) closed systems.
Figure 5.
Ideal cut-out switch. As the stimulus increases (horizontal axis), the response of the ON state (green) increases up to a threshold value (blue) and then drops to the OFF state (red) where it remains even when the stimulus is decreased.
In both models 1 and 2, the coexistence of two stable steady states is generally associated with a phenomenon of hysteresis where a system can jump back and forth between two different stable states for different critical values of the control parameter, corresponding to two limit points. However, the parameter gap between the limit points can take a significant magnitude, depending on the values of the other rate constants, such that Rab5-to-Rab7 conversion does not reverse under fluctuations of the control parameter. Reversibility may occur only by changing it systematically further away from the bistability range. However, returning the system back to the conditions of early endosome generation and Rab5 domain assembly, that is, a reversible transition from Rab7 to Rab5, would be extremely unlikely to achieve upon variation of a single parameter. Moreover, after conversion, Rab7-positive endosomes rapidly undergo fusion with late endosomes and lysosomes, thus irreversibly committing cargo to degradation (Rink et al, 2005)
Both models 1 and 2 explain Rab repression through a Rab-dependent negative feedback loop. Interestingly, negative feedback loops are present in many biological decision-making units. However, they commonly occur in pairs of mutual inhibition. For example, the decision upon lysis versus lysogeny in phage lambda is taken by the corepressive circuit of the cro and cI genes (Ptashne et al, 1982). The function of the GAP gene network during Drosophila embryogenesis that selects among mutually exclusive cell fates is best modelled by 13 inhibitory interactions but no activating interaction among the GAP genes (Perkins et al, 2006). By contrast, only a few inhibitory regulatory interactions but many more activating interactions have so far been described for Rab proteins (see below). Our analysis has shown that interacting Rab modules can take decisions despite a limited number of inhibitions, just one in the case of early and late endosomal identities. The model described here allows one to make a number of testable predictions. One of the most important predictions is that a negative feedback loop on Rab5 must be initiated by Rab7. This could be brought about by Rab5-GAPs or, interestingly, by an inhibitor of Rab5 GEF activity, both under the control of Rab7. It will be interesting to see which of these predictions can be substantiated at the molecular level through the identification of new regulatory components.
An important feature of the modelling strategy used here is that we considered compartmentalization of the endocytic pathway in Rab domains as a modular system (Zerial and McBride, 2001). The concept of modularity has far-reaching implications for biological functions as well as modelling approaches (Hartwell et al, 1999; Hofmann et al, 2006). The current picture of how individual modules are linked to form an integrated regulatory system assumes a hierarchical organization of parent modules distributing activity among daughter modules (Hartwell et al, 1999; Ravasz et al, 2002). The cut-out switch described here sequentially grants activity to interacting daughter modules without interference by a parent module. This alternative mechanism seems especially suited for cargo progression along the endocytic pathway, which is confronted with highly variable cargo content and therefore requires local, decentralized decisions about module activation, performance and deactivation.
We propose that the design principle shown here is not limited to Rab conversion but may underlie other modules posing a similar paradox. This proposal is supported by a series of experimental evidences for the necessary components of predicted cut-out switches in other modules centred on Rab as well as other GTPases, for example, Rho proteins, in intracellular transport. A common feature shared by these GTPases is the cooperativity in GEF and effector recruitment, which is one of the key components of the cut-out switch. For example, the cascade of activation of Rab GTPases that regulates exocytosis of Golgi-derived vesicles in yeast strikingly resembles the Rab5–Rab7 sequential activation and, thus, may follow a cut-out switch as described here. In this system, the GTPase Ypt31p/Ypt32p recruits the effector Sec2p, which in turn acts as a GEF for the subsequent GTPase Sec4p. Activation of Sec4p fosters the recruitment of Sec15p, which binds to Sec2p and displaces the previous GTPase Ypt32p (Grosshans et al, 2006). Moreover, the maturation of Golgi cisternae in yeast may represent a further modular system (Losev et al, 2006; Matsuura-Tokita et al, 2006). Our study, in general, highlights the cooperativity in GEF–effector interactions, which is widespread among small GTPases of the Ras superfamily. For example, bud assembly in yeast is regulated by the Rho GTPase Cdc42p. Its activation is catalysed by the GEF Cdc24p, which is complexed to the Cdc42p effector Bem1p. Here, GEF–effector cooperativity is exploited to concentrate proteins required for cell polarity and bud assembly at a discrete site (Butty et al, 2002).
For further studies of the above systems, it is interesting to note that a comparison between the two models (Figure 2) indicates that only model 1 belongs to the class of monotone systems (Angeli et al, 2004). Monotone systems are characterized by a positive parity of any embedded feedback loop. The parity of a loop is given by the product of the signs of the edges that make up the respective loop. Note that the single inhibition in model 1 is not part of any closed loop and just connects parts that contain closed loops. Monotone systems are known to possess bistability for appropriately adjusted kinetics and parameter values. The mechanism whereby an irreversible transition of Rab domains can be brought about in a network of reversible biochemical reactions is also based on bistability. Hence, the toggle switch of model 1 is an expected solution to the problem. However, model 2 contains a single inhibition in the central feedback loop and is therefore a non-monotone system. Generally, for the latter class of systems, an oscillatory behaviour is expected, which, at first sight, would question their utility in controlling the unidirectional transition between Rab domains. However, in addition to the expected oscillatory behaviour, we found a large area in parameter space of our model 2 that again yields bistability in such a non-monotone system. We therefore believe that physiological parameter values correspond to the model's bistable regime and that physiological parameter changes exploit the model's functionality as a cut-out switch.
In conclusion, the description of the Rab5–Rab7 system as small functional units, or modules, represented by individual Rab domains offers the possibility to further develop a more comprehensive model of the entire endocytic pathway, taking into account the recycling branch as well as further molecular components of the endocytic machinery, for example, coats and SNARE proteins (Heinrich and Rapoport, 2005). It is conceivable that the cut-out switch described here could be a design principle shared by other regulatory cascades from a broad range of biological processes.
Materials and methods
Modelling
See Supplementary information for the types of Rab-GEF and Rab-GAP kinetics and corresponding nonlinear rate laws.
Model screening and phase-plane analysis
Qualitative model screening has been performed by using the numerical analysis software MatLab 7.4.0 (Quarteroni, 2006). Graphics and data visualization have been realized with SigmaPlot (SigmaPlot, 2005).
Bifurcation analysis and simulation
To perform the bifurcation analysis, we used the software package AUTO (Doedel et al, 1991). Numerical simulations were performed with Copasi (Hoops et al, 2006).
The model of the Rab5–Rab7 anti-phase cut-out switch is available in an SBML format in Supplementary information and has been submitted to the biomodels database (http://www.ebi.ac.uk/biomodels/) with the accession number MODEL5937037510.
Statistical analysis of single-endosome tracking data
See Supplementary information for details.
Experimental procedure
For details on cell culture, transfection, DNA constructs, antibodies, LDL labelling and uptake, and RNA interference, see Rink et al (2005). All image processing and analysis were carried out with the Motiontracking/Kalaimoscope 6.7 program (Transinsight, www.kalaimoscope.com).
Supplementary Material
Supplementary Information 1
Supplementary Information 2
Acknowledgments
We are grateful to F Jülicher and J Howard for fruitful discussions. We acknowledge support by the systems biology network HepatoSys of the German Ministry for Education and Research through grant 0313082J. Andreas Deutsch is a member of the DFG Research Center for Regenerative Therapies Dresden—Cluster of Excellence—and gratefully acknowledges support by the Center. This work was supported by grants of the BMBF Hepatosys programme, the EU Endotrack, the DFG and the Max Planck Society. The authors declare that they do not have any competing commercial interests.
References
- Angeli D, Ferrell JE Jr, Sontag ED (2004) Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proc Natl Acad Sci USA 101: 1822–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butty AC, Perrinjaquet N, Petit A, Jaquenoud M, Segall JE, Hofmann K, Zwahlen C, Peter M (2002) A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J 21: 1565–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M (1999) The Rab5 effector EEA1 is a core component of endosome docking. Nature 397: 621–625 [DOI] [PubMed] [Google Scholar]
- de Renzis S, Sonnichsen B, Zerial M (2002) Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes (see comment). Nat Cell Biol 4: 124–133 [DOI] [PubMed] [Google Scholar]
- Doedel EJ, Keller HB, Kernévez JP (1991) Numerical analysis and control of bifurcation problems: (I) Bifurcation in finite dimensions. Int J Bif Chaos 1: 493–520 [Google Scholar]
- Dumas JJ, Merithew E, Sudharshan E, Rajamani D, Hayes S, Lawe D, Corvera S, Lambright DG (2001) Multivalent endosome targeting by homodimeric EEA1. Mol Cell 8: 947–958 [DOI] [PubMed] [Google Scholar]
- Ferrell JE Jr (2002) Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14: 140–148 [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Hopfield JJ, Leibler S, Murray AW (1999) From molecular to modular cell biology. Nature 402: C47–C52 [DOI] [PubMed] [Google Scholar]
- Heinrich R, Rapoport TA (2005) Generation of nonidentical compartments in vesicular transport systems. J Cell Biol 168: 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M (2005) Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell 121: 437–450 [DOI] [PubMed] [Google Scholar]
- Hofmann KP, Spahn CM, Heinrich R, Heinemann U (2006) Building functional modules from molecular interactions. Trends Biochem Sci 31: 497–508 [DOI] [PubMed] [Google Scholar]
- Hoops S, Sahle S, Gauges R, Lee C, Pahle J, Simus N, Singhal M, Xu L, Mendes P, Kummer U (2006) COPASI—a COmplex PAthway SImulator. Bioinformatics 22: 3067–3074 [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M (1997) A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90: 1149–1159 [DOI] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X (2006) Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124: 997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe R, Horiuchi H, Runge A, Zerial M (2001a) Expression, purification, and characterization of Rab5 effector complex, rabaptin-5/rabex-5. Methods Enzymol 329: 132–145 [DOI] [PubMed] [Google Scholar]
- Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M (2001b) Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell 12: 2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS (2006) Golgi maturation visualized in living yeast. Nature 441: 1002–1006 [DOI] [PubMed] [Google Scholar]
- Markgraf DF, Peplowska K, Ungermann C (2007) Rab cascades and tethering factors in the endomembrane system. FEBS Lett 581: 2125–2130 [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A (2006) Live imaging of yeast Golgi cisternal maturation. Nature 441: 1007–1010 [DOI] [PubMed] [Google Scholar]
- McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 98: 377–386 [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Zerial M (2002) Mosaic organization of the endocytic pathway. Exp Cell Res 272: 8–14 [DOI] [PubMed] [Google Scholar]
- Morecoft JH, Hehre FW (1933) Electrical Circuits and Machinery. London: Wiley; London: Chapman & Hall [Google Scholar]
- Oliver KG (1990) Basic Industrial Electricity: A Training and Maintenance Manual. New York, NY: Industrial Press [Google Scholar]
- Pal A, Severin F, Lommer B, Shevchenko A, Zerial M (2006) Huntingtin–HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease. J Cell Biol 172: 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini E, Satin B, Bucci C, de Bernard M, Telford JL, Manetti R, Rappuoli R, Zerial M, Montecucco C (1997) The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J 16: 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins TJ, Jaeger J, Reinitz J, Glass L (2006) Reverse engineering the gap gene network of Drosophila melanogaster. PLoS Comp Biol 2: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Aivazian D (2004) Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol 5: 886–896 [DOI] [PubMed] [Google Scholar]
- Ptashne M, Johnson AD, Pabo CO (1982) A genetic switch in a bacterial virus. Sci Am 247: 128–140 [DOI] [PubMed] [Google Scholar]
- Quarteroni A, Saleri F (2006) Scientific Computing with MATLAB and Octave (Texts in Computational Science and Engineering). Milan: Springer-Verlag [Google Scholar]
- Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabasi AL (2002) Hierarchical organization of modularity in metabolic networks. Science 297: 1551–1555 [DOI] [PubMed] [Google Scholar]
- Rieder SE, Emr SD (1997) A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell 8: 2307–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122: 735–749 [DOI] [PubMed] [Google Scholar]
- Rybin V, Ullrich O, Rubino M, Alexandrov K, Simon I, Seabra MC, Goody R, Zerial M (1996) GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature 383: 266–269 [DOI] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA 97: 9402–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SigmaPlot (2005) SigmaPlot Exact Graphs with Advisory Stats. San Jose, CA: SigmaPlot [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M (2000) Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149: 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JJ, Chen KC, Novak B (2003) Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol 15: 221–231 [DOI] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294: 1299–1304 [DOI] [PubMed] [Google Scholar]
- Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB, Bucci C (1997) Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem 272: 4391–4397 [DOI] [PubMed] [Google Scholar]
- Vonderheit A, Helenius A (2005) Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol 3: e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD (2000) New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol 151: 551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1
Supplementary Information 2