Abstract
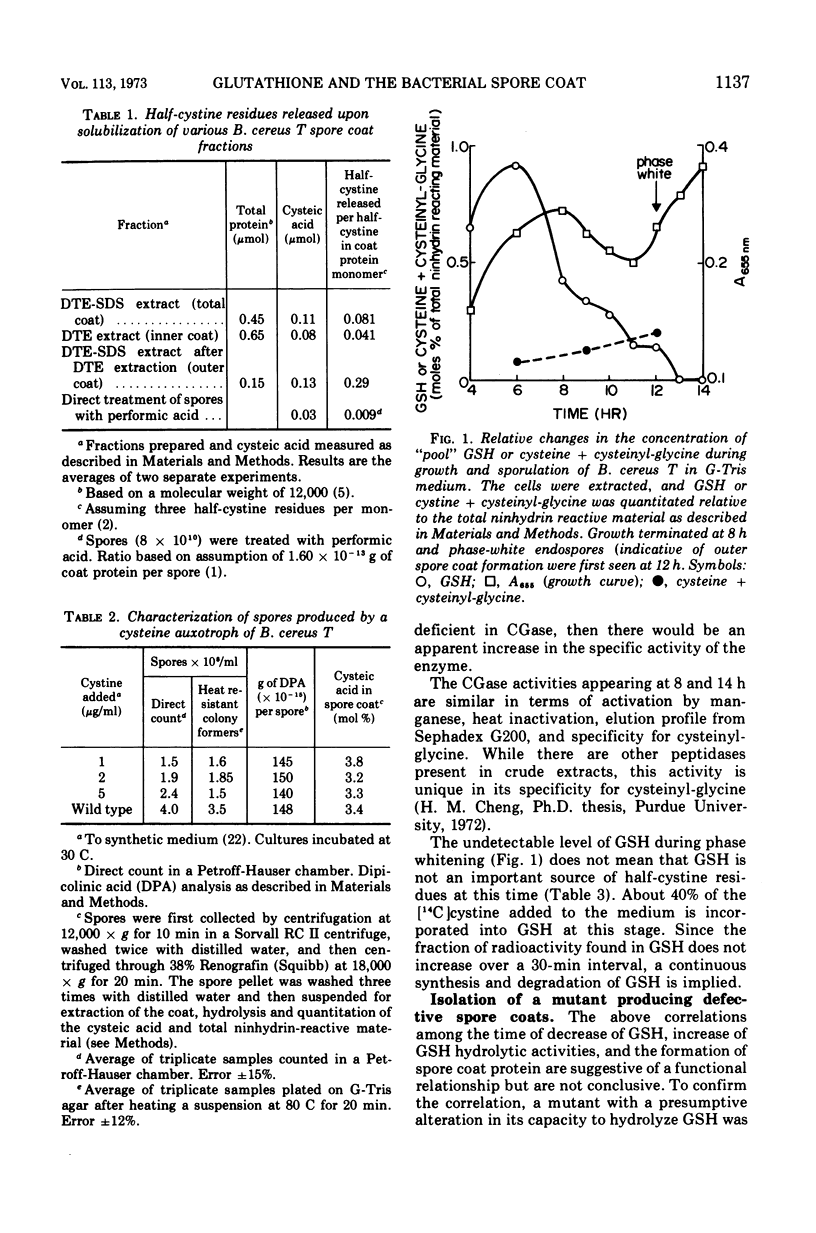
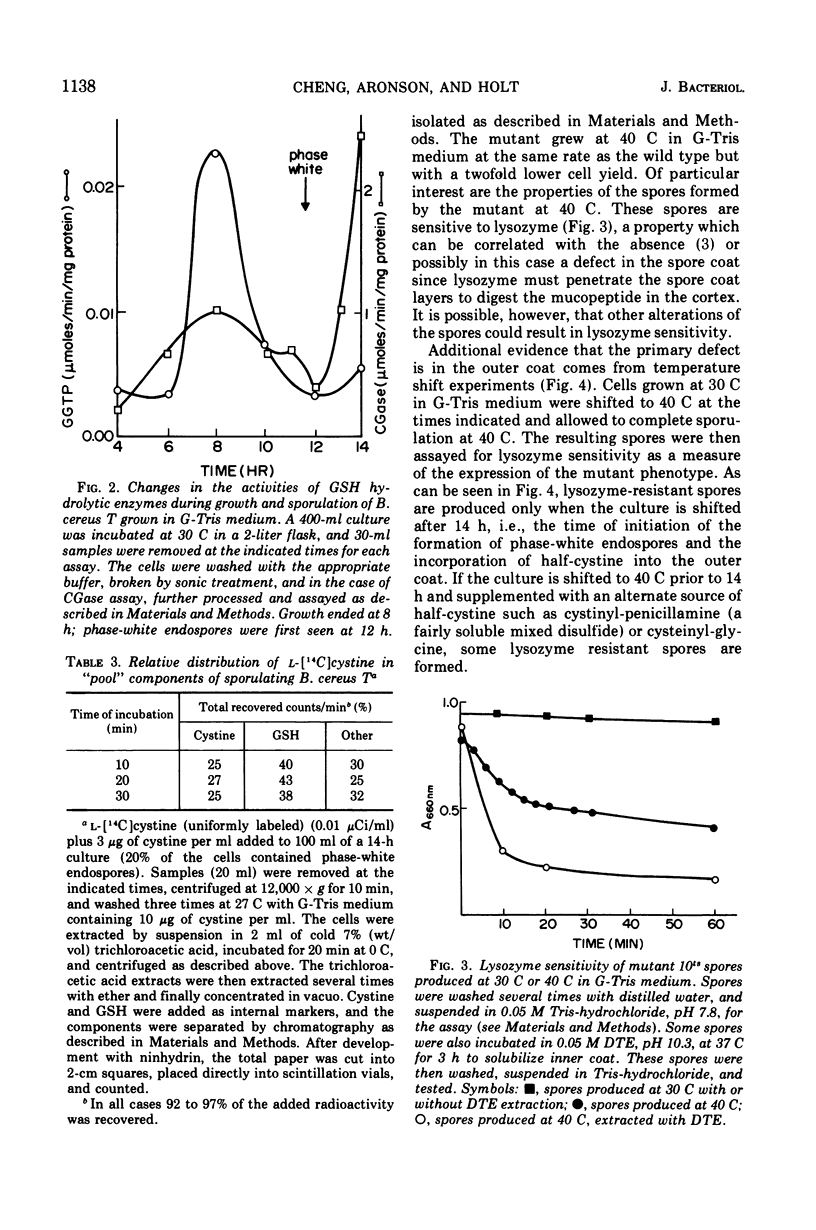
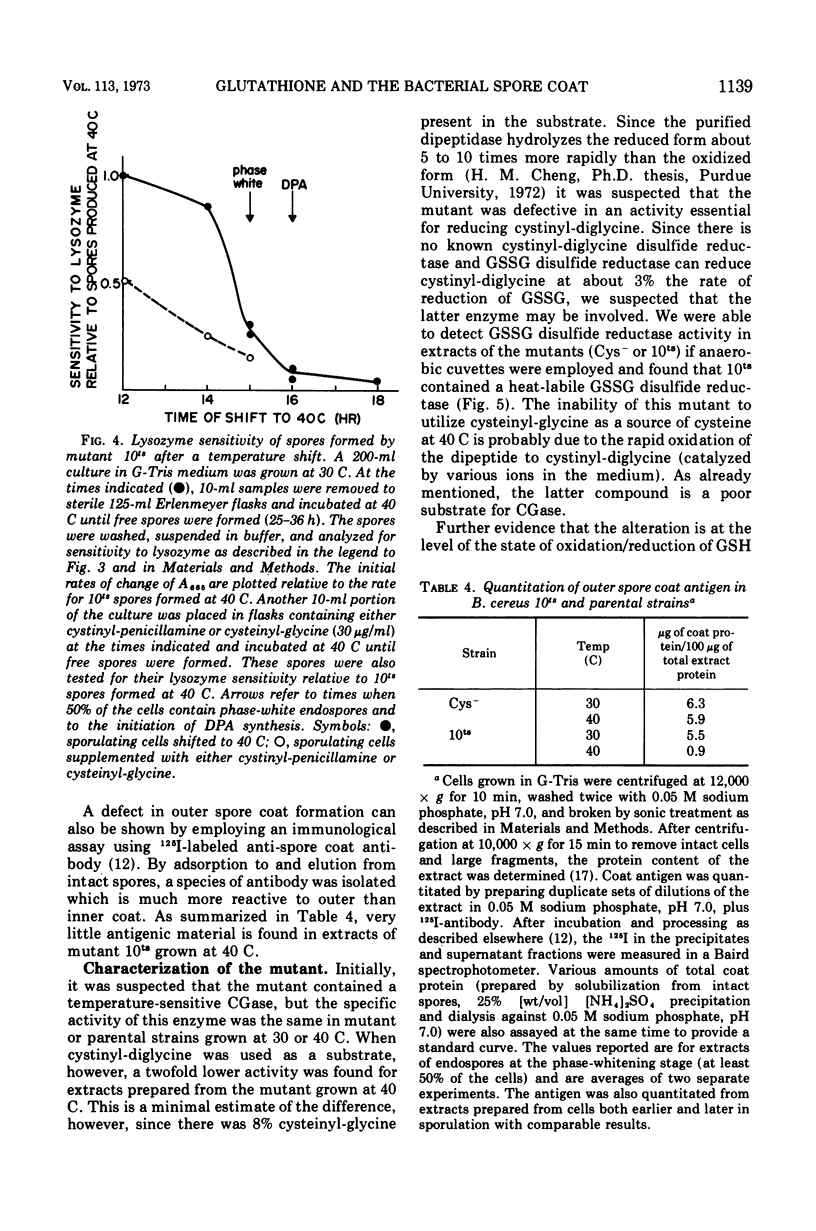
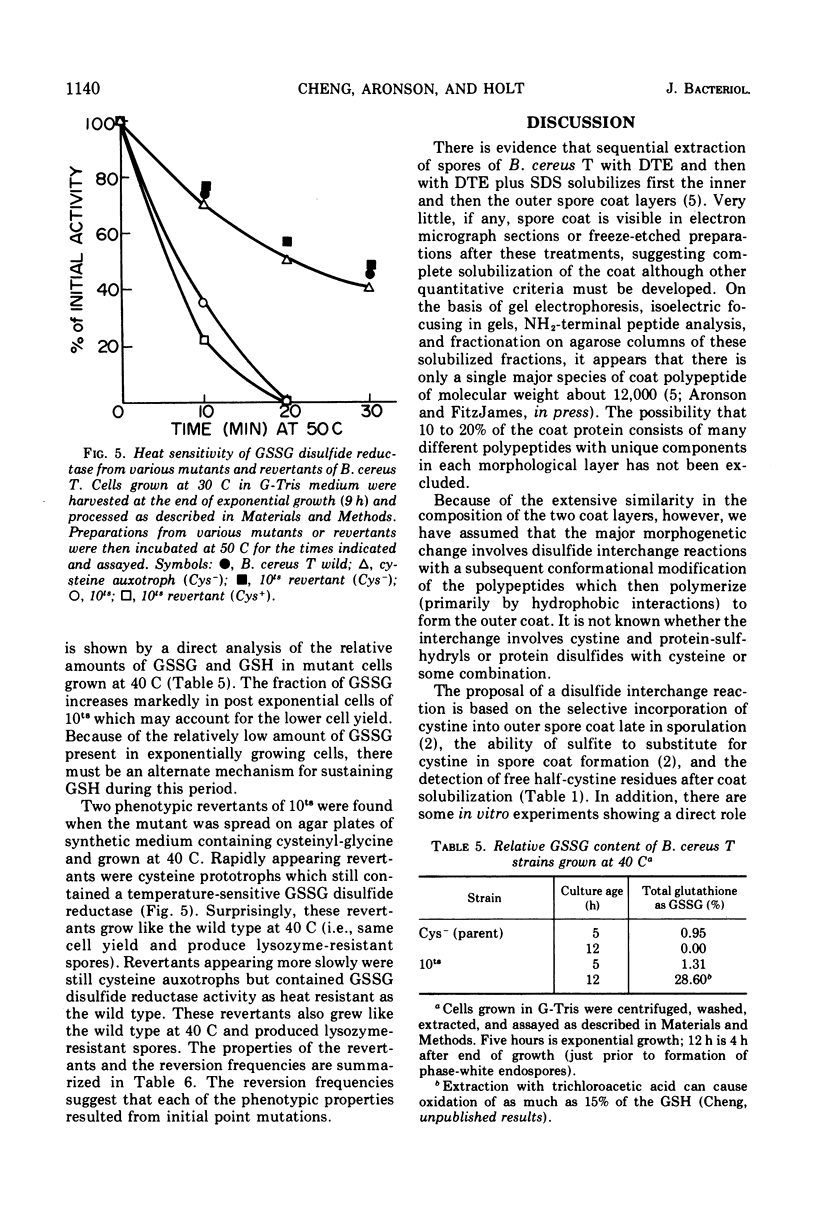
There is a marked increase in the half-cystine content of bacterial spores, especially the coat layers at the time of formation of the outer coat. When a cysteine auxotroph of Bacillus cereus T is grown on limiting cysteine, the spores contain the normal content of half-cystine, suggesting an alternate source. Glutathione appears to be such a supply of cysteine since it is hydrolyzed during sporulation and there are increased activities of the hydrolyzing enzymes at the same time. In addition, a cysteine auxotroph with a second alteration, a temperature-sensitive glutathione disulfide reductase, produces lysozyme-sensitive spores at 40 C. These spores appear to be defective in the formation of outer spore coat. During sporulation at 40 C, the double mutant accumulates oxidized glutathione which is a poor substrate for the hydrolytic enzymes. As a result, sporulating cells are deficient in half-cystines which are essential for outer spore coat morphogenesis. This alteration can be overcome by a shift to 30 C or by addition of cystinyl-pencillamine or cysteinyl-glycine to cultures sporulating at 40 C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Reconstitution of bacterial spore coat layers in vitro. J Bacteriol. 1971 Oct;108(1):571–578. doi: 10.1128/jb.108.1.571-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWHALL J. C., SCOWEN E. F., WATTS R. W. FURTHER OBSERVATIONS ON USE OF D-PENICILLAMINE IN CYSTINURIA. Br Med J. 1964 May 30;1(5395):1411–1413. doi: 10.1136/bmj.1.5395.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S., De Lorenzo F., Anfinsen C. B. Studies on the mechanism of the enzymic catalysis of disulfide interchange in proteins. J Biol Chem. 1967 Feb 10;242(3):398–402. [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hashimoo T., Conti S. F. Ultrastructural changes associated with activation and germination of Bacillus cereus T spores. J Bacteriol. 1971 Jan;105(1):361–368. doi: 10.1128/jb.105.1.361-368.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D., Aronson A. I., Golub E. S. Development of a quantitative immunological assay for the study of spore coat synthesis and morphogenesis. J Bacteriol. 1973 Jan;113(1):313–321. doi: 10.1128/jb.113.1.313-321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Kondo M., Foster J. W. Chemical and electron microscope studies on fractions prepared from coats of Bacillus spores. J Gen Microbiol. 1967 May;47(2):257–271. doi: 10.1099/00221287-47-2-257. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levisohn S., Aronson A. I. Regulation of extracellular protease production in Bacillus cereus. J Bacteriol. 1967 Mar;93(3):1023–1030. doi: 10.1128/jb.93.3.1023-1030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHESON A. T., TIGANE E., HANES C. S. Quantitative chromatographic methods. 5. An improved ninhydrin-hydrindantin reagent. Can J Biochem Physiol. 1961 Feb;39:417–425. doi: 10.1139/o61-040. [DOI] [PubMed] [Google Scholar]
- MCCORQUODALE D. J. SOME PROPERTIES OF A RIBOSOMAL CYSTEINYLGLYCINASE OF ESCHERICHIA COLI B. J Biol Chem. 1963 Dec;238:3914–3920. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- OLSON C. K., BINKLEY F. Metabolism of glutathione. III. Enzymatic hydrolysis of cysteinylglycine. J Biol Chem. 1950 Oct;186(2):731–735. [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. GAMMA-GLUTAMYL-P-NITROANILIDE: A NEW CONVENIENT SUBSTRATE FOR DETERMINATION AND STUDY OF L- AND D-GAMMA-GLUTAMYLTRANSPEPTIDASE ACTIVITIES. Biochim Biophys Acta. 1963 Aug 6;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. ISOLATION OF GAMMA-GLUTAMYL TRANSPEPTIDASE FROM HOG KIDNEY. J Biol Chem. 1965 Jan;240:338–347. [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- Schneider J. A., Bradley K. H., Seegmiller J. E. Colorimetric assay of cystine using noradrenochrome. Anal Biochem. 1968 Apr;23(1):129–131. doi: 10.1016/0003-2697(68)90017-1. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germination. VI. Origin of spore core and coat proteins. J Biol Chem. 1968 Sep 10;243(17):4588–4599. [PubMed] [Google Scholar]
- Thompson E. D., Nakata H. M. Reduction of activity of reduced nicotinamide adenine dinucleotide oxidase by divalent cations in cell-free extracts of Bacillus cereus T. J Bacteriol. 1971 Feb;105(2):494–497. doi: 10.1128/jb.105.2.494-497.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell P. L. Measurement of oxidized glutathione and total glutathione in the perfused rat heart. Biochem J. 1970 May;117(4):661–665. doi: 10.1042/bj1170661. [DOI] [PMC free article] [PubMed] [Google Scholar]


