Abstract
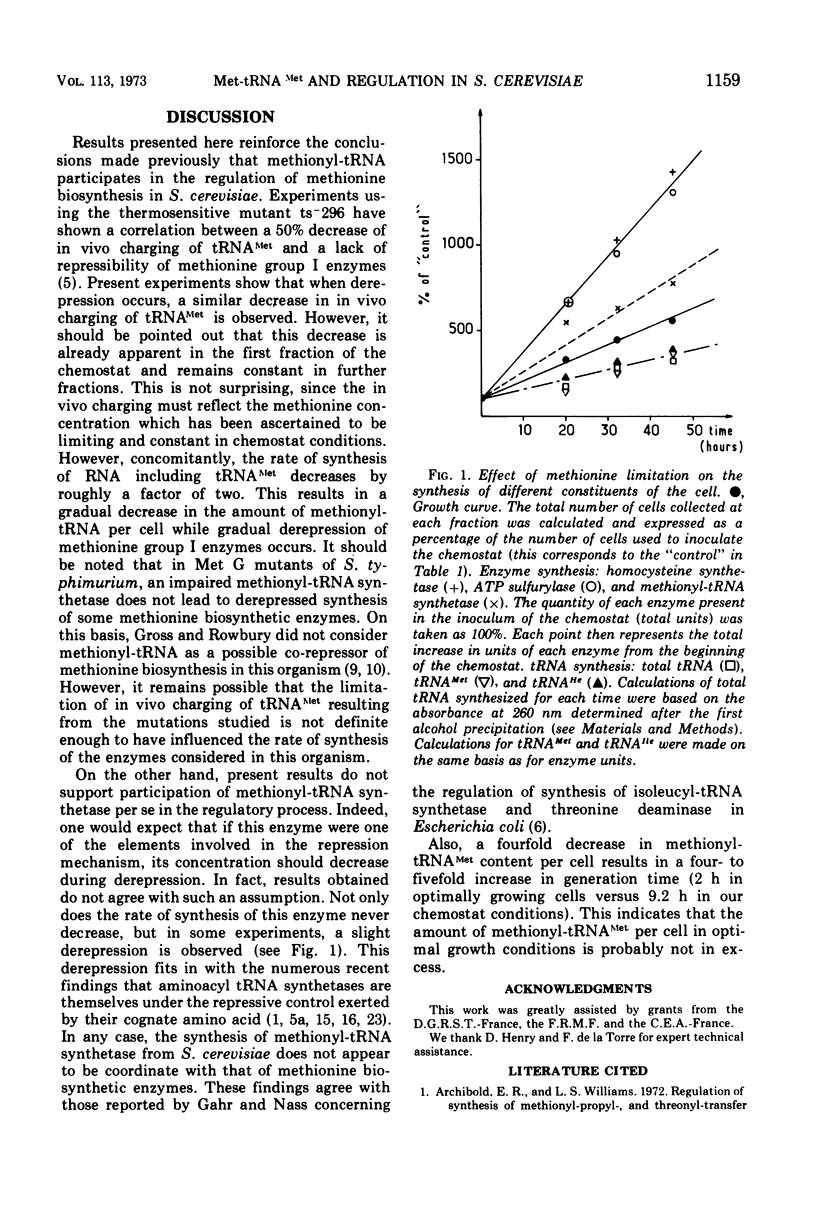
Derepression of some methionine biosynthetic enzymes (methionine group I enzymes) obtained in methionine limitation has been found to be accompanied by a significant lack of in vivo charging of bulk methionine transfer ribonucleic acid (tRNAMet) and in addition by a decreased rate of synthesis of all tRNAs. Under the same conditions, methionyl-tRNA synthetase (MTS) was derepressed rather than repressed. These results are in agreement with those previously published based on studies of a mutant with an impaired MTS (5) and reinforce the idea that the rate of synthesis of methionine group I enzymes can be related to the total content of methionyl (Met)-tRNA Met per cell. They also render unlikely that MTS could be a constituent of the regulatory signal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Cherest H., Eichler F., Robichon-Szulmajster H. Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jan;97(1):328–336. doi: 10.1128/jb.97.1.328-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Robichon-Szulmajster H. Methionine-mediated repression in Saccharomyces cerevisiae: a pleiotropic regulatory system involving methionyl transfer ribonucleic acid and the product of gene eth2. J Bacteriol. 1971 Jun;106(3):758–772. doi: 10.1128/jb.106.3.758-772.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVITO P. C., DREYFUSS J. METABOLIC REGULATION OF ADENOSINE TRIPHOSPHATE SULFURYLASE IN YEAST. J Bacteriol. 1964 Nov;88:1341–1348. doi: 10.1128/jb.88.5.1341-1348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann B., Karst F., Weil J. H. Regulation of the biosynthesis of valyl-tRNA synthetase in yeast. Biochim Biophys Acta. 1971 Dec 16;254(2):226–236. doi: 10.1016/0005-2787(71)90831-8. [DOI] [PubMed] [Google Scholar]
- GALZY P., SLONIMSKI P. P. Evolution de la constitution enzymatique de la levure cultivée sur acide lactique ou sur glucose comme seule source de carbone. C R Hebd Seances Acad Sci. 1957 Dec 23;245(26):2556–2558. [PubMed] [Google Scholar]
- Gahr M., Nass G. Regulation of the formation of isoleucyl-tRNA-synthetase and the level of isoleucine biosynthetic enzymes in E. coli K12. Mol Gen Genet. 1972;116(4):348–359. doi: 10.1007/BF00270091. [DOI] [PubMed] [Google Scholar]
- Gross T. S., Rowbury R. J. Biochemical and physiological properties of methionyl-sRNA synthetase mutants of Salmonella typhimurium. J Gen Microbiol. 1971 Jan;65(1):5–21. doi: 10.1099/00221287-65-1-5. [DOI] [PubMed] [Google Scholar]
- Gross T. S., Rowbury R. J. Methionyl transfer RNA synthetase mutants of Salmonella typhimurium which have normal control of the methionine biosynthetic enzymes. Biochim Biophys Acta. 1969 Jun 17;184(1):233–236. doi: 10.1016/0304-4165(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Kjellin-Stråby K., Phillips J. H. Methyl-deficient transfer ribonucleic acid and macromolecular synthesis in methionine-starved Saccharomyces cerevisiae. J Bacteriol. 1969 Nov;100(2):679–686. doi: 10.1128/jb.100.2.679-686.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- McGinnis E., Williams L. S. Regulation of synthesis of the aminoacyl-transfer ribonucleic acid synthetases for the branched-chain amino acids of Escherichia coli. J Bacteriol. 1971 Oct;108(1):254–262. doi: 10.1128/jb.108.1.254-262.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Experiments with the Chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci U S A. 1950 Dec;36(12):708–719. doi: 10.1073/pnas.36.12.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Grell E. H., Jacobson K. B. Mechanism of suppression in Drosophila: a change in tyrosine transfer RNA. J Mol Biol. 1971 Apr 28;57(2):231–245. doi: 10.1016/0022-2836(71)90343-3. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]


