Abstract
HL-60 human leukemia cells, differentiated into a neutrophil lineage by all-trans retinoic acid (ATRA) treatment, express three members of the carcinoembryonic antigen (CEA) gene family, CEA-related cell adhesion molecule 1 (CEACAM1; CD66a), CEACAM3 (CD66d), and CEACAM6 (CD66c). CD66d is a neutrophil lineage-specific marker, and CD66a and CD66c are found on epithelial and other cells. HL-60 cells continuously treated with ATRA underwent apoptosis, and cells transiently treated for 1 day underwent cell-cycle arrest, entered into senescence, and exhibited reduced apoptosis with CD66-positive cells accounting for the majority of live cells. CD66 antigens were also induced in NB4 leukemic cells upon continuous treatment with ATRA. NB4 cells underwent apoptosis with a higher frequency in transient versus continuous-treated cells (38% vs. 19% at Day 5), in contrast to HL-60 cells that underwent cell-cycle arrest and senescence when transiently treated with ATRA. CD66 antigens were not induced in transient, ATRA-treated NB4 cells compared with HL-60 cells. Cell-cycle arrest in HL-60 cells involved reduction in expression levels of p21, cyclins D and E, while Rb1 exhibited reduction in protein levels without changes in mRNA levels over the time course of ATRA treatment. Analysis of several proapoptotic proteins implicated the activation of calpain and cleavage of Bax in the intrinsic apoptotic pathway, similar to published studies about the apoptosis of neutrophils. CD1d expression was also induced by ATRA in HL-60 cells and ligation with anti-CD1d antibody-induced apoptosis. In contrast, CD1d-positive primary monocytes were protected from spontaneous apoptosis by CD1d ligation. These studies demonstrate distinct cell fates for ATRA-treated HL-60 cells that provide new insights into ATRA-induced cell differentiation.
Keywords: CEACAM1, CD1d
INTRODUCTION
Retinoids, natural and synthetic derivatives of vitamin A, regulate various cellular functions in the body through specific nuclear retinoic acid and retinoid X receptors, which are encoded by separate genes [1]. All-trans retinoic acid (ATRA), the natural isomer of 13-cis retinoic acid, has shown to be an effective agent in the treatment of acute promyelocytic leukemia with induction of complete remissions in the majority of patients [2, 3]. Although the mechanism of its action is still under active investigation, it is widely accepted that ATRA induces differentiation and apoptosis in these leukemic cells. It can readily be understood that either of these outcomes can induce a remission, and resistance to ATRA is a major problem [3]. In addition, ATRA-induced cell senescence has been demonstrated in certain tumors, including neuroblastoma and keratinocyte and breast carcinomas, making ATRA treatment an attractive additive to chemotherapy [4]. At least two types of cell senescence have been described. The first, replicative senescence, has been defined as a telomere-dependent cell growth arrest [5], and the second, telomere-independent, senescence-associated β-galactosidase (SA-β-Gal)-positive cell senescence, has been defined as accelerated senescence. Although its role in senescence is not defined, β-Gal staining at pH 6.0 has been used as a surrogate marker of senescence [6]. In addition, senescent cells are characterized by G1 cell-cycle arrest and the inability to enter S-phase in response to physiologic mitogens, and the cells remain metabolically active and resist apoptotic death for long periods of time [4].
The HL-60 cell line, established from the peripheral blood leukocytes of a patient with acute promyelocytic leukemia [7] and later shown to resemble acute myeloblastic leumekia [8], responds to various stimuli, including ATRA, and accordingly, differentiates into mature, functional cells, similar to granulocytes or monocytes [9]. Among the various surface markers for granulocytes, CD66 is used for immunophenotyping in myelopoiesis [10] and is of special interest to this laboratory. HL-60 cells have long been used as a model of granulocyte differentiation [11] and are known to express CD66a under differentiating conditions [12].
Differentiated HL-60 cells have many advantages over studying fresh human granulocytes, including the fact that human granulocytes represent a spectrum of cells undergoing apoptosis when isolated from the blood and must be re-isolated on a daily basis for interventional studies. For this study, we chose ATRA differentiation of HL-60 into CD66-positive granulocytes as a model for granulocyte differentiation.
CD66 antigens are members of the Ig superfamily and mediate cell–cell interactions including cell recognition in immunity or pathogen infection (see review, refs. [13, 14], for details). CD66a [carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1)] is constitutively expressed on neutrophils and induced in activated T, NK, and B cells [15]. Although there is a single gene for CD66a in humans (two in mice), many gene products are produced by alternative mRNA splicing, making analysis of its function a challenge. For example, one, three, or four Ig-like extracellular domains may be expressed with short or long cytoplasmic domains [16]. However, in lymphocytes, isoforms with the long cytoplasmic domain are exclusively expressed, and as the long cytoplasmic domain possesses two ITIMs, recent interest has focused on this isoform for an inhibitory role in the immune system [17]. CD66a has also attracted interest in the immune system, as it is a cell-surface receptor in humans for many pathogenic strains of bacteria, and in mice, for the murine hepatitis virus [18]. Thus, it is thought that its immune inhibitory role has been usurped by pathogens to further their host–pathogen relationship. With the current interest in the innate immune system and the observation that the highest expression of CD66a is found in neutrophils [19], it is worth speculating that CD66a may play a major role in controlling the various functions of neutrophils. In this regard, apoptosis has been shown to be a major mechanism of neutrophil regulation in that delayed or accelerated apoptosis can have immune consequences [19, 20]. As recent studies in our lab have shown that CD66a directs lumen formation in breast epithelial cells via an apoptotic mechanism [21], we speculate that it may play a role in controlling neutrophil apoptosis. Indeed, recent studies of rat neutrophils indicated that this is a possibility [22]. Moreover, an antiapoptosis role of CD66a has also been shown in monocytes [23].
Although conducting these studies, we also found that ATRA induced CD1d (CD1D) on the cell surface of HL-60 cells. CD1d, a MHC-like molecule, is a nonpeptide, antigen-presenting molecule, broadly distributed in hematopoietic and nonhematopoietic cells, including intestinal epithelial cells [24]. In the myeloid lineage, monocytes or monocyte-derived dendritic cells, but not granulocytes, express CD1d [25,26,27]. Recently, CD1d mRNA induction with retinoic acid was shown in HL-60 cells [28]. Furthermore, Metelitsa et al. [29] reported that Jurkat human T cell leukemia expresses CD1d constitutively. However, the biological role of CD1d in leukemia cells has not been investigated.
In this study, we show that continuous treatment of HL-60 cells with ATRA induces cell-cycle arrest at G1 and apoptosis with concomitant differentiation into a granulocytic lineage, as evidenced by expression of CD66a, CD66d (a lineage-specific marker), and CD66c. In contrast, HL-60 cells transiently treated with ATRA result in cell senescence with positive staining of β-Gal at pH 6.0, a prominent marker of cell senescence. Interestingly, double-staining for apoptotic cells with FITC-annexin V and propidium iodide (PI) revealed that CD66a-positive cells were more resistant to apoptosis than CD66a-negative cells. As transient treatment with ATRA for a single day induces cell senescence without apoptosis, the duration of ATRA treatment may have different outcomes in the treatment of promyelocytic leukemia, thus providing a novel insight into the cell-cycle regulation of these cells. Furthermore, ATRA induced CD1d expression, and its ligation with anti-CD1d antibodies caused apoptosis on HL-60 cells, whereas monocytes were protected from apoptosis with CD1d ligation.
MATERIALS AND METHODS
Cell culture
HL-60 cells [American Type Culture Collection (ATCC), Manassas, VA, USA] and NB4 cells (kindly gifted from Dr. Phillip Koeffler, Cedar-Sinai Medical Center, University of California Los Angeles School of Medicine, Los Angeles, CA, USA) were maintained with RPMI 1640 containing 10% FBS and antibiotics/antimycotics at 5% CO2 incubator, 37°C. Cells were treated with 2 μM ATRA (Fisher Scientific, Pittsburgh, PA, USA). Cell number was counted using a Z1 Coulter counter (Beckman Coulter, Fullerton, CA, USA). Jurkat cells were purchased from ATCC. CEACAM1-4 long form (CEACAM1-4L)-transfected Jurkat cells were established as described previously [30]. Immunomagnetic cell separation of primary monocytes was carried out with human monocyte isolation kit II using AutoMACS separator (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s method. Cells were stimulated with anti-human CD1d antibody (BD Biosciences, San Jose, CA, USA) or MG1-45 control antibody (BioLegend, San Diego, CA, USA).
Cell proliferation analysis
Cells were washed with PBS and suspended in PBS containing 0.1% BSA. CFSE (Invitrogen, Carlsbad, CA, USA) stock solution (2.5 mM in DMSO) was diluted with PBS containing 0.1% BSA to the final concentration, 2.5 μM, and mixed with the same volume of cell suspension. After the incubation for 5 min at 37°C, cells were washed with complete medium and cocultured overnight for further experiments. Fluorescence intensity was measured using a FACSCalibur (BD Biosciences).
Cell-cycle analysis
Cells (1×106) were washed with PBS and suspended in 0.5 ml PBS. Cell suspension was transferred into 4.5 ml ice-cold 70% ethanol and fixed. After washing with PBS, cells were suspended in 5 ml PBS, incubated for 1 min at room temperature. The cell pellet was suspended in 1 ml PI staining solution (4% PI, 2 μg/ml RNase, 0.1% Triton X-100 in PBS) and incubated for 15 min at 37°C. Fluorescence intensity was measured using FACSCalibur.
Apoptosis assay
Apoptosis was analyzed by double-staining with FITC-annexin V (early apoptosis) and PI (late apoptosis) using the apoptosis detection kit II from BD Biosciences.
Electron microscopy (EM)
Cells were washed with PBS, incubated in 2% glutaraldehyde in 0.1 M cacodylate buffer at 4°C overnight, treated with 1% OsO4 for 30 min at 4°C, washed again, and stored at 4°C overnight. Cells were dehydrated with step-wise ethanol–water treatments and embedded in Eponate. Thin sections were stained for 15 min with aqueous 5% uranyl acetate followed by 2 min staining with Sato’s lead. Sections were observed and photographed with a FEI Tecnai G2 12 twin transmission electron microscope equipped with a Gatan US 1000 2K charged-coupled device camera.
Cell-surface marker detection
Cell-surface marker molecules were probed with fluorescent-labeled mAb or nonlabeled mAb and fluorescent-labeled secondary antibody (anti-mouse IgG) in PBS containing 1% BSA at 4°C. Fluorescence intensity was analyzed using FACSCalibur. The antibodies used were as follows: FITC anti-human β2-microgloblin (B2M) from BioLegend, PE anti-human CD1d from BD Biosciences, and mouse IgG1 from the MOPC21 tumor line (Sigma Chemical Co., St. Louis, MO, USA) used as isotype control. T84.1 mAb was raised against CD66e and characterized as anti-CD66a, -c, -d, and -e [31, 32].
Cytokine secretion
Cytokine levels in samples were analyzed in the Clinical Immunobiology Correlative Laboratory at City of Hope (Duarte, CA, USA) and the Human Cytokine Twenty-Five-Plex Antibody Bead Kit (Biosource International, Camarillo, CA, USA), essentially as per the manufacturer’s protocol. The Bio-Plex Luminex 100 XYP instrument and Bio-Plex Manager 3.0 software (Bio-Rad Laboratories, Hercules, CA, USA) were used for sample acquisition and analysis. Samples were tested in duplicate, and the results were reported in pg/ml. Percent coefficient of variation was, in most cases, less than 10%.
Western blotting
Proteins were extracted with radioimmunoprecipitation assay buffer containing Complete Mini (Roche, Indianapolis, IN, USA) as protease inhibitor. Extracted proteins were separated by SDS-PAGE and blotted onto Immobilon-P polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA), which were blocked with 10 mM Tris-buffered saline (pH 7.4) containing 5% skim milk and 0.1% Tween-20. Proteins were probed with appropriate antibody in the same buffer, and then membranes were washed with Tris-buffered saline containing 0.1% Tween 20. The signal was detected with HRP-conjugated anti-mouse or rabbit IgG antibody using the chemiluminescent reaction. Antibodies used in this experiments were the following: anti-β-actin (ACTB) mAb, anti- apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC; PYCARD) polyclonal antibody (pAb), anti-cyclin E1 (CCNE1) mAb, anti-p27 (CDKN1B) pAb, anti-Rb1 (RB1) mAb, and anti-Src homology 2-containing tyrosine phosphatase 1 (SHP1; PTPN6) pAb from Abcam (Cambridge, MA, USA); anti-Bax (BAX) pAb and anti-caspase 3 (CASP3) pAb from BD Biosciences; anticalpain small subunit (CAPNS1) mAb from Chemicon (Pittsburg, PA, USA); and anticaspase 1/ICE (CASP1) pAb and anticyclin D1 (CCND1) mAb from Upstate (Charlottesville, VA, USA).
Estimation of CD66a isoforms
RNA was prepared with Trizol reagent (Invitrogen), according to the manufacturer, following DNase treatment (Promega Biosciences, San Luis Obispo, CA, USA). cDNA was prepared using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Primer sequences were as follows: CD66a forward primer: 5′-AACGTCACCCAGAATGACA-3′, reverse primer: 5′-TCATTGGAGTGGTCCTGAG-3′; β-actin forward primer: 5′-CTGGCCGGGACCTGACTGACTACCTC-3′, reverse primer: 5′-AAACAAATAAAGCCATGCCAATCTCA-3′.
Cell staining
HL-60 cells were treated with ATRA transiently for 1 day and maintained in ATRA-free complete medium following 3 days. At Day 4, cells were stained with PI and applied to Moflo MLS (Dako, Carpinteria, CA, USA) for separation. PI-negative cells were cytospun and stained with Giemsa’s stain. Detail structure of PI-negative cells was analyzed by EM. Lipid particles in ATRA-treated HL60 cells were visualized with dipyrromethene boron difluoride (BODIPY) 493/503 (Invitrogen) at 1 μM final concentration, according to the manufacturer’s method. SA-β-Gal at pH 6.0 was estimated as a senescent cell marker according to the method of Dimri et al. [6]. Images were acquired with an Olympus AX70 automated upright microscope equipped with a Retiga Exi camera.
In vivo calpain activity assay
Rhodamine 110-labeled bis-(t-BOC-L-leucyl-L-methionine amide) calpain substrate (Invitrogen) was added to the cells at the final concentration 10 μM and incubated for 1.5 h at 37°C. After washing the cells with PBS, fluorescence intensity was measured by FACSCalibur.
Quantitative RT-PCR (qRT-PCR)
mRNA expression was measured using the Bio-Rad iQ5 real-time PCR detection system (Bio-Rad Laboratories). Data were analyzed according to the method of Pfaffl [33], using iQ5 Optical System Software Version 2.0, using β-actin expression for normalization. Primers used in this paper are shown in Supplementary Table 1.
RESULTS
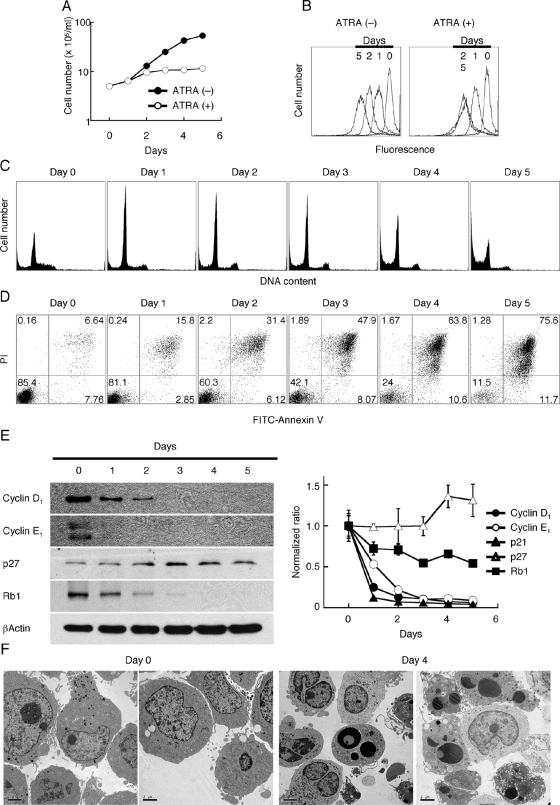
Cell growth arrest and apoptosis in ATRA-treated HL-60 leukemia cells
Previously, Breitman et al. [11] showed that ATRA treatment of HL-60 cells caused cell growth arrest and differentiation into a neutrophil lineage. In our study, HL-60 cells exhibited growth arrest after 1–2 days of ATRA treatment (Fig. 1A), and when labeled with CFSE, no further cell division was observed from Days 2 to 5 (Fig. 1B). Cell-cycle analysis by DNA content showed the accumulation of G0/G1 cells starting at Day 1, with no further changes until Days 4–5, when significant apoptosis decreased overall DNA content (Fig. 1C). This suggests that ATRA caused G1/S cell-cycle arrest; however, G2/M cells were also observed at the same level throughout the treatment period. Apoptosis analysis using annexin V and PI staining revealed <15% early apoptotic cells (annexin V-positive, PI-negative) through the treatment period; however, double-positive cells steadily increased, reaching 75% of the cells by Day 5 (Fig. 1D). Expression of several key cell-cycle regulatory proteins was analyzed by Western blotting in ATRA-treated HL-60 cells. Cyclins D1 and E1, major cyclins involved in the G1/S cell-cycle checkpoint, were decreased dramatically and rapidly, and p27 exhibited a different expression pattern (Fig. 1E). p27 remained at the same level as untreated cells or slightly increased by Days 4–5. qRT-PCR analysis revealed a similar profile, with cyclin D1 and p21 showing an early and dramatic decrease in mRNA levels, followed closely by cyclin E1. Rb1 levels also decreased with treatment but not as dramatically as cyclins D1 and E1. Also in agreement with the Western blot analysis, p27 and Rb1 mRNA levels show little change over the time course of treatment. Although p21, an inhibitor of cyclin-dependent kinases, may be expected to increase during cell-cycle arrest, p21 has multiple functions, including regulation of apoptosis and cell fate [37], and its decrease over the course of ATRA treatment may reflect its role in these other functions. Thus, it appears that decreased cyclin D1 levels, followed by cyclin E1, are primarily responsible for the cell-cycle arrest seen in ATRA-treated HL-60 cells.
Fig. 1.
Induction of apoptosis in HL-60 cells treated with ATRA. HL-60 cells (5×105 cells/ml) were treated with 2 μM ATRA for up to 5 days and analyzed for (A) cell number by Coulter counter analysis, (B) cell proliferation by CFSE staining, (C) cell cycle by permeabilization and PI staining, (D) apoptosis by double-staining with FITC-annexin V and PI, and (E) expression of cell-cycle regulatory molecules by Western blotting and real-time RT-PCR. These experiments were repeated at least twice, and the representative results are shown. (F) EM (original magnification, ×1100) was performed on ATRA-treated HL-60 cells at Days 0 and 4. Note the presence of bilobed nuclei and abundant cytoplasmic granules in treated cells, as well as apoptotic cells with condensed nuclei with intact plasma membranes.
Apoptosis, rather than necrosis, was confirmed using EM, demonstrating apoptotic cells with intact plasma membranes, nuclei with condensed chromatin, and abundant apoptotic bodies (Fig. 1F). Moreover, the formation of cells with bi- and trilobed nuclei and abundant granules was observed by Day 4. These results are similar to those reported in earlier studies [38, 39]. Evidence for differentiation of ATRA-treated HL-60 cells into a neutrophil lineage was provided by EM; we also confirmed neutrophilic differentiation by cell-surface marker antigen (data not shown) and secretion of cytokines, revealing prominent secretion of IL-1β (IL-1B), MIP-1β (CCL4), and MCP-1 (CCL2; Table 1).
TABLE 1.
Cytokine Analysis for ATRA-Treated HL-60 Cellsa
| Day/cytokine
|
No treatment
|
ATRA-treated
|
||
|---|---|---|---|---|
| Day 2 | Day 4 | Day 2 | Day 4 | |
| IL-1β (IL1B) | 72 (27) | 107 (25) | 202 (29) | – |
| IL-7 (IL7) | 46 (50) | 42 (58) | – | – |
| IL-8 (IL8) | 182 (8) | 78 (4) | 156 (8) | 133 (3) |
| IL-15 (IL15) | – | 21 (4) | 19 (42) | – |
| IL-17 (IL17) | 7 (8) | 13 (20) | 12 (14) | – |
| TNF-α (TNF) | 20 (0) | – | – | – |
| MIP-1α (CCL3) | – | 41 (28) | 34 (35) | 30 (20) |
| MIP-1β (CCL4) | – | – | 713 (4) | – |
| MIG (CXCL9) | 32 (35) | – | – | – |
| RANTES (CCL5) | 314 (3) | 200 (6) | 135 (34) | – |
| MCP-1 (CCL2) | 10 (8) | 7 (12) | 149 (3) | 57 (5) |
| IP-10 (CXCL10) | 18 (6) | 17 (18) | 20 (36) | 8 (139) |
All values in pg/ml analyzed in triplicate (±sd). –, Values below detectable limit of assay. MIG, Monokine induced by IFN-γ; IP-10, IFN-inducible protein 10.
Differentiation into neutrophil lineage
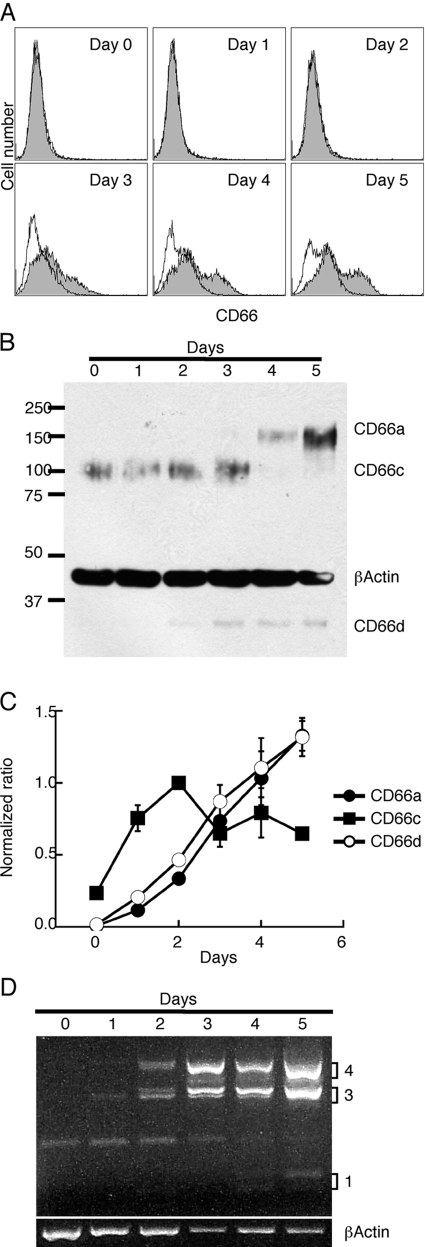
In terms of neutrophil cell-surface markers, CD66 expression is one of the most dominantly expressed classes of proteins and includes at least one member (CD66d or CEACAM3) in which expression is found exclusively in granulocytes [32, 40, 41]. The expression of CD66 cell-surface antigens was performed using mAb T84.1, which detects CD66a (CEACAM1) as well as CD66c (CAECAM6), CD66d (CEACAM3), and CD66e (CEA). CD66a and CD66d also function as receptors for Opa-positive strains of Neisseria [42]. It can be seen that cell-surface expression of the CD66 antigens in ATRA-treated HL-60 cells begins only after Day 3, reaching a maximum at Day 5 (Fig. 2A). As CD66 antigens may also be stored in cytoplasmic granules [43, 44], we followed CD66 expression by Western blotting. In this analysis, small amounts of CD66c were found in untreated cells and decreased to undetectable amounts by Day 4, and CD66a and CD66d revealed an opposite expression profile (Fig. 2B). Further evidence that the CD66 antigens were likely present in cytoplasmic granules was provided by FACS analysis after membrane permeabilization, revealing high levels as early as Day 2 after ATRA treatment (data not shown). In addition, we followed CD66 mRNA expression levels by qRT-PCR. This analysis shows the induction of CD66a and CD66d with ATRA treatment (Fig. 2C), in agreement with the Western blot analysis. CD66c mRNA expression differed from CD66a and CD66d in that its mRNA was still detectable late and higher than that in control cells, although protein expression was undetectable by Days 4–5. As CD66a can be expressed as multiple isoforms with the long cytoplasmic domain isoform predominant in leukocytes, PCR analysis was performed using isoform-specific primers. This analysis shows that CEACAM1-3L and -4L are the predominant isoforms expressed in ATRA-treated HL-60 cells (Fig. 2D).
Fig. 2.
CD66 expression in ATRA-treated HL-60 cells. Time course of expression of (A) CD66a, -c, and -d by FACS analysis; (B) CD66a, -c, and -d by Western blot analysis; (C) CD66a, -c, and -d by qRT-PCR; and (D) CD66a isoforms by RT-PCR on ATRA-treated HL-60 cells. RT-PCR was normalized to β-actin expression. All experiments were repeated at least twice with similar results.
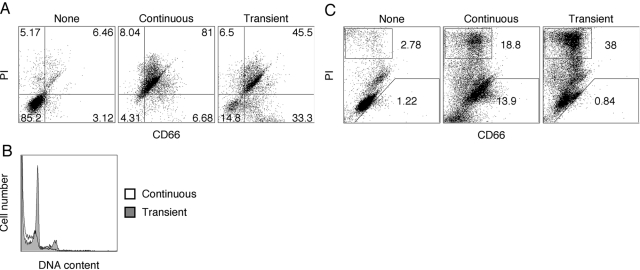
Requirement of continuous retinoic acid stimulation for apoptosis in HL-60 and NB4 cells
In most previous studies about HL-60 cells, the cells were treated continuously with differentiation agents for 5–7 days, at which time, the majority of the cells became apoptotic and died. As apoptosis after 24–48 h is the normal fate of cultured neutrophils, these results were interpreted as further proof of differentiation into the neutrophil lineage. However, when we stimulated HL-60 cells with ATRA for 1 day, followed by its removal from the culture, the cells underwent cell-cycle arrest without subsequent apoptosis. Under the conditions of continuous exposure to ATRA, 90% of HL-60 cells undergo significant apoptosis (PI-positive) by Day 9, and transiently treated cells are 50% PI-positive (Fig. 3A), although these cells did undergo cell-cycle arrest (Fig. 3B). Furthermore, CD66 expression correlated with increased cell survival in the transiently ATRA-treated HL-60 cells (Fig. 3A), suggesting that the more differentiated cells were more resistant to apoptosis. In comparing continuous versus transient treatment with ATRA, the CD66-positive/PI-negative cells increased from 6% to 33%, demonstrating that CD66 expression correlates to resistance to apoptosis, in agreement with the studies about rat neutrophils by Singer and coworkers [22].
Fig. 3.
Comparison of continuous versus transient ATRA treatment of HL-60 cells, which (A) were untreated (None), continuously treated with ATRA (2 μM) for 9 days, or transiently treated with ATRA for 1 day, followed by culturing in ATRA-free medium for an additional 8 days. All cells were double-stained for CD66 and PI and analyzed by FACS at Day 9. (B) Cell-cycle analysis (permeabilized, PI) was performed at Day 11 on transiently (shaded) versus continuously ATRA-treated (open) HL-60 cells. (C) NB4 cells were untreated (None), continuously treated with ATRA (2 μM) for 5 days, or transiently treated with ATRA for 1 day, followed by culturing in ATRA-free medium for an additional 4 days. All cells were double-stained for CD66 and PI and analyzed by FACS at Day 5. All experiments were repeated at least twice to ensure the validity of results.
Although ATRA-stimulated HL-60 cells are a well-characterized model for the neutrophilic lineage, these cells lack the chromosomal translocation t(15;17), which is a characteristic marker for acute promyelocytic leukemia. Therefore, we also investigated the effect of ATRA stimulation on CD66 induction and cell fate in the t(15;17)-positive leukemia cell line NB4. Although CD66 antigens (14%) were also induced in NB4 cells with continuous ATRA stimulation, no CD66 antigens were detected after transient ATRA stimulation (Fig. 3C). Furthermore, the number of PI-positive cells (38%, Day 5) was higher for transient ATRA treatment of NB4 cells compared with continuous treatment (19%, Day 5). These results indicate major differences between the two cell lines when treated with ATRA but also indicate that in both cases, CD66 expression protects cells from apoptosis.
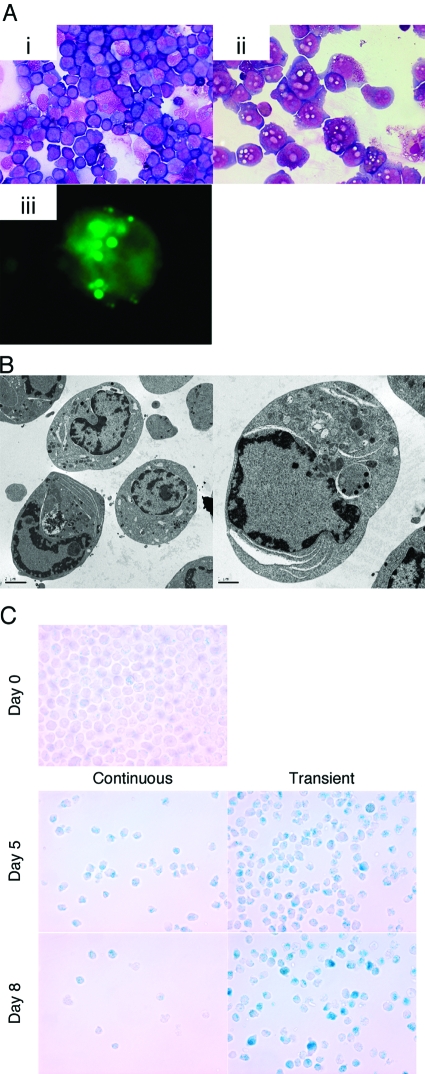
In HL-60 cells, transient ATRA treatment caused cell-cycle arrest, and the cells survived longer without further cell proliferation. At Day 4, we observed many particles in the cytosol by Giemsa’s staining (Fig. 4, A, i and ii).). These particles were stained with the hydrophobic fluorescent dye BODIPY, indicating that they were lipid-containing (Fig. 4, A, iii). Moreover EM analysis showed the presence of large vacuoles in the cytosol and an unusual staining pattern in the nucleus (Fig. 4B). Jouni et al. [45] reported similar vacuoles with a foamy appearance in Vitamin D3-induced HL-60 macrophages. Furthermore, the transiently treated cells were SA-β-Gal-positive (Fig. 4C), suggesting that transient ATRA treatment caused a senescence-like phenotype in the apoptosis-resistant cells.
Fig. 4.
Microscopic analysis of ATRA-treated HL-60 cells, which (A) when treated transiently with ATRA, were stained with PI at Day 4. PI-negative cells were separated by Moflo and stained with Giemsa’s stain. (i) Untreated cells; (ii) PI-negative cells; (iii) lipid particles in cytosol were detected by BODIPY staining on transiently treated HL-60 cells at Day 4. (B) Further structure was analyzed by EM. (C) Untreated (Day 0), transiently treated, or continuously ATRA-treated HL-60 cells were stained for β-Gal using X-Gal at pH 6.0 for the presence of SA-β-Gal at Days 5 and 8. Note the decreased cell numbers in the continuously treated cells and the presence of blue-staining cells in the transiently treated cells. This experiment was repeated at least twice with similar results.
Apoptosis pathway
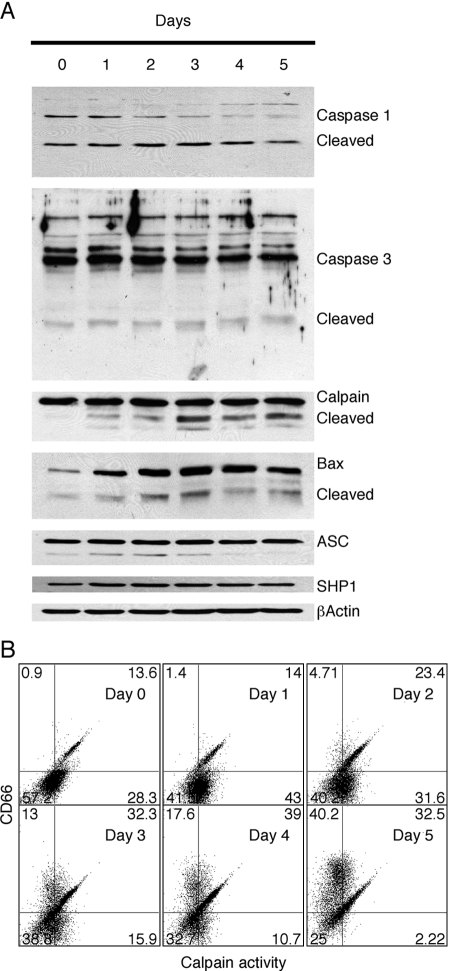
The mechanism of ATRA-induced apoptosis in HL-60 cells has not been investigated extensively [46]. However, in human neutrophils, the apoptosis pathway has been found to involve the intrinsic, mitochondrial pathway, including activation of calpain and several caspases [20, 47, 48]. When we analyzed the activation of several caspases, we found that caspase 1, but not caspase 3, was activated by ATRA treatment (Fig. 5A). The lack of activation of caspase 3 argues against activation of the extrinsic, receptor-mediated pathway, and the activation of caspase 1 may be associated with the production of IL-1β (see Table 1) rather than apoptosis. On the other hand, calpain activation was observed by its cleavage and activity in vivo, although the maximum activity was observed prior to its cleavage, suggesting that other events such as Ca2+ signaling may also be involved (Fig. 5B). The report that calpain activation plays a major role in the apoptosis pathway of human neutrophils [47] suggests that a similar mechanism is involved in the apoptosis of ATRA-treated HL-60 cells.
Fig. 5.
Analysis of ATRA-treated HL-60 cell for markers of apoptosis. (A) HL-60 cells were treated with 2 μM ATRA for 0–5 days and analyzed by Western blot analysis for expression of caspase-1, caspase-3, calpain, Bax, ASC, and SHP1. In each case, note the presence or absence of the cleaved, activated form of the proapoptotic protein. (B) FACS analysis of ATRA-treated cells was performed by double-staining with the calpain substrate rhodamine 110, bis-(t-BOC-L-leucyl-L-methionine amide), and CD66 antibody T84.1 All experiments were repeated at least twice, and similar results were obtained.
In the mitochondrial pathway of apoptosis, Bax cleavage by calpain plays a major role, in that calpain-cleaved Bax is a more potent inducer of apoptosis than intact Bax [49, 50]. Indeed, Western blot analysis of ATRA-treated HL-60 cells shows that Bax expression and Bax cleavage increased and reached a maximum at Day 3 (Fig. 5A). Taken together, these data strongly suggest that ATRA treatment of HL-60 cells induces the mitochondrial pathway of apoptosis and that it closely mimics the apoptosis pathway found in human neutrophils.
In an attempt to identify other molecules involved in the apoptotic pathway, we followed the levels of ASC by Western blots analysis, as ASC is a known Bax adaptor protein that regulates the p53-Bax mitochondrial apoptosis pathway and was reported to be induced in ATRA-treated HL-60 cells [51]. We found that ASC was constitutively expressed together with low amounts of a lower molecular weight isoform (Fig. 5A). Thus, it appears that regulation of the expression levels of ASC does not play a role in apoptosis of these cells.
As we observed that CEACAM1-4L colocalized with SHP1 in pervanadate-treated Jurkat cells [30], SHP1 expression under ATRA stimulation condition in HL-60 cells was investigated. SHP1 expression was induced at Days 1–3 and returned to a normal level at Days 4–5, as reported by Uesugi et al. [52] (Fig. 5A).
CD1d induction and apoptosis with its stimulation
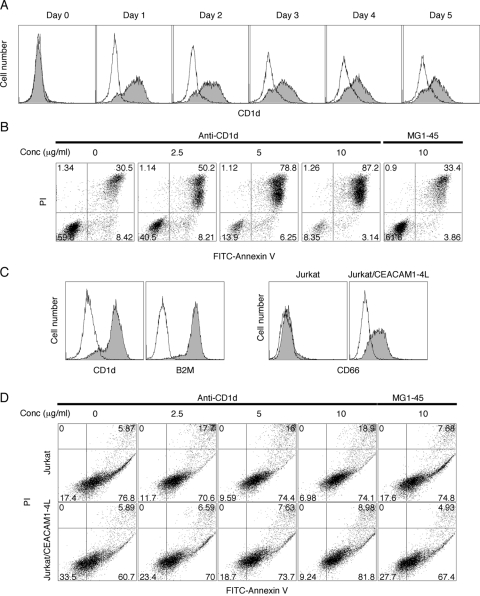
Chen and Ross [28] studied CD1d transcriptional regulation with retinoic acid and observed its mRNA induction in HL-60 cells. As shown in Figure 6A, CD1d expression was induced with ATRA at Day 1. Interestingly, CD1d stimulation with its antibody caused apoptosis in a dose-dependent manner (Fig. 6B).
Fig. 6.
CD1d induction and apoptosis with its stimulation on leukemic cell lines. HL-60 cells were treated with 2 μM ATRA for 0–5 days, continuously or transiently. (A) CD1d expression (shaded) was determined by FACS. The isotype control is shown (open). (B) ATRA-treated HL-60 cells were stimulated with anti-CD1d antibody at Day 1 for an additional 2 days. Apoptosis was analyzed by FITC-annexin V/PI double-staining. MG1-45 isotype antibody was used as negative control. (C) CD1d, B2M, and CD66 expressions in Jurkat cells were measured by FACS. (D) Jurkat cells transfected with or without CEACAM1-4L were stimulated with anti-CD1d antibody for 2 days, and apoptosis was analyzed with FITC-annexin V/PI double-staining. All experiments were repeated at least twice with consistent results.
For further confirmation of apoptosis caused by CD1d stimulation, Jurkat cells, a CD1d-positive leukemic cell line [29], were transfected with CEACAM1-4L and stimulated with anti-CD1d antibody, and apoptosis was measured (Fig. 6C). Although Jurkat cells did not respond to anti-CD1d stimulation to the same extent as ATRA-treated HL-60 cells, the percentage of apoptotic cells increased by twofold with significance in the CEACAM1-4L-transfected cells (Fig. 6D). Interestingly, Jurkat cells but not CD1d-positive HL-60 cells were highly agglutinated by the anti-CD1d antibody (data not shown). Noteworthy, CD1d stimulation caused less apoptosis in CEACAM1-4L-transfected Jurkat cells compared with parental Jurkat cells, similar to that observed in CD66-positive HL-60 cells. Thus, CEACAM1 expression correlates with protection from spontaneous and anti-CD1d antibody-induced apoptosis.
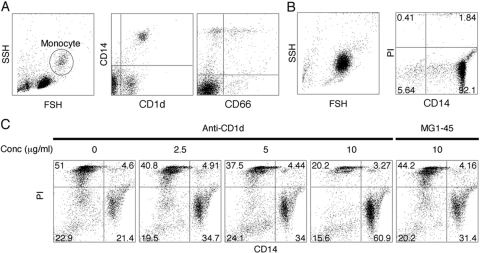
CD1d expression was induced in neutrophilic HL-60 cells as shown above; however, no CD1d expression was observed in human primary neutrophils. In general, monocytes were CD1d-positive. Interestingly, we observed a foam-like vacuole appearance in ATRA-treated HL-60 cells similar to Vitamin D3-treated HL-60 cells thought to differentiate into macrophages. This suggests that ATRA-simulated HL-60 cells have characteristics including neutrophil and monocyte/macrophage lineage in myelopoiesis. Based on these facts, we stimulated CD1d-positive human primary monocytes with anti-CD1d antibody to investigate if CD1d ligation accelerated spontaneous apoptosis as seen on ATRA-treated HL-60 cells or Jurkat cells. As reported previously, monocytes express CD1d and CD66 (Fig. 7). In contrast to ATRA-treated HL-60 cells or Jurkat cells, CD1d ligation protected monocytes from spontaneous apoptosis. This functional difference requires further study.
Fig. 7.
CD1d stimulation on human primary monocytes. (A) CD1d and CD66 expression on human primary monocytes, which (B) were isolated by immunomagnetic purification. Purity and viability were evaluated by CD14/PI staining. (C) Isolated monocytes were stimulated with anti-CD1d antibody overnight, and then cell viability was determined by CD14/PI staining. All experiments were repeated at least twice, and a typical result is shown here. SSH, side scatter height; FSH, forward scatter height.
DISCUSSION
Although ATRA-differentiated HL-60 cells have been used as a surrogate cell line for neutrophil studies, the expression of typical neutrophil markers in terms of cell fate is less studied. With respect to cell surface markers, CD66 is an important cell surface marker for neutrophils. In fact, several studies have shown that retinoic acid is an inducer of CD66a in a number of cell lines [12, 53, 54]. In our own study, using a pan-specific anti-CD66 mAb for Western blotting, we found that CD66c was expressed in untreated HL-60 cells and decreased to low levels after 4 days of ATRA treatment, and CD66a and CD66d were only seen after 4–5 days of ATRA treatment. As the expression of CD66d is known to be lineage-specific [42, 55], these data suggest that ATRA induces the granulocytic lineage in HL-60 cells. Furthermore, CD66a is highly expressed in mature neutrophils but not in neutrophil precursors, in agreement with our data. Finally, EM analysis reveals a typical neutrophil morphology for these cells, convincingly demonstrating that ATRA-treated cells are an excellent model for the study of neutrophils.
As neutrophils are terminally differentiated cells, it was important to examine the cell fate of ATRA-treated HL-60 cells. We found that ATRA treatment caused the majority of cells to accumulate at the G1/S checkpoint with a minority population at G2/M. In agreement with G1/S cell-cycle arrest, cyclins D1 and E1 promptly decreased at the protein and mRNA levels after ATRA treatment. However, p27 remained at the same level or higher during the 4- to 5-day time-course treatment. Thus, the G1/S cyclins must play a major role in the G1/S cell-cycle arrest seen in ATRA-treated HL-60 cells. Although ATRA is well-known to cause G1/S cell-cycle arrest, the presence of cells at G2/M requires mention. Suppression of Rb1 expression at the protein and mRNA levels was seen in our study. As this gene is also involved in the G2/M checkpoint, these data suggest that Rb1 suppression may be responsible for arrest of a subpopulation at the G2/M transition.
Freshly isolated neutrophils succumb to apoptosis after 24–48 h in culture. However, treatment with agents such as LPS or GM-CSF can prolong their life in culture [47], and long-lived neutrophils are thought to be protective during acute infections and detrimental in chronic inflammatory diseases such as rheumatoid arthritis. As ATRA-treated HL-60 cells are mostly apoptotic by Days 4–5, ATRA-treated HL-60 cells have been thought to mimic the lifespan of mature neutrophils. However, in studies about other cell lines, ATRA treatment causes a cell senescent-like phenotype [4]. In our study, we observed induction of SA-β-Gal, a marker of cell senescence, especially in transiently ATRA-treated HL-60 cells. Furthermore, we showed that although continuous ATRA treatment resulted in only apoptosis, transient treatment allowed the cells to survive for up to 11 days with a senescence-like phenotype. Interestingly, the majority of the apoptosis-resistant cells expressed CD66a in HL-60 cells and NB4 cells (the CD66-positive, PI-negative population in Figure , 3A and 3C). Indeed, CD66a has been shown to be an antiapoptotic molecule in rat neutrophils [22]. Furthermore, a correlation between CD66a and the cell-cycle regulator molecules Rb1 and p27 in cancer has also been reported [56]. Thus, CD66a may play a key role in determining cell fate.
It is noteworthy that the expression of distinct CD66a isoforms correlates to cell-survival signaling. For example, the ratio of long and short cytoplasmic domain isoforms affects cell density-dependent survival in epithelial cells [57]. Although short- and long-form cytoplasmic domain isoforms are seen in most epithelial cells, only the ITIM-containing, long-form isoform is found in leukocytes. In this study, we observed the time-dependent expression of the long cytoplasmic domain CD66a isoforms in ATRA-treated HL-60 cells and that its expression correlated with the apoptosis-resistant senescent cells. We conclude that CD66a was expressed as an antiapoptotic molecule in ATRA–HL-60 cells and that cells with the senescence-like phenotype survive longer. These results may have important implications in following the course of ATRA treatment of promyelocytic leukemia.
After cell-cycle arrest, the proapoptotic protein Bax was induced and activated by proteolytical cleavage as shown in Figure 5. The results suggest the high activity of calpain rather than caspases is responsible for Bax cleavage and that the mitochondrial rather than the receptor-mediated is the major apoptotic pathway in ATRA-treated HL-60 cells. As mentioned above, CD66a cell-surface protein was detected on surviving cells after activation of the proapoptotic pathway, suggesting that it exerts an antiapoptotic effect, which was more prominent on cells transiently treated with ATRA, suggesting that the antiapoptotic effects of CD66a dominate in the senescent-like phenotype but fail to overcome apoptosis in HL-60 cells continuously treated with ATRA. It should be noted that ATRA-treated cells also produce cytokines IL-1β, MIP-1β, and MCP-1, which may have an affect on their survival and depending on the culture conditions, may reach higher levels in the continuously treated cells.
Upon analysis of cell-surface markers induced by ATRA, we found a remarkable induction of CD1d expression, which agrees with the previous report of its mRNA expression [28]. In the present study, we observed CD1d induction with ATRA on HL-60 along with B2M, which is required for a functional heterodimer with CD1d. To test the possibility that CD1d may be functional, we treated these cells with anti-CD1d antibodies. Previously, Metelitsa et al. [29] found strong NKT cell cytotoxicity against α-galactosylceramide-pulsed, CD1d-positive Jurkat cells. The effect of CD1d ligation with antibodies has also been studied and showed that anti-CD1d antibody stimulation induced cytokine expression, IL-10 in intestinal epithelial cells, or IL-12 through NF-κB activation in monocytes [58, 59]. Moreover, tyrosine phosphorylation in its cytoplasmic tail was shown to be required for downstream signaling. Herein, we observed cell death caused by CD1d ligation on CD1d-positive HL-60 cells. On the other hand, anti-CD1d antibody-treated monocytes prevented spontaneous apoptosis. If antibody-induced CD1d ligation mimics recognition by NKT cells, these results would suggest that NKT cells are capable of killing CD1d-positive leukemic cells. The expression of CD1d and CD66 in ATRA-treated HL-60 cells, together with their different cell fate, suggests new opportunities for functional studies about these cells.
Acknowledgments
Research was supported by National Institutes of Health grant CA84202. M. O. designed and performed the experiments, analyzed the data, and wrote the paper. J. E. S. obtained funding, provided oversight, and wrote the paper. The authors declare no competing financial interests. We thank Dr. Shu Mi and Dr. Michael Kalos of the Clinical Immunobiology Correlative Studies Laboratory of City of Hope National Medical Center for Luminex assays, Dr. Mariko Lee and Dr. Brian Armstrong for support and advice about fluorescence microscope experiments, Dr. Sofia Loela for pathological analysis, and Dr. John Hardy for his EM analysis.
References
- Mehta K. Retinoids as regulators of gene transcription. J Biol Regul Homeost Agents. 2003;17:1–12. [PubMed] [Google Scholar]
- Puccetti E, Ruthardt M. Acute promyelocytic leukemia: PML/RARα and the leukemic stem cell. Leukemia. 2004;18:1169–1175. doi: 10.1038/sj.leu.2403367. [DOI] [PubMed] [Google Scholar]
- Tallman M S, Nabhan C, Feusner J H, Rowe J M. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- Roninson I B, Dokmanovic M. Induction of senescence-associated growth inhibitors in the tumor-suppressive function of retinoids. J Cell Biochem. 2003;88:83–94. doi: 10.1002/jcb.10320. [DOI] [PubMed] [Google Scholar]
- Wright W E, Shay J W. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr Opin Genet Dev. 2001;11:98–103. doi: 10.1016/s0959-437x(00)00163-5. [DOI] [PubMed] [Google Scholar]
- Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S J, Gallo R C, Gallagher R E. Continuous growth and differentiation of human myeloid leukemic cells in suspension culture. Nature. 1977;270:347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Dalton W T, Jr, Ahearn M J, McCredie K B, Freireich E J, Stass S A, Trujillo J M. HL-60 cell line was derived from a patient with FAB-M2 and not FAB-M3. Blood. 1988;71:242–247. [PubMed] [Google Scholar]
- Tsiftsoglou A S, Pappas I S, Vizirianakis I S. Mechanisms involved in the induced differentiation of leukemia cells. Pharmacol Ther. 2003;100:257–290. doi: 10.1016/j.pharmthera.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hansen I, Meyer K, Hokland P. Flow cytometric identification of myeloid disorders by asynchronous expression of the CD14 and CD66 antigens. Eur J Haematol. 1998;61:339–346. doi: 10.1111/j.1600-0609.1998.tb01098.x. [DOI] [PubMed] [Google Scholar]
- Breitman T R, Selonick S E, Collins S J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelic M, Chen I, Parker J, Zhang P, Grunert F, Chen T. Retinoic acid treated HL60 cells express CEACAM1 (CD66a) and phagocytose Neisseria gonorrhoeae. FEMS Immunol Med Microbiol. 2004;42:261–266. doi: 10.1016/j.femsim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarstrom S, Holmes K V, Karlsson A, Kuroki M. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- Gray-Owen S D, Blumberg R S. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- Barnett T R, Kretschmer A, Austen D A, Goebel S J, Hart J T, Elting J J, Kamarck M E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989;108:267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaishi T, Iijima H, Nakajima A, Chen D, Blumberg R S. Role of CEACAM1 as a regulator of T cells. Ann N Y Acad Sci. 2006;1072:155–175. doi: 10.1196/annals.1326.004. [DOI] [PubMed] [Google Scholar]
- Kuespert K, Pils S, Hauck C R. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–571. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoevska V, Horst A, Klampe B, Lucka L, Wagener C, Nollau P. CEACAM1, an adhesion molecule of human granulocytes, is fucosylated by fucosyltransferase IX and interacts with DC-SIGN of dendritic cells via Lewis x residues. Glycobiology. 2006;16:197–209. doi: 10.1093/glycob/cwj057. [DOI] [PubMed] [Google Scholar]
- Simon H U. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Kirshner J, Chen C J, Liu P, Huang J, Shively J E. CEACAM1–4S, a cell–cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci USA. 2003;100:521–526. doi: 10.1073/pnas.232711199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B B, Klaile E, Scheffrahn I, Muller M M, Kammerer R, Reutter W, Obrink B, Lucka L. CEACAM1 (CD66a) mediates delay of spontaneous and Fas ligand-induced apoptosis in granulocytes. Eur J Immunol. 2005;35:1949–1959. doi: 10.1002/eji.200425691. [DOI] [PubMed] [Google Scholar]
- Yu Q, Chow E M, Wong H, Gu J, Mandelboim O, Gray-Owen S D, Ostrowski M A. CEACAM1 (CD66a) promotes human monocyte survival via a phosphatidylinositol 3-kinase- and AKT-dependent pathway. J Biol Chem. 2006;281:39179–39193. doi: 10.1074/jbc.M608864200. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner M B. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Exley M, Garcia J, Wilson S B, Spada F, Gerdes D, Tahir S M, Patton K T, Blumberg R S, Porcelli S, Chott A, Balk S P. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada F M, Borriello F, Sugita M, Watts G F, Koezuka Y, Porcelli S A. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur J Immunol. 2000;30:3468–3477. doi: 10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Gerlini G, Hefti H P, Kleinhans M, Nickoloff B J, Burg G, Nestle F O. Cd1d is expressed on dermal dendritic cells and monocyte-derived dendritic cells. J Invest Dermatol. 2001;117:576–582. doi: 10.1046/j.0022-202x.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Chen Q, Ross A C. Retinoic acid regulates CD1d gene expression at the transcriptional level in human and rodent monocytic cells. Exp Biol Med (Maywood) 2007;232:488–494. [PMC free article] [PubMed] [Google Scholar]
- Metelitsa L S, Naidenko O V, Kant A, Wu H W, Loza M J, Perussia B, Kronenberg M, Seeger R C. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- Chen C J, Shively J E. The cell–cell adhesion molecule carcinoembryonic antigen-related cellular adhesion molecule 1 inhibits IL-2 production and proliferation in human T cells by association with Src homology protein-1 and down-regulates IL-2 receptor. J Immunol. 2004;172:3544–3552. doi: 10.4049/jimmunol.172.6.3544. [DOI] [PubMed] [Google Scholar]
- Neumaier M, Fenger U, Wagener C. Monoclonal antibodies for carcinoembryonic antigen (CEA) as a model system: identification of two novel CEA-related antigens in meconium and colorectal carcinoma tissue by Western blots and differential immunoaffinity chromatography. J Immunol. 1985;135:3604–3609. [PubMed] [Google Scholar]
- Skubitz K M, Campbell K D, Skubitz A P. CD66a, CD66b, CD66c, and CD66d each independently stimulate neutrophils. J Leukoc Biol. 1996;60:106–117. doi: 10.1002/jlb.60.1.106. [DOI] [PubMed] [Google Scholar]
- Pfaffl M W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla-Martinez L, Kremer M, Specht K, Calzada-Wack J, Nathrath M, Schaich R, Hofler H, Fend F. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol. 2003;162:1449–1461. doi: 10.1016/S0002-9440(10)64278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Tidow C, Metzger R, Kugler K, Diederichs S, Idos G, Thomas M, Dockhorn-Dworniczak B, Schneider P M, Koeffler H P, Berdel W E, Serve H. Cyclin E is the only cyclin-dependent kinase 2-associated cyclin that predicts metastasis and survival in early stage non-small cell lung cancer. Cancer Res. 2001;61:647–653. [PubMed] [Google Scholar]
- Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 2001;158:419–429. doi: 10.1016/S0002-9440(10)63985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A, Dowdy S F, Roberts J M. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Bobichon H, Mayer P, Carpentier Y, Desoize B. HL60 cells exhibit particular ultrastructural features during all-trans retinoic acid-induced apoptosis. Int J Oncol. 1998;12:649–653. doi: 10.3892/ijo.12.3.649. [DOI] [PubMed] [Google Scholar]
- Di Francesco A, Desnoyer R W, Covacci V, Wolf F I, Romani A, Cittadini A, Bond M. Changes in magnesium content and subcellular distribution during retinoic acid-induced differentiation of HL60 cells. Arch Biochem Biophys. 1998;360:149–157. doi: 10.1006/abbi.1998.0937. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Abe H, Imakiirei T, Liao S, Uchida H, Yamauchi Y, Oikawa S, Kuroki M. Identification and comparison of residues critical for cell-adhesion activities of two neutrophil CD66 antigens, CEACAM6 and CEACAM8. J Leukoc Biol. 2001;70:543–550. [PubMed] [Google Scholar]
- McCaw S E, Schneider J, Liao E H, Zimmermann W, Gray-Owen S D. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol. 2003;49:623–637. doi: 10.1046/j.1365-2958.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- Hauck C R, Meyer T F. “Small” talk: Opa proteins as mediators of Neisseria-host-cell communication. Curr Opin Microbiol. 2003;6:43–49. doi: 10.1016/s1369-5274(03)00004-3. [DOI] [PubMed] [Google Scholar]
- Ducker T P, Skubitz K M. Subcellular localization of CD66, CD67, and NCA in human neutrophils. J Leukoc Biol. 1992;52:11–16. doi: 10.1002/jlb.52.1.11. [DOI] [PubMed] [Google Scholar]
- Zhao L, Furebring M, Xu S, Venge P. Subcellular localization and mobilization of carcinoembryonic antigen-related cell adhesion molecule 8 in human neutrophils. Br J Haematol. 2004;125:666–673. doi: 10.1111/j.1365-2141.2004.04963.x. [DOI] [PubMed] [Google Scholar]
- Jouni Z E, Winzerling J J, McNamara D J. 1,25-Dihydroxyvitamin D3-induced HL-60 macrophages: regulation of cholesterol and LDL metabolism. Atherosclerosis. 1995;117:125–138. doi: 10.1016/0021-9150(95)05569-i. [DOI] [PubMed] [Google Scholar]
- Otake Y, Sengupta T K, Bandyopadhyay S, Spicer E K, Fernandes D J. Retinoid-induced apoptosis in HL-60 cells is associated with nucleolin down-regulation and destabilization of Bcl-2 mRNA. Mol Pharmacol. 2005;67:319–326. doi: 10.1124/mol.104.006080. [DOI] [PubMed] [Google Scholar]
- Akgul C, Moulding D A, Edwards S W. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–322. doi: 10.1016/s0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- Maianski N A, Maianski A N, Kuijpers T W, Roos D. Apoptosis of neutrophils. Acta Haematol. 2004;111:56–66. doi: 10.1159/000074486. [DOI] [PubMed] [Google Scholar]
- Wood D E, Thomas A, Devi L A, Berman Y, Beavis R C, Reed J C, Newcomb E W. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J. Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wild-type Bax. Cancer Lett. 2003;189:221–230. doi: 10.1016/s0304-3835(02)00552-9. [DOI] [PubMed] [Google Scholar]
- Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T, Sagara J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- Uesugi Y, Fuse I, Toba K, Kishi K, Furukawa T, Koike T, Aizawa Y. Involvement of SHP-1, a phosphotyrosine phosphatase, during myeloid cell differentiation in acute promyelocytic leukemia cell lines. Eur J Haematol. 1999;62:239–245. doi: 10.1111/j.1600-0609.1999.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Watt S M, Fawcett J, Murdoch S J, Teixeira A M, Gschmeissner S E, Hajibagheri N M, Simmons D L. CD66 identifies the biliary glycoprotein (BGP) adhesion molecule: cloning, expression, and adhesion functions of the BGPc splice variant. Blood. 1994;84:200–210. [PubMed] [Google Scholar]
- Botling J, Oberg F, Nilsson K. CD49f (α 6 integrin) and CD66a (BGP) are specifically induced by retinoids during human monocytic differentiation. Leukemia. 1995;9:2034–2041. [PubMed] [Google Scholar]
- Chen T, Gotschlich E C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger A M, Kappes H, Methner C, Rieck G, Brummer J, Wagener C, Loning T, Milde-Langosch K. Expression of the adhesion molecule CEACAM1 (CD66a, BGP, C-CAM) in breast cancer is associated with the expression of the tumor-suppressor genes Rb, Rb2, and p27. Virchows Arch. 2002;440:139–144. doi: 10.1007/s00428-001-0554-0. [DOI] [PubMed] [Google Scholar]
- Scheffrahn I, Singer B B, Sigmundsson K, Lucka L, Obrink B. Control of density-dependent, cell state-specific signal transduction by the cell adhesion molecule CEACAM1, and its influence on cell cycle regulation. Exp Cell Res. 2005;307:427–435. doi: 10.1016/j.yexcr.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Colgan S P, Hershberg R M, Furuta G T, Blumberg R S. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci USA. 1999;96:13938–13943. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S C, Shaulov A, Wang R, Balk S P, Exley M A. CD1d ligation on human monocytes directly signals rapid NF-κB activation and production of bioactive IL-12. Proc Natl Acad Sci USA. 2005;102:11811–11816. doi: 10.1073/pnas.0503366102. [DOI] [PMC free article] [PubMed] [Google Scholar]