Abstract
This study provides a detailed description of immunolocalization of two oxygen-binding proteins, neuroglobin (Ngb) and cytoglobin (Cygb), in the anterior segment of healthy human and canine eyes. Specific antibodies against Ngb and Cygb were used to examine their distribution patterns in anterior segment structures including the cornea, iris, trabecular meshwork, canal of Schlemm, ciliary body, and lens. Patterns of immunoreactivity (IR) were imaged with confocal scanning laser and conventional microscopy. Analysis of sectioned human and canine eyes showed Ngb and Cygb IR in the corneal epithelium and endothelium. In the iris, Ngb and Cygb IR was localized to the anterior border and the stroma, iridal sphincter, and dilator muscle. In the iridocorneal angle, Ngb and Cygb were detected in endothelial cells of the trabecular meshwork and canal of Schlemm in human. In the ciliary body, Ngb and Cygb IR was localized to the non-pigmented ciliary epithelium of the pars plana and pars plicata and in ciliary body musculature. Ngb and Cygb distribution was similar and colocalized within the same structures of healthy human and canine anterior eye segments. Based on their immunolocalization and previously reported biochemical features, we hypothesize that Ngb and Cygb may function as scavengers of reactive oxygen species. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 56:863–872, 2008)
Keywords: globin, oxygen-binding, nitric oxide scavenging
Globins are a family of heme-containing proteins that reversibly bind gaseous ligands such as oxygen (O2), nitric oxide (NO), and carbon monoxide (CO). Their presence has been described in bacteria, fungi, protists, plants, and animals (Hardison 1996). Four mammalian globins have thus far been identified: hemoglobin, myoglobin, neuroglobin, and cytoglobin (Burmester et al. 2000,2002; Trent et al. 2001; Pesce et al. 2002; Trent and Hargrove 2002). Neuroglobin has been identified in the brain (Burmester et al. 2000; Awenius et al. 2001; Mammen et al. 2002; Reuss et al. 2002; Zhang et al. 2002; Wystub et al. 2003; Fuchs et al. 2004; Kugelstadt et al. 2004), in retinal neurons (Schmidt et al. 2003; Fuchs et al. 2004; Kugelstadt et al. 2004), and in different endocrine tissues (Geuens et al. 2003). Functional roles that have been proposed for Ngb include enhancement of oxygen delivery to mitochondria (Schmidt et al. 2003; Burmester and Hankeln 2004; Fuchs et al. 2004; Wystub et al. 2004), detoxification of NO (Mammen et al. 2002; Herold et al. 2004; Nienhaus et al. 2004; Vallone et al. 2004), and hypoxia sensing (Couture et al. 2001; Dewilde et al. 2001; Wakasugi et al. 2003). In addition, it has been shown that Ngb expression is increased in vivo and in vitro during acute neuronal hypoxia and that Ngb overexpression leads to enhanced survival of cortical neurons (Sun et al. 2001,2003). Cytoglobin was detected in virtually all tissues in humans, mice and zebrafish (Burmester et al. 2002; Trent and Hargrove 2002). Proposed roles for Cygb include facilitation of oxygen diffusion, NO scavenging (Trent and Hargrove 2002), and O2 supply for enzymatic reactions such as hydroxylation of proline residues during collagen synthesis (Schmidt et al. 2004).
The anatomical structures of the anterior segment of the mammalian eye are composed of the cornea, iris, iridocorneal angle, ciliary body, and lens (Gould et al. 2004). Basic physiological requirements that need to be fulfilled for anterior eye segment structures to function efficiently are sufficient O2 supply (Hoper et al. 1989; Pfister 1991; Helbig et al. 1993; Fitch et al. 2000; McNulty et al. 2004), the existence of mechanisms for scavenging of reactive oxygen species (ROS) (Ishimoto et al. 1996; Rose et al. 1998; Zhou et al. 1999; Cejková et al. 2004; Parker et al. 2004; Ohia et al. 2005), and the regulation of NO levels (Becquet et al. 1997; Wu et al. 1997; Bosch-Morell et al. 2002; Kim et al. 2002; Schneemann et al. 2003). Based on the immunolocalization of Ngb and Cygb and their previously reported biochemical features, we hypothesize that Ngb and Cygb may serve important functions as scavengers of reactive oxygen species in the anterior eye segment. Therefore, the purpose of this study was to use IHC procedures and confocal microscopy to study the detailed localization of the two gaseous ligand-binding globins, Ngb and Cygb, within anterior eye segment structures.
Materials and Methods
Human Tissue
Globes from six donors with no medical history of ocular diseases or histopathological evidence of ocular diseases (ages: 1.5 and 6 months and 60, 62, 78, and 82 years) were fixed for 48 hr in 10% neutral-buffered formalin immediately after tissue procurement. They were transferred to an ethanol-formaldehyde mixture (Penfix; Richard-Allan Scientific, Kalamazoo, MI), embedded in paraffin, and sectioned at 4 μm thickness. The eyes were obtained through the Iowa Lions Eye Bank in Iowa City and from the F.C. Blodi Eye Pathology Laboratory at the University of Iowa. Tissue collection adhered to the tenets of the Declaration of Helsinki and University of Iowa guidelines.
Canine Tissue
Eyes from nine healthy adult beagles (2–4 years of age) were collected immediately after euthanasia. Each animal was euthanized for reasons other than use in this study. Before euthanasia, all eyes were examined for signs of ocular abnormalities (slit-lamp examination, fundus examination) and the presence of elevated intraocular pressure (tonometry). Eyes with detectable abnormalities of the anterior segment, lens, or the fundus and/or presence of elevated intraocular pressure (>25 mmHg) were not collected for use in this study. Globes were fixed for 12 hr at 4C in 4% paraformaldehyde in 0.1 M PBS. After fixation, globes were embedded in paraffin, and sections were cut at 7 μm thickness. All research conducted in this study was in full compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and Iowa State University Committee on Animal Care regulations.
Antibodies
Full-length human neuroglobin and cytoglobin recombinant proteins were synthesized and purified as described previously (Trent et al. 2001; Trent and Hargrove 2002). Polyclonal antisera against synthesized Ngb or Cygb proteins were raised in rabbits and antibodies (Abs) were affinity purified from the serum using the recombinant proteins coupled to a SulfoLink column (Pierce Biotechnology; Rockford, IL). Mouse monoclonal anti-Ngb antibody and the human recombinant Ngb protein used to produce this antibody were kindly provided by BioVendor (Candler, NC). Antibody specificity was verified using Western blot analysis on recombinant proteins, canine tissue samples (Ostojic et al. 2006), and human retinal tissue samples (Ostojic et al. in press). Anti-Ngb Abs detected recombinant Ngb protein at ∼17 kDa, and a band of similar molecular mass was detected in protein samples from dog (Ostojic et al. 2006) and human retinas (Ostojic et al. in press). Anti-Cygb Ab detected recombinant Cygb protein at ∼21 kDa and also detected Cygb in protein extracts from dog retina at ∼29 kDa (Ostojic et al. 2006) and a single ∼26 kDa protein band from the human retinal extract (Ostojic et al. in press). The difference in the molecular mass between human recombinant Cygb (∼21 kDa) and Cygb detected in the protein extract from dog and human retina (∼29 kDa and ∼26 kDa, respectively) is likely the result of posttranslational modification of the protein (Ostojic et al. in press). Primary Abs used in this study and their dilutions are summarized in Supplemental Table 1.
IHC
Fluorescent IHC was performed as described previously (Ostojic et al. 2006). Briefly, tissue sections were deparaffinized, rehydrated in a graded alcohol series, processed for antigen retrieval, and incubated for 2 hr in blocking solution. Sections were double-labeled with a mixture of the primary Abs overnight and incubated in one of the following secondary Ab mixtures: goat anti-mouse Cy5 (Jackson ImmunoResearch; West Grove, PA) and goat anti-rabbit Alexa 488 Ab (Molecular Probes; Eugene, OR) or goat anti-rabbit Cy5 (Jackson ImmunoResearch) and goat anti-mouse Alexa 488 Ab (Molecular Probes) diluted to 1:200. After the 2-hr incubation, sections were washed in potassium PBS (KPBS) with Triton X-100. Finally, sections were counterstained with 1 μg/ml of 4′,6-diamino-2-phenylindole (DAPI; Molecular Probes), washed in KPBS, and coverslipped. Negative controls were run in parallel during all processing and included the omission of the primary Ab or secondary Ab.
Analysis of Tissue Sections
Human and canine tissue sections labeled with fluorescent antibodies were visualized and images were captured using a Leica confocal scanning laser microscope (TCS-NT; Leica Microsystems, Exton, PA). A Sony DXC-S500 color digital camera (Labtek; Campbell, CA) was used for the brightfield images in Supplemental Figure 2. All figures were prepared using Photoshop (version 7.0; Adobe, San Jose, CA) and Freehand (version10.0; Macromedia, San Francisco, CA).
Results
The distribution pattern of Ngb and Cygb was examined in human and canine anterior eye segments. The specificity of the antibodies for Ngb and Cygb was verified in canine and human eye tissues using immunoblot analyses in previous studies (Ostojic et al. 2006; Ostojic et al. in press). In addition, antibody specificity was verified by preadsorbing each of the antibodies with their respective recombinant Ngb and Cygb proteins before incubation on tissue sections, in which case no specific staining was observed (Ostojic et al. 2006; Ostojic et al. in press). A mouse monoclonal and rabbit polyclonal anti-Ngb Abs were used in this analysis. Both Abs displayed identical labeling patterns in all structures examined. Cytoglobin distribution in human and canine anterior eye segments was determined using a rabbit polyclonal antibody. In double-labeling studies to investigate the colocalization of Ngb and Cygb in the same cells, a mouse monoclonal anti-Ngb antibody was used in combination with the rabbit polyclonal anti-Cygb antibody.
Distribution of Ngb and Cygb in the Cornea
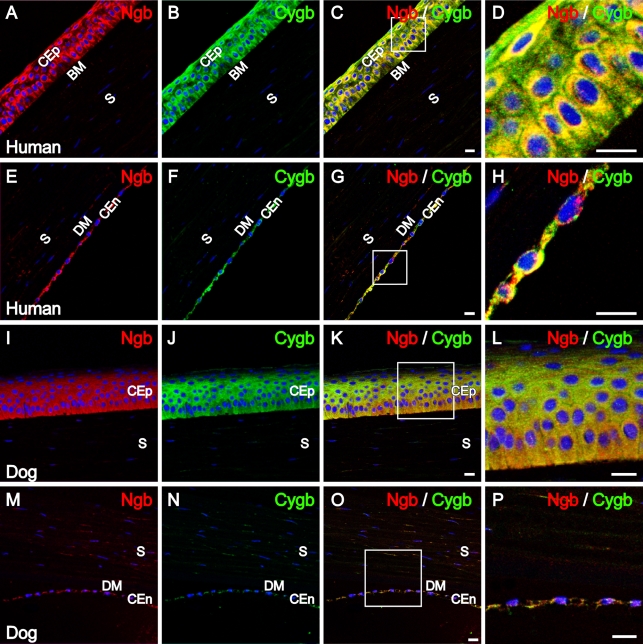
To study the distribution of Ngb and Cygb within the anterior eye segment, double labeling was performed on human and canine cornea. As shown in Figure 1, Ngb immunoreactivity (IR) and Cygb IR was detected in the corneal epithelium and corneal endothelium. Weak IR was present in corneal stromal keratocytes. No IR was detectable in human Bowman's membrane (Figures 1A–1D) and human and canine Descemet's membrane (Figures 1E–1H and 1M–1P, respectively).
Figure 1.
Neuroglobin (Ngb) and cytoglobin (Cygb) immunolocalization in the human and canine cornea. Confocal images of the human cornea double-labeled for (A) Ngb (red) and (B) Cygb (green) displayed immunoreactivity (IR) of both proteins in the corneal epithelium shown in C and D. Note the absence of IR in Bowman's membrane. (D) Enlarged region of the corneal epithelium indicated by the box from C. (E) Ngb IR (red) and (F) Cygb IR (green) in human corneal endothelial cells colocalized, as shown in G and H; note the absence of IR in Descemet's membrane. (H) Enlarged region of the corneal endothelium indicated by the box from G. (I) Confocal images of Ngb IR (red) and (J) Cygb IR (green) in the canine corneal epithelium; IR colocalized, as shown in merged images (K,L). (L) Enlarged region of the corneal epithelium indicated by the box from K. (M) Ngb IR (red) and (N) Cygb IR (green) in canine corneal endothelial cells colocalized in O and P. (P) Enlarged region of the corneal endothelium indicated by the box from O. In all images, cell nuclei (blue) were labeled with 4′,6-diamino-2-phenylindole (DAPI). CEp, corneal epithelium; BM, Bowman's membrane; S, stroma; DM, Descemet's membrane; CEn, corneal endothelium. Bars: A–C,E–G,I–K,M–O = 25 μm; D,H,L,P = 12.5 μm.
Distribution of Ngb and Cygb in the Iris
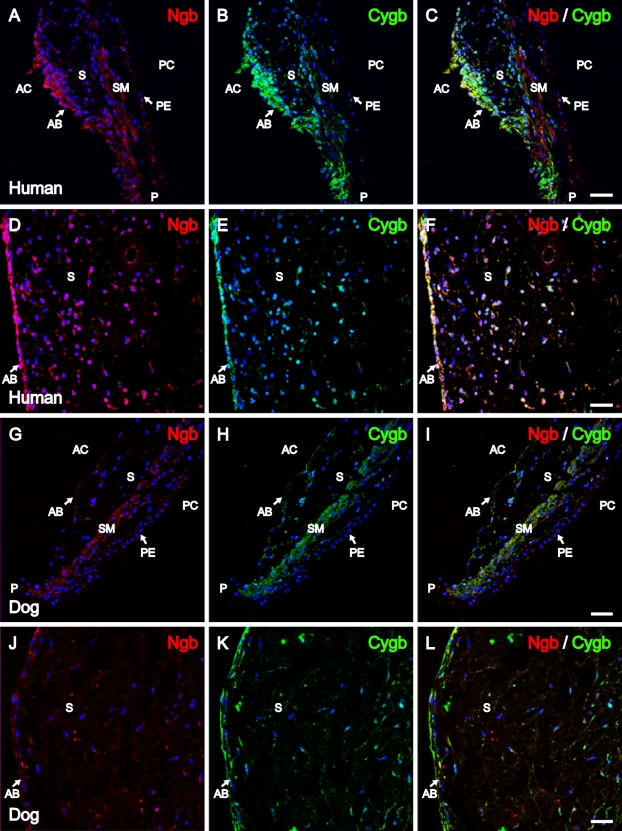
Double-labeling with mouse monoclonal anti-Ngb and rabbit polyclonal anti-Cygb was performed to examine the distribution patterns of Ngb and Cygb in human and canine iris. As shown in Figures 2A–2F, Ngb IR and Cygb IR in the human iris was colocalized to the anterior border layer, stroma, and iridal sphincter muscle. In addition, Ngb IR and Cygb IR was detected in the iridal dilator muscle (data not shown). Similar patterns of distribution for Ngb and Cygb were observed in the canine iris, as shown in Figures 2G–2L.
Figure 2.
Ngb and Cygb immunolocalization in the human and canine iris. Confocal images of the human iris showing patterns of distribution for (A) Ngb IR (red) and (B) Cygb IR (green) in cells of the anterior border layer and the stroma, as well as in the iridal sphincter. (C) Ngb and Cygb IR colocalized within the same cells. Images of the area close to the root of the iris show (D) Ngb IR (red) and (E) Cygb IR (green) in cells of the anterior border layer and stroma; proteins are colocalized, as shown in merged image (F). Confocal images of the canine iris show (G) Ngb IR (red) and (H) Cygb IR (green) in cells of the anterior border layer and the stroma, as well as in the iridal sphincter; proteins were found to colocalize, as shown in I. Images of the area close to the root of the iris showing (J) Ngb IR (red) and (K) Cygb IR (green) in cells of the anterior border layer and stroma. Ngb and Cygb colocalize within the cells, as shown in the merged image (L). In all images, the cell nuclei (blue) were labeled with DAPI. AC, anterior chamber; AB, anterior border layer; S, stroma; SM, sphincter muscle; PE, pigmented epithelium; PC, posterior chamber; P, pupillary border. Bar = 25 μm.
The anterior border layer and stroma of the iris is composed of two cell types: fibroblasts and melanocytes (Fine and Yanoff 1979; Gelatt 1999). To determine whether Ngb and Cygb are found in both cell types of the iris anterior border layer and stroma, double-labeling was performed with an anti-vimentin antibody (which labels both cell types) and light microscopic images (which show melanin pigmentation) were captured along with the confocal images of the fluorescent labeling (Supplemental Figure 1).
Distribution of Ngb and Cygb in the Iridocorneal Angle
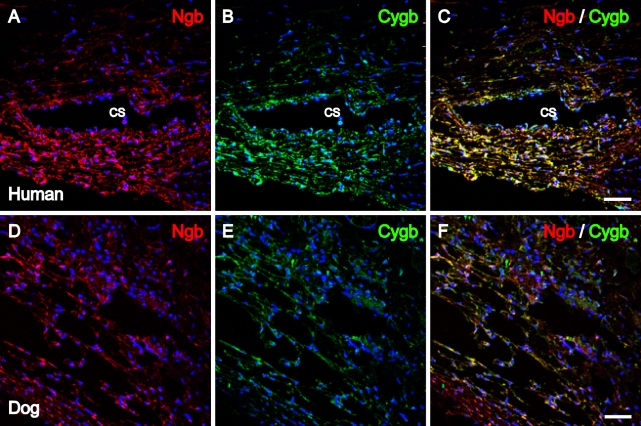
The iridocorneal angle of human and canine eyes was examined for the presence and possible colocalization of Ngb and Cygb. As shown in Figure 3, Ngb IR and Cygb IR were detected in the same cells of the trabecular meshwork in human and canine eyes. In addition, Ngb and Cygb colocalization was detected in the human iridocorneal angle in the cells lining the canal of Schlemm, as shown in the merged image of Figure 3C.
Figure 3.
Ngb and Cygb immunolocalization in the human and canine iridocorneal angle. Images were captured with confocal microscopy. Human iridocorneal angle with (A) Ngb IR (red) and (B) Cygb IR (green) in the cells of the trabecular meshwork and the cells lining the canal of Schlemm. Immunoreactivity for both proteins was found to colocalize within the same cells, as shown in C. (D) Ngb IR (red) and (E) Cygb IR (green) colocalized within the cells of the canine trabecular meshwork (F). In all images, cell nuclei (blue) were labeled with DAPI. CS, canal of Schlemm. Bar = 12.5 μm.
Distribution of Ngb and Cygb in the Ciliary Body
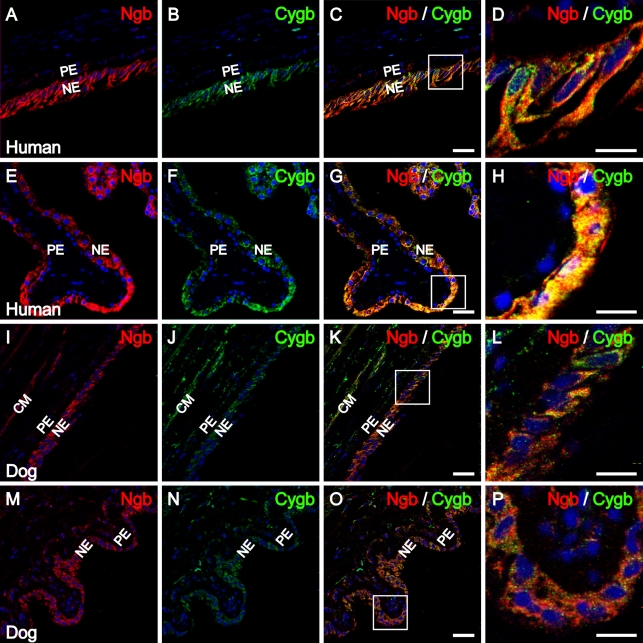
Double-labeling immunofluorescence studies were performed to investigate the distribution and possible colocalization of Ngb and Cygb within the ciliary body and lens of human and canine eyes. As shown in Figures 4A–4D, in the pars plana of the human ciliary body, Ngb and Cygb IR was colocalized to the non-pigmented ciliary epithelium. Similarly, as shown in Figures 4E and 4F, in the pars plicata of the human ciliary body, Ngb and Cygb IR were colocalized to the non-pigmented ciliary epithelium. In addition, both Ngb and Cygb IR were detected in human ciliary muscle (Supplemental Figure 2). In the canine and human ciliary body, Ngb and Cygb IR were localized in the non-pigmented ciliary epithelium of the pars plana and pars plicata and in the ciliary muscle (Figures 4I–4P). These results show extensive colocalization of Ngb and Cygb within the ciliary body.
Figure 4.
Ngb and Cygb immunolocalization in the human and canine ciliary body. Confocal images showing distribution patterns for (A) Ngb IR (red) and (B) Cygb IR (green) in non-pigmented epithelial cells of the human pars plana of the ciliary body. (C,D) Merged images show that Ngb and Cygb IR colocalize within the same cells. (D) Enlarged region of non-pigmented epithelial cells indicated by the box from C. (E) Ngb IR (red) and (F) Cygb IR (green) in non-pigmented epithelial cells of the human pars plicata of the ciliary body colocalized in merged images (G,H). (H) Enlarged region of non-pigmented epithelial cells indicated by the box from G. Confocal images showing (I) Ngb IR (red) and (J) Cygb IR (green) in non-pigmented epithelial cells of the canine pars plana of the ciliary body and ciliary muscle; IR colocalized in K and L. (L) Enlarged region of non-pigmented epithelial cells indicated by the box from K. (M) Ngb IR (red) and (N) Cygb IR (green) in non-pigmented epithelial cells of the canine pars plicata of the ciliary body colocalized in O. (P) Enlarged region of non-pigmented epithelial cells indicated by the box from O. In all images, cell nuclei (blue) were labeled with DAPI. NE, non-pigmented epithelium; PE, pigmented epithelium; CM, ciliary muscle. Bars: A–C,E–G,I–K,M–O = 25 μm; D,H,L,P = 12.5 μm.
In the lens, as shown in Supplemental Figure 3, weak Ngb and Cygb IR were colocalized to the lens epithelium.
Supplemental Table 2 summarizes the regional distribution of Ngb and Cygb in the human and canine anterior segment.
Discussion
The highly differentiated tissues of the anterior eye segment, comprised of the ciliary body, iris, iridocorneal angle, cornea, and lens, are critical for normal vision. To function properly, the anterior segment of the eye requires a sufficient supply of O2 (Hoper et al. 1989; Pfister 1991; Helbig et al. 1993; Fitch et al. 2000), mechanisms for ROS scavenging (Ishimoto et al. 1996; Rose et al. 1998; Ohia et al. 2005), and the ability to regulate levels of NO (Becquet et al. 1997; Wu et al. 1997; Bosch-Morell et al. 2002; Kim et al. 2002; Schneemann et al. 2003). A detailed comparative study of the patterns of distribution of Ngb and Cygb in the anterior segment of the human and canine eye is warranted because of the advancements in canine research models for studying human disease. The recent completion of the genome sequence in dogs (http://www.ncbi.nih.gov/Genomes/), together with long-term inbreeding that has resulted in reduced genetic diversity within breeds, represents a solid foundation for the canine as a promising model for studying human genetic diseases. Furthermore, dogs seem to be a viable large animal model for study of ocular disorders (Bedford and Grierson 1986; Cideciyan et al. 2005), increasing the possibility that using a canine model in future studies would advance our current insight into key features of human ocular physiology and disease.
The presence of Ngb and Cygb has been studied in considerable detail in the retinas of mice, rats, dogs, and humans (Schmidt et al. 2003,2005; Bentmann et al. 2005; Ostojic et al. 2006; Ostojic et al. in press), and both proteins were found in neurons but not in glial cells. In addition, Ngb has been detected in many endocrine tissues (Reuss et al. 2002; Geuens et al. 2003), and Cygb has been reported in hepatic stellate cells (Kawada et al. 2001), connective tissue fibroblasts, osteoblasts, and chondroblasts (Schmidt et al. 2004). Our study detected both Ngb and Cygb IR in the diverse tissues of the anterior eye segment. To our knowledge, this is the first study to comprehensively investigate the presence and distribution of Ngb and Cygb, two globins that have the ability to bind gaseous ligands, in the anterior segment of the eye. Previous to this report, a study by Schmidt et al. (2005) described the presence of Cygb IR in the iris. The findings reported in the iris are similar to those obtained in this study. In contrast, these investigators did not detect Cygb IR in the mouse ciliary epithelium. Possible explanations for the difference between their results and our finding of Cygb IR in the cells of the non-pigmented ciliary epithelium may be caused by species-specific differences and the different source of primary antibodies used in these studies. Although the anti-Cygb antibody used in our study was generated against whole recombinant human protein, Schmidt et al. (2005) used antibodies generated against different Cygb peptides. Furthermore, specificity of the antibodies used in this study were confirmed on recombinant proteins, canine tissue samples, and on human retinal tissue samples (Ostojic et al. 2006).
Because the exact role of Ngb and Cygb has yet to be determined, it is difficult to hypothesize on the functional significance of the distribution patterns of these proteins. From a developmental perspective, the presence of Ngb and Cygb in diverse tissues such as ciliary epithelium, ciliary muscle, iris stroma, sphincter and dilator muscles, endothelium of the trabecular meshwork, and corneal endothelium may be caused by the neuroectoderm/neural crest (Foets et al. 1992; Gelatt 1999) origin of these tissues. On the other hand, this does not explain the presence of Ngb in corneal epithelium, which originates from the surface ectoderm, nor in cells lining the canal of Schlemm, which are of mesodermal origin (Foets et al. 1992; Gelatt 1999). Even though Ngb has been detected in tissues/cells other than neurons (β cells of the islets of Langerhans in pancreas (Geuens et al. 2003), spermatogonia and spermatocytes in testes, endocrine cells of the anterior pituitary, medulla, and glomerular zone of the cortex of the adrenal gland (Reuss et al. 2002), it remains to be determined what role Ngb might have in these particular tissues.
It is possible that Ngb and Cygb function in the anterior segment of the eye is related to free radical/NO scavenging. The data from kinetic studies support the idea that Ngb is involved in scavenging of reactive oxygen and nitrogen species (Fago et al. 2004; Brunori et al. 2005). Also, Cygb has been shown to have peroxidase activity in stellate cells of the liver (Kawada et al. 2001). Based on these previous studies and these findings, we hypothesize that a similar scavenging role is maintained in the anterior eye segment. Indeed, anterior eye segment structures have been found to have diverse NOS activity under both physiological and pathological conditions (Becquet et al. 1997). It has also been reported that NO acts as a vasodilator to regulate aqueous production in the ciliary body (Nathanson and McKee 1995; Haufschild et al. 1996) and can influence aqueous outflow in the trabecular meshwork and ciliary muscle (Nathanson and McKee 1995; Geyer et al. 1997; Schneemann et al. 2003). In addition, NOS activity was detected in corneal epithelium, stromal fibroblasts, and endothelium during experimental inflammation (Becquet et al. 1997; Kim et al. 2002) and in the iris because of endotoxin-induced uveitis (Becquet et al. 1997). Furthermore, hydrogen peroxide (H2O2) is present in tissues of the anterior uvea and in mammalian aqueous humor at concentrations between 30 and 70 μM (Rose et al. 1998). To protect against oxidative stress induced by H2O2, different mechanisms are used. Catalase, the enzyme that metabolizes H2O2 to water and oxygen, has been found in the cornea, aqueous humor, trabecular meshwork, and ciliary body (Rose et al. 1998). It is possible that, in addition to other mechanisms, Ngb and/or Cygb may serve in protecting the eye from damage induced by reactive oxygen species and excess NO.
In summary, our double-immunolabeling study describes the presence and colocalization of two gaseous ligand-binding molecules, Ngb and Cygb in the anterior segment of human and canine eyes. These results provide a foundation for future studies designed to investigate human ocular regulation of oxidative metabolism.
Supplementary Material
Acknowledgments
This work was supported in part by NINDS 44007; the Glaucoma Foundation, New York; the Carver Trust; and Research to Prevent Blindness.
We thank Jeffrey Orasky and Matt Harper for technical assistance during preparation of this manuscript and Daria Pospisilova, Petr Kasparek, and Jiri Havlasek from BioVendor Laboratory Medicine for providing us with mouse monoclonal anti-Ngb Ab.
References
- Awenius C, Hankeln T, Burmester T (2001) Neuroglobins from the zebrafish Danio rerio and the pufferfish Tetraodon nigroviridis. Biochem Biophys Res Commun 287:418–421 [DOI] [PubMed] [Google Scholar]
- Becquet F, Courtois Y, Goureau O (1997) Nitric oxide in the eye: multifaceted roles and diverse outcomes. Surv Ophthalmol 42:71–82 [DOI] [PubMed] [Google Scholar]
- Bedford PG, Grierson I (1986) Aqueous drainage in the dog. Res Vet Sci 41:172–186 [PubMed] [Google Scholar]
- Bentmann A, Schmidt M, Reuss S, Wolfrum U, Hankeln T, Burmester T (2005) Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J Biol Chem 280:20660–20665 [DOI] [PubMed] [Google Scholar]
- Bosch-Morell F, Romá J, Marín N, Romero B, Rodriguez-Galietero A, Johnsen-Soriano S, Díaz-Llopis M, et al. (2002) Role of oxygen and nitrogen species in experimental uveitis: anti-inflammatory activity of the synthetic antioxidant ebselen. Free Radic Biol Med 33:669–675 [DOI] [PubMed] [Google Scholar]
- Brunori M, Giuffrè A, Nienhaus K, Nienhaus GU, Scandurra FM, Vallone B (2005) Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc Natl Acad Sci USA 102:8483–8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester T, Ebner B, Weich B, Hankeln T (2002) Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol 19:416–421 [DOI] [PubMed] [Google Scholar]
- Burmester T, Hankeln T (2004) Neuroglobin: a respiratory protein of the nervous system. News Physiol Sci 19:110–113 [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T (2000) A vertebrate globin expressed in the brain. Nature 407:520–523 [DOI] [PubMed] [Google Scholar]
- Cejková J, Stípek S, Crkovská J, Ardan T, Pláteník J, Cejka C, Midelfart A (2004) UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res 53:1–10. [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Aleman TS, Gu D, Pearce-Kelling SE, Sumaroka A, Acland GM, et al. (2005) In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc Natl Acad Sci USA 102:5233–5238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture M, Burmester T, Hankeln T, Rousseau DL (2001) The heme environment of mouse neuroglobin. Evidence for the presence of two conformations of the heme pocket. J Biol Chem 276:36377–36382 [DOI] [PubMed] [Google Scholar]
- Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden MC, et al. (2001) Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem 276:38949–38955 [DOI] [PubMed] [Google Scholar]
- Fago A, Hundahl C, Malte H, Weber RE (2004) Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins. IUBMB Life 56:689–696 [DOI] [PubMed] [Google Scholar]
- Fine BS, Yanoff M (1979) Ocular Histology. Hagerstown, MD, Harper & Row
- Fitch CL, Swedberg SH, Livesey JC (2000) Measurement and manipulation of the partial pressure of oxygen in the rat anterior chamber. Curr Eye Res 20:121–126 [PubMed] [Google Scholar]
- Foets B, van den Oord J, Engelmann K, Missotten L (1992) A comparative immunohistochemical study of human corneotrabecular tissue. Graefes Arch Clin Exp Ophthalmol 230:269–274 [DOI] [PubMed] [Google Scholar]
- Fuchs C, Heib V, Kiger L, Haberkamp M, Roesner A, Schmidt M, Hamdane D, et al. (2004) Zebrafish reveals different and conserved features of vertebrate neuroglobin gene structure, expression pattern, and ligand binding. J Biol Chem 279:24116–24122 [DOI] [PubMed] [Google Scholar]
- Gelatt KN (1999) Veterinary Ophthalmology. Philadelphia, Lippincott Williams & Wilkins
- Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP, Moens L (2003) A globin in the nucleus! J Biol Chem 278:30417–30420 [DOI] [PubMed] [Google Scholar]
- Geyer O, Podos SM, Mittag T (1997) Nitric oxide synthase activity in tissues of the bovine eye. Graefes Arch Clin Exp Ophthalmol 235:786–793 [DOI] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW (2004) Anterior segment development relevant to glaucoma. Int J Dev Biol 48:1015–1029 [DOI] [PubMed] [Google Scholar]
- Hardison RC (1996) A brief history of hemoglobins: plant, animal, protist, and bacteria. Proc Natl Acad Sci USA 93:5675–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufschild T, Nava E, Meyer P, Flammer J, Lüscher TF, Haefliger IO (1996) Spontaneous calcium-independent nitric oxide synthase activity in porcine ciliary processes. Biochem Biophys Res Commun 222:786–789 [DOI] [PubMed] [Google Scholar]
- Helbig H, Hinz JP, Kellner U, Foerster MH (1993) Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol 2:161–164 [PubMed] [Google Scholar]
- Herold S, Fago A, Weber RE, Dewilde S, Moens L (2004) Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem 279:22841–22847 [DOI] [PubMed] [Google Scholar]
- Hoper J, Funk R, Zagorski Z, Rohen JW (1989) Oxygen delivery to the anterior chamber of the eye–a novel function of the anterior iris surface. Curr Eye Res 8:649–659 [DOI] [PubMed] [Google Scholar]
- Ishimoto S, Wu GS, Hayashi S, Zhang J, Rao NA (1996) Free radical tissue damages in the anterior segment of the eye in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 37:630–636 [PubMed] [Google Scholar]
- Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K (2001) Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem 276:25318–25323 [DOI] [PubMed] [Google Scholar]
- Kim JC, Cheong TB, Park GS, Park MH, Kwon NS, Yoon HY (2002) The role of nitric oxide in ocular surface diseases. Adv Exp Med Biol 506:687–695 [DOI] [PubMed] [Google Scholar]
- Kugelstadt D, Haberkamp M, Hankeln T, Burmester T (2004) Neuroglobin, cytoglobin, and a novel, eye-specific globin from chicken. Biochem Biophys Res Commun 325:719–725 [DOI] [PubMed] [Google Scholar]
- Mammen PP, Shelton JM, Goetsch SC, Williams SC, Richardson JA, Garry MG, Garry DJ (2002) Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J Histochem Cytochem 50:1591–1598 [DOI] [PubMed] [Google Scholar]
- McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S (2004) Regulation of tissue oxygen levels in the mammalian lens. J Physiol 559:883–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JA, McKee M (1995) Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci 36:1765–1773 [PubMed] [Google Scholar]
- Nienhaus K, Kriegl JM, Nienhaus GU (2004) Structural dynamics in the active site of murine neuroglobin and its effects on ligand binding. J Biol Chem 279:22944–22952 [DOI] [PubMed] [Google Scholar]
- Ohia SE, Opere CA, Leday AM (2005) Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res 579:22–36 [DOI] [PubMed] [Google Scholar]
- Ostojic J, Grozdanic SD, Syed NA, Hargrove MS, Trent JT III, Kuehn MH, Kwon YH, et al. (In Press) Patterns of distribution of oxygen-binding globins, neuroglobin and cytoglobin, in human retina. Arch Ophthalmol [DOI] [PubMed]
- Ostojic J, Sakaguchi DS, de Lathouder Y, Hargrove MS, Trent JT III, Kwon YH, Kardon RH, et al. (2006) Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest Ophthalmol Vis Sci 47:1016–1023 [DOI] [PubMed] [Google Scholar]
- Parker NR, Jamie JF, Davies MJ, Truscott RJ (2004) Protein-bound kynurenine is a photosensitizer of oxidative damage. Free Radic Biol Med 37:1479–1489 [DOI] [PubMed] [Google Scholar]
- Pesce A, Bolognesi M, Bocedi A, Ascenzi P, Dewilde S, Moens L, Hankeln T, et al. (2002) Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin family. EMBO Rep 3:1146–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister RR (1991) The intraocular changes of anterior segment necrosis. Eye 5:214–221 [DOI] [PubMed] [Google Scholar]
- Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T (2002) Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience 115:645–656 [DOI] [PubMed] [Google Scholar]
- Rose RC, Richer SP, Bode AM (1998) Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med 217:397–407 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Gerlach F, Avivi A, Laufs T, Wystub S, Simpson JC, Nevo E, et al. (2004) Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. J Biol Chem 279:8063–8069 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T (2003) How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem 278:1932–1935 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Laufs T, Reuss S, Hankeln T, Burmester T (2005) Divergent distribution of cytoglobin and neuroglobin in the murine eye. Neurosci Lett 374:207–211 [DOI] [PubMed] [Google Scholar]
- Schneemann A, Leusink-Muis A, van den Berg T, Hoyng PF, Kamphuis W (2003) Elevation of nitric oxide production in human trabecular meshwork by increased pressure. Graefes Arch Clin Exp Ophthalmol 241:321–326 [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA (2001) Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci USA 98:15306–15311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA (2003) Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA 100:3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent JT III, Hargrove MS (2002) A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem 277:19538–19545 [DOI] [PubMed] [Google Scholar]
- Trent JT III, Watts RA, Hargrove MS (2001) Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J Biol Chem 276:30106–30110 [DOI] [PubMed] [Google Scholar]
- Vallone B, Nienhaus K, Brunori M, Nienhaus GU (2004) The structure of murine neuroglobin: Novel pathways for ligand migration and binding. Proteins 56:85–92 [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Nakano T, Morishima I (2003) Oxidized human neuroglobin acts as a heterotrimeric Galpha protein guanine nucleotide dissociation inhibitor. J Biol Chem 278:36505–36512 [DOI] [PubMed] [Google Scholar]
- Wu GS, Zhang J, Rao NA (1997) Peroxynitrite and oxidative damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 38:1333–1339 [PubMed] [Google Scholar]
- Wystub S, Ebner B, Fuchs C, Weich B, Burmester T, Hankeln T (2004) Interspecies comparison of neuroglobin, cytoglobin and myoglobin: sequence evolution and candidate regulatory elements. Cytogenet Genome Res 105:65–78 [DOI] [PubMed] [Google Scholar]
- Wystub S, Laufs T, Schmidt M, Burmester T, Maas U, Saaler-Reinhardt S, Hankeln T, et al. (2003) Localization of neuroglobin protein in the mouse brain. Neurosci Lett 346:114–116 [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang C, Deng M, Li L, Wang H, Fan M, Xu W, et al. (2002) Full-length cDNA cloning of human neuroglobin and tissue expression of rat neuroglobin. Biochem Biophys Res Commun 290:1411–1419 [DOI] [PubMed] [Google Scholar]
- Zhou L, Li Y, Yue BY (1999) Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol 180:182–189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.