Abstract
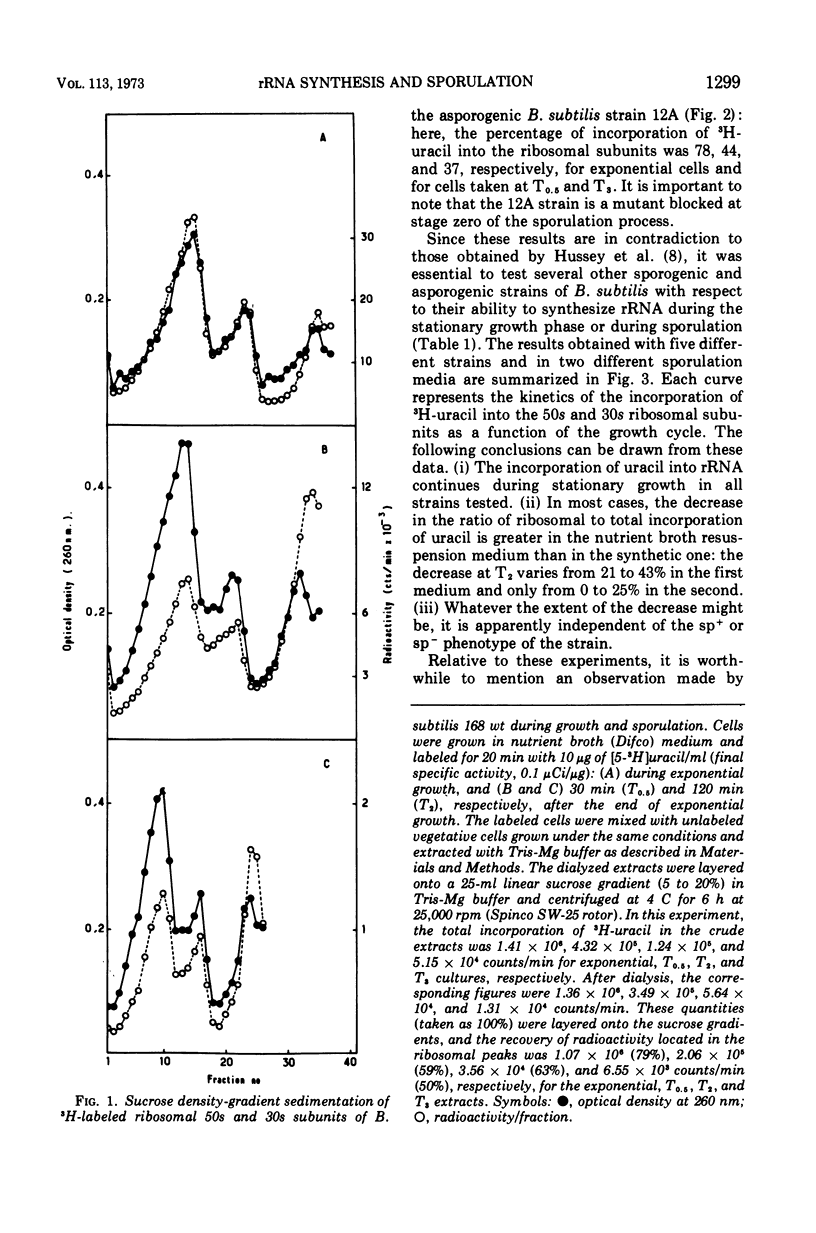
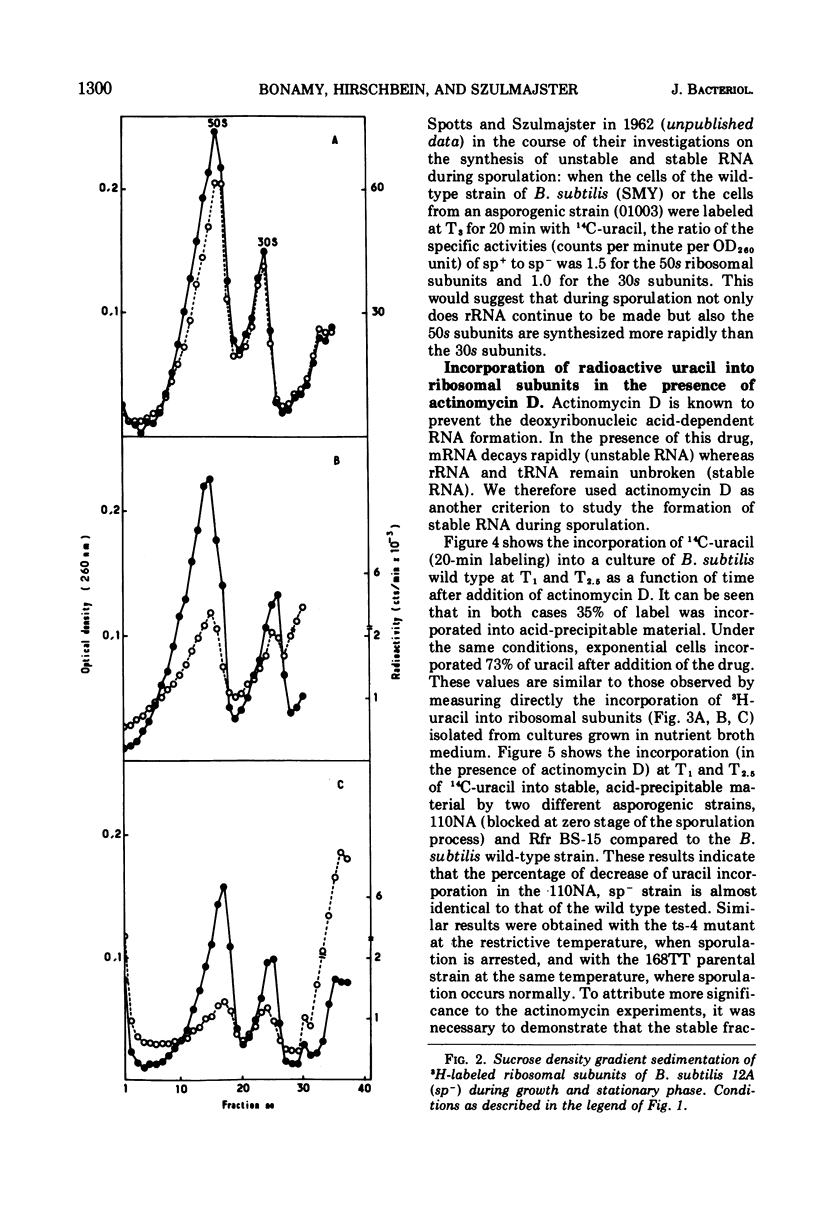
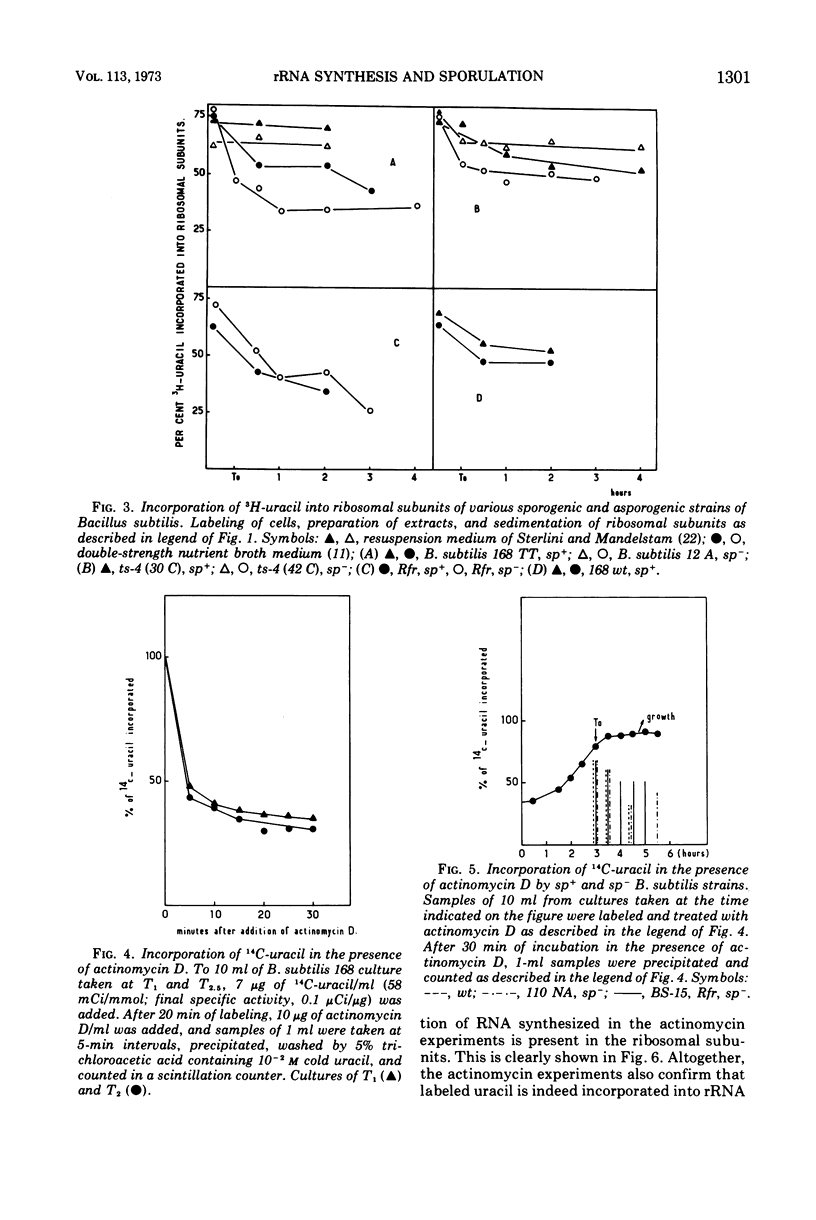
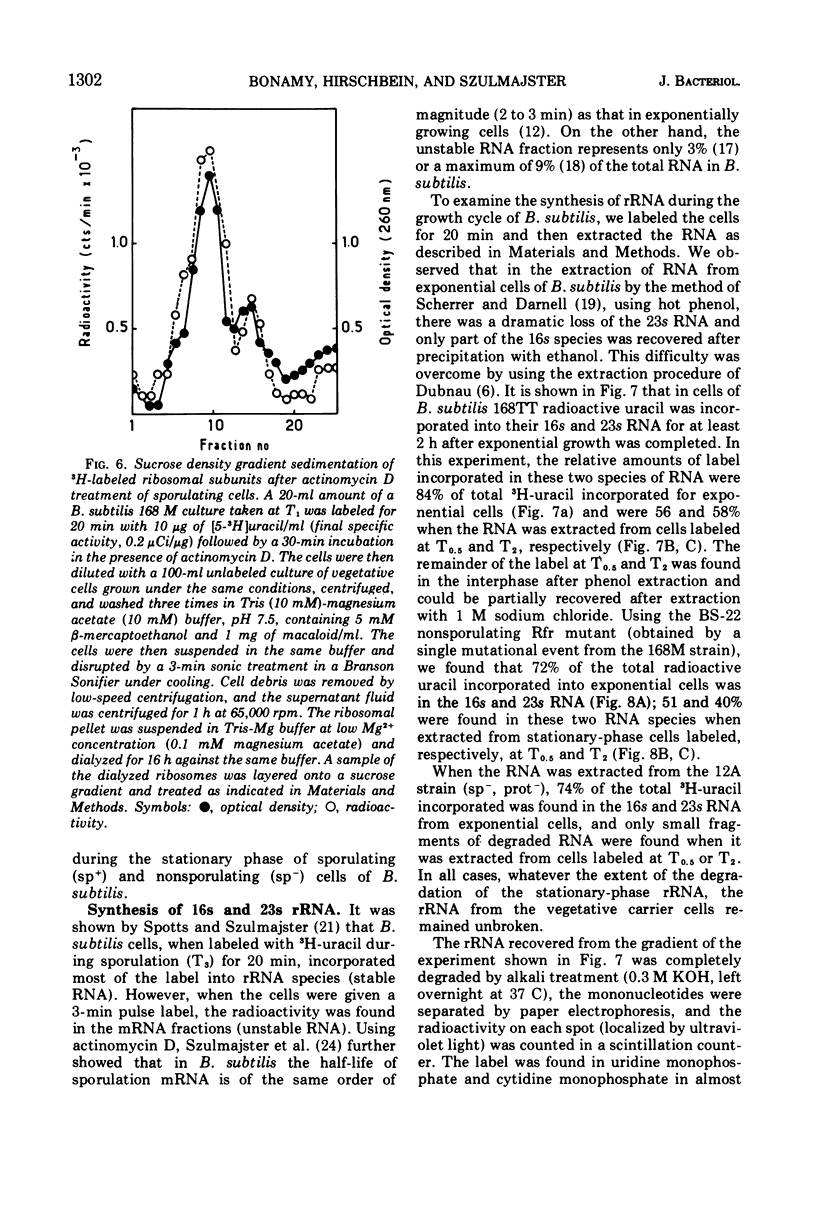
The incorporation of radioactive uracil into 50s and 30s ribosomal subunits and ribosomal ribonucleic acid (rRNA) was studied during the growth cycle of different sporogenic and asporogenic strains of Bacillus subtilis. It was found that partially synchronized cultures of the strains examined incorporated labeled uracil into the two ribosomal subunit species and rRNA during sporulation and during the stationary phase of the asporogenic strains. Kinetic studies have shown that, compared to vegetative cells, the percentage of uracil incorporated into the ribosomal subunits of cells taken 30 min after the end of exponential growth was decreased by about 25 to 35%. This decrease, however, appeared to be a general characteristic of stationary-phase cells and seems to depend on the nature of the sporulation medium and to some extent on the nature of the strain but not on the sp+ or sp− phenotype of the strain. Moreover, by use of actinomycin D it was shown that the labeled uracil incorporated, in the presence of the drug, during the sporulation period was located in the ribosomal subunits (stable RNA). Based on these results, we concluded that during sporulation ribosomal genes are transcribed and consequently rRNA continues to be synthesized, although to a lesser extent than during vegetative growth. These results are discussed in the light of those obtained by Hussey et al.
Full text
PDF


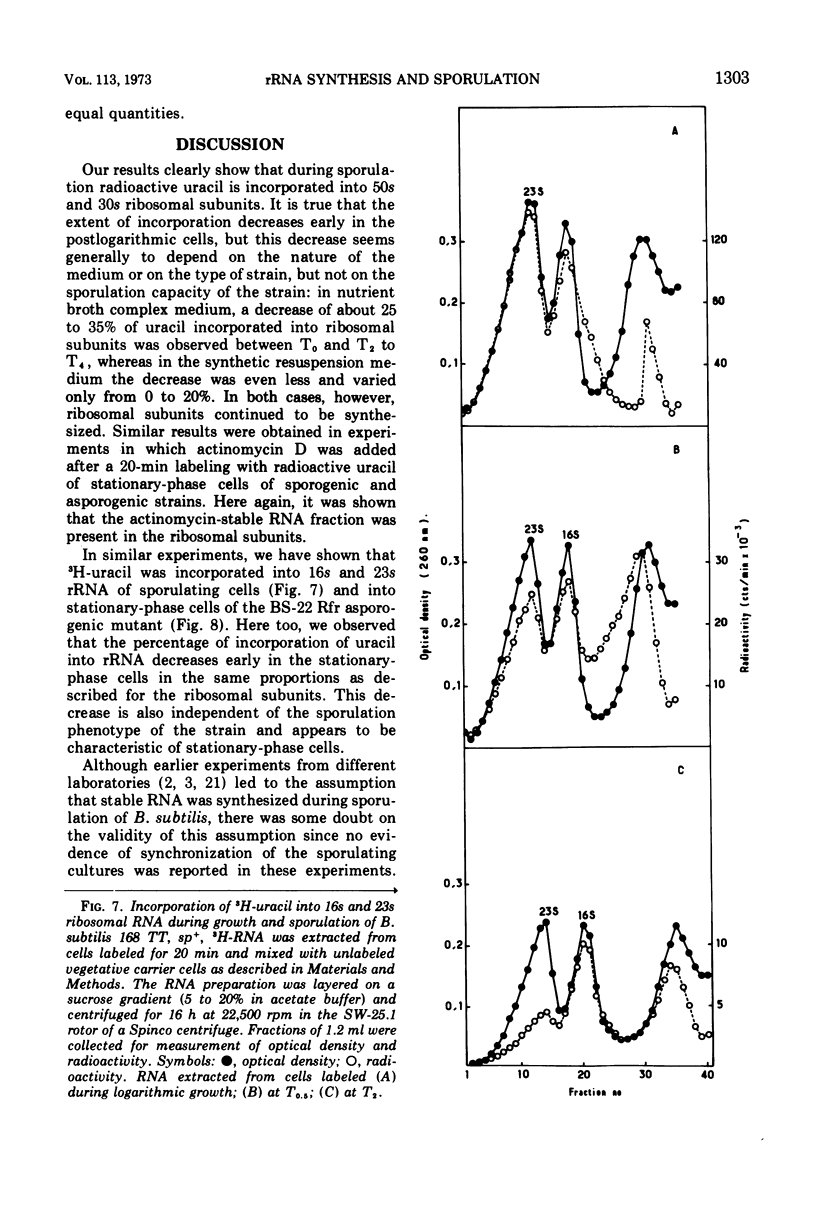


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALASSA G. RENOUVELLEMENT DE L'ACIDE RIBONUCL'EIQUE AU COURS DE LA SPORULATION DE BACILLUS SUBTILIS. Biochim Biophys Acta. 1963 Nov 22;76:410–416. doi: 10.1016/0006-3002(63)90060-x. [DOI] [PubMed] [Google Scholar]
- Balassa G. Synthèse et fonction des ARN messagers au cours de la sporulation de Bacillus subtilis. Ann Inst Pasteur (Paris) 1966 Feb;110(2):175–191. [PubMed] [Google Scholar]
- Cocucci S. M., Sussman M. RNA in cytoplasmic and nuclear fractions of cellular slime mold amebas. J Cell Biol. 1970 May;45(2):399–407. doi: 10.1083/jcb.45.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. Degradation of ribonucleic acid in Escherichia coli in phosphorus-deficient culture. Biochim Biophys Acta. 1959 Feb;31(2):570–572. doi: 10.1016/0006-3002(59)90044-7. [DOI] [PubMed] [Google Scholar]
- Hussey C., Losick R., Sonenshein A. L. Ribosomal RNA synthesis is turned off during sporulation of Bacillus subtilis. J Mol Biol. 1971 Apr 14;57(1):59–70. doi: 10.1016/0022-2836(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Hussey C., Pero J., Shorenstein R. G., Losick R. In vitro synthesis of ribosomal RNA by Bacillus subtilis RNA polymerase. Proc Natl Acad Sci U S A. 1972 Feb;69(2):407–411. doi: 10.1073/pnas.69.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Nakata K., Winslow R. M. Coordinate control of ribonucleic acid synthesis during uracil deprivation. J Biol Chem. 1969 Jun 10;244(11):3092–3100. [PubMed] [Google Scholar]
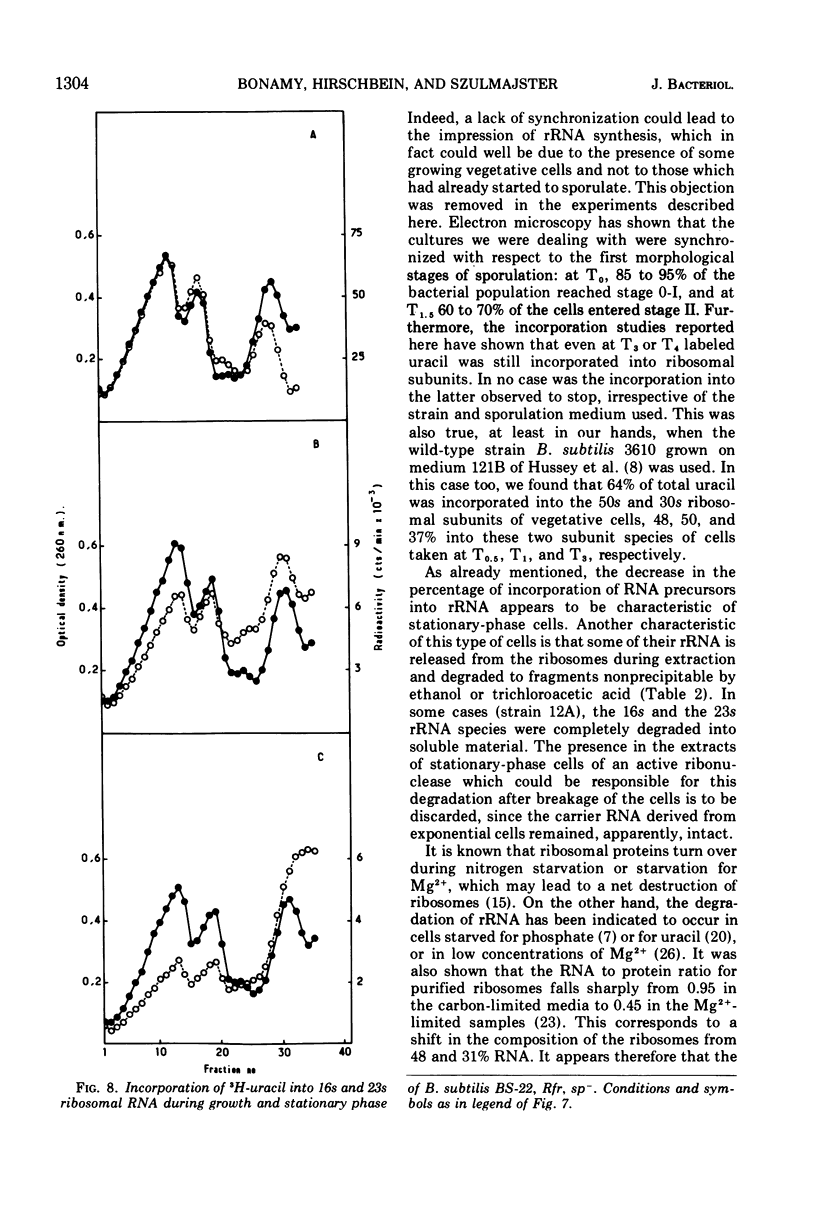
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Michel J. F., Cami B. Sélection de mutants de Bacillus subtilis bloqués au début de la sporulation. Nature des mutations sélectionnées. Ann Inst Pasteur (Paris) 1969 Jan;116(1):3–18. [PubMed] [Google Scholar]
- Midgley J. E. The messenger ribonucleic acid content of Bacillus subtilis 168. Biochem J. 1969 Nov;115(2):171–181. doi: 10.1042/bj1150171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- SYKES J., TEMPEST D. W. THE EFFECT OF MAGNESIUM AND OF CARBON LIMITATION ON THE MACROMOLECULAR ORGANISATION AND METABOLIC ACTIVITY OF PSEUDOMONAS SP., STRAIN C-IB. Biochim Biophys Acta. 1965 May 11;103:93–108. doi: 10.1016/0005-2787(65)90543-5. [DOI] [PubMed] [Google Scholar]
- SZULMAJSTER J., CANFIELD R. E., BLICHARSKA J. [Action of actinomycin D on the sporulation of Bacillus subtilis]. C R Hebd Seances Acad Sci. 1963 Feb 25;256:2057–2060. [PubMed] [Google Scholar]
- Salser W., Janin J., Levinthal C. Measurement of the unstable RNA in exponentially growing cultures of Bacillus subtilis and Escherichia coli. J Mol Biol. 1968 Jan 28;31(2):237–266. doi: 10.1016/0022-2836(68)90442-7. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade H. E., Robinson H. K. The distribution of ribosomal ribonucleic acids among subcellular fractions from bacteria and the adverse effect of the membrane fraction on the stability of ribosomes. Biochem J. 1965 Sep;96(3):753–765. doi: 10.1042/bj0960753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk-Salkinoja M. S., Planta R. J. Formation and life cycle of ribosomal subunits, mono- and polyribosomes in Bacillus licheniformis. Arch Biochem Biophys. 1970 Dec;141(2):477–488. doi: 10.1016/0003-9861(70)90165-7. [DOI] [PubMed] [Google Scholar]


