Abstract
It has been proposed that palmitoylation of the N-terminal segment of surfactant protein SP-C is important for maintaining association of pulmonary surfactant complexes with interfacial films compressed to high pressures at the end of expiration. In this study, we examined surfactant membrane models containing palmitoylated and nonpalmitoylated synthetic peptides, based on the N-terminal SP-C sequence, in dipalmitoylphosphatidylcholine (DPPC)/egg phosphatidylglycerol (7:3, w/w) by 2H-NMR. Perturbations of lipid properties by the peptide versions were compared in samples containing chain- and headgroup-deuterated lipid (DPPC-d62 and DPPC-d4 respectively). Also, deuterated peptide palmitate chains were compared with those of DPPC in otherwise identical lipid-protein mixtures. Palmitoylated peptide increased average DPPC-d62 chain orientational order slightly, particularly for temperatures spanning gel and liquid crystalline coexistence, implying penetration of palmitoylated peptide into ordered membrane. In contrast, the nonpalmitoylated peptide had a small disordering effect in this temperature range. Both peptide versions perturbed DPPC-d4 headgroup orientation similarly, suggesting little effect of palmitoylation on the largely electrostatic peptide-headgroup interaction. Deuterated acyl chains attached to the SP-C N-terminal segment displayed a qualitatively different distribution of chain order, and lower average order, than DPPC-d62 in the same membranes. This likely reflects local perturbation of lipid headgroup spacing by the peptide portion interacting with the bilayer near the peptide palmitate chains. This study suggests that SP-C-attached acyl chains could be important for coupling of lipid and protein motions in surfactant bilayers and monolayers, especially in the context of ordered phospholipid structures such as those potentially formed during exhalation, when stabilization of the respiratory surface by surfactant is the most crucial.
INTRODUCTION
Pulmonary surfactant is a lipid/protein complex essential to facilitate the work of breathing. It is synthesized by the respiratory epithelium and secreted into the thin water layer covering the alveolar surface, where surfactant reduces surface tension and so prevents alveolar collapse at the end of expiration (1). Lack of an operative surfactant is associated with severe respiratory pathologies (2,3). On the other hand, several components of surfactant play important defense functions at the respiratory surface, contributing to maintaining a low pathogenic load in the large surface the lungs expose to environment (4). The different components of pulmonary surfactant contribute in different ways to the properties of the surfactant film. Surfactant contains ∼80%, by weight, of phospholipids, including dipalmitoylphosphatidylcholine (DPPC), which accounts for ∼40% of the total mass. Negatively-charged phospholipids, usually phosphatidylglycerol (PG) and minor amounts of phosphatidylinositol (PI), account for up to 10%–15% of surfactant in most animal species (5,6). Hydrophilic surfactant proteins SP-A and SP-D are important for host defense (7–9) and have less impact than hydrophobic surfactant proteins SP-B and SP-C on the surface active properties of surfactant bilayers and monolayers.
The presence of SP-B and SP-C is critical for optimizing the ability of surfactant to produce dramatic reductions of surface tension (10–12). In particular, SP-C has been shown to promote transfer of phospholipids from surfactant bilayers into the air-liquid interface (13–15). Also, SP-C has been shown to promote compression-driven formation and stabilization of multilayered surfactant reservoirs associated with the interfacial films (16,17). These surface-associated surfactant structures would favor rapid replenishment of the interface with surface active molecules during expansion, at inspiration, whereas, in addition, providing stability to the film in the highest compressed states. The ability of SP-C to promote stabilization of compressed surfactant phases would explain the fact that surfactant from SP-C knocked-out mice forms interfacial films with a reduced stability (18).
SP-C is a highly hydrophobic lipopeptide containing 35 amino acids, which adopt a mainly α-helical secondary structure (19,20). A very regular α-helix formed by residues 9–34 adopts a transmembrane orientation in phospholipid bilayers, which perfectly matches the thickness of a fluid DPPC membrane (21,22). The NMR structure of SP-C as determined in organic solvents shows that the N-terminal segment of SP-C, comprising residues 1–9, shows no defined conformation (22,23). However, studies with synthetic peptides designed to mimic this segment suggested that the N-terminal segment could adopt a β-turn structure (24). Native SP-C is modified posttranslationally in most species by two palmitic chains, covalently bound to Cys-5 and Cys-6 residues. In some animals, SP-C contains a single cysteine, which is also stoichiometrically palmitoylated. Covalently-attached palmitic chains are present in a broad group of proteins, many of which are membrane-anchored and involved in signaling events (25–27). In the case of SP-C, palmitoylation is thought to stabilize the α-helical structure (21,28) and also to be important to sustain association of surfactant reservoirs with the interfacial film (29–31). The lack of covalently bound palmitoyl groups reduces the compressibility and the surface tension lowering properties of dynamically cycled films, the mechanical stability of SP-C-containing surface structures, and the re-spreading on expansion of phospholipids from the reservoir back into the air-liquid interface (30,32–34). This clearly indicates that palmitoylation is required for optimal surface activity of SP-C.
Numerous studies have approached the characterization of lipid-protein interactions of SP-C, in an attempt to understand the molecular mechanisms by which SP-C modulates surface activity in surfactant. Analyses of the ways in which SP-C affects thermotropic properties, along with order and packing of phospholipids in membranes, by differential scanning calorimetry (35,36), infrared spectroscopy (21,37) or electron spin resonance (38), has shown that the behavior of SP-C is dominated by the interactions of the hydrophobic transmembrane helix. Deuterium-NMR has been also used extensively to examine the nature and extent of the perturbations introduced by SP-C in surfactant bilayers. Incorporation of SP-C into membranes containing acyl chain-deuterated or headgroup-deuterated phospholipids showed that the protein had no noticeable effects on the acyl chain orientational order of the bilayers in the liquid-crystalline phase (36,39,40), although it considerably broadened the thermotropic gel-to-liquid crystalline phase transition in zwitterionic (36) and anionic (39) membranes. SP-C was found to affect the first spectral moment more strongly for DPPG-d62 than for DPPC-d62, which was attributed to SP-C having some preferential interaction with negatively charged lipids. Deuterium-NMR also allowed a detailed examination of the effect of SP-C on conformation of the headgroup region in phospholipid membranes (40).
Recent attention has been paid to the structure and lipid-protein dynamics of the palmitoylated N-terminal segment of SP-C, the only region of the protein likely protruding out of bilayers and monolayers and therefore prone to support and modulate membrane-membrane or membrane-interface interactions. The study of synthetic peptides mimicking the sequence of the N-terminal segment of SP-C has shown that this motif has an intrinsic propensity to interact with bilayers (41) and monolayers (42), even in the absence of cysteine palmitoylation. However, the contribution of the N-terminal sequence to the membrane-perturbing properties of SP-C and the effects of palmitoylation on the ability of this region of the protein to modify membrane behavior has not been studied widely. In an attempt to understand how the N-terminal segment of SP-C interacts with and perturbs phospholipid membranes, two peptides with sequences corresponding to the 13-residue N-terminal segment of the protein have been synthesized, either with free or palmitoylated cysteines, and reconstituted into bilayers of acyl chain-deuterated (DPPC-d62) or choline-labeled (DPPC-d4) phospholipids. The perturbations induced by palmitoylated and nonpalmitoylated peptides on the bilayers have been inferred from comparison, at various temperatures, of 2H-NMR spectra from deuterated lipids in peptide-free membranes and membranes containing the peptides. The motional state of the palmitate chains on the palmitoylated peptide has also been investigated by comparing the spectra of DPPC-d62 with that of deuterated peptide palmitate chains into otherwise identical mixtures of DPPC/egg-PG plus palmitoylated SP-C N-terminal segment peptide.
MATERIALS AND METHODS
Materials
Chloroform (Chl) and methanol (MeOH) were HPLC-grade solvents from Scharlau (Barcelona, Spain). The chain-perdeuterated phospholipids DPPC-d62, DPPC deuterated at the α and β positions of the choline headgroup (DPPC-d4) and egg-PG were obtained from Avanti Polar Lipids (Alabaster, AL) and were used without further purification. The average molecular weight calculated for egg-PG was 771. The labels α and β of DPPC-d4 refer to the choline methylene groups nearest and next-nearest, respectively, to the phosphate group.
Peptide synthesis and purification
Three 13-residue peptides were synthesized by Fmoc (fluoren-9-ylmethoxycarbonyl) chemistry, based on the sequence of porcine SP-C. Peptide pSP-C(free) (NH2-LRIPCCPVNLKRL-CONH2) has the two cysteines of the native sequence bearing free thiol groups, whereas the peptide pSP-C(palm)2 (NH2-LRIPCPalmCPalmPVNLKRL-CONH2) has the two cysteines palmitoylated via thioester bonds. This peptide was prepared by treatment of pSP-C(free) with palmitoyl chloride in trifluoroacetic acid for 10 min (69), followed by HPLC purification. The molecular weights of the nonpalmitoylated and palmitoylated peptides were 1525 and 2002 respectively, and both were assumed to carry three positive charges under the conditions of these experiments. A version of pSP-C(Palm)2 with fully deuterated (C15D31) fatty acid chains was also produced. All peptides were purified to >95% by HPLC, and their purity was checked by matrix-assisted laser desorption-ionization time-of flight (MALDI-TOF) MS analysis.
Sample preparation and analysis
Samples of lipid and peptide were mixed in chloroform/methanol 2:1 (v/v). The solvent was removed by rotary evaporation followed by evacuation overnight. Dried peptide-lipid mixtures containing 15 mg of DPPC-d62/PG 7:3 (w/w) or DPPC-d4/PG 7:3 (w/w) in the absence or in the presence of 10% (w/w) of palmitoylated or nonpalmitoylated peptides were dispersed in 10 mL of buffer (Tris, 5 mM; NaCl, 150 mM; pH 7.0) at 50°C for 1 h to form multilamellar suspensions. The samples were then centrifuged at 50,000 × g for 30 min at 4°C and the pellet was resuspended in 300 μL of supernatant from the centrifugation and transferred into an NMR tube of 8 mm. Lipid concentrations were determined by phosphorus analysis (44). After hydration, each sample was warmed to 46°C and allowed to equilibrate for ∼15 min before starting data collection. Temperatures were lowered in 2°C steps except near the main liquid-crystal to gel transition for which 1°C steps were used. After each temperature change, the sample was allowed to equilibrate for at least 15 min before the beginning of data collection. 2H-NMR spectra were collected in a 9.4 T superconducting solenoid operating at 61.4 MHz using the quadrupole echo sequence with π/2 pulses of 4.3–5.5 μs separated by 50 μs. For each spectrum of a sample containing DPPC-d62/PG 7:3 (w/w), 8,000 transients were averaged. For samples containing DPPC-d4/PG, 20,000 transients were averaged. For the sample containing peptide having deuterated palmitic chains attached at the cysteines, 40,000 transients were collected. To facilitate calculation of first spectral moments, spectra were symmetrized by zeroing residual noise in the imaginary channel. The narrow peak obtained in the center of most spectra likely arises from natural abundance deuterium in the water or from small particles generated during the preparation of samples. This was confirmed by rehydrating selected samples in deuterium-depleted water and rescanning after this series of experiments was completed.
RESULTS
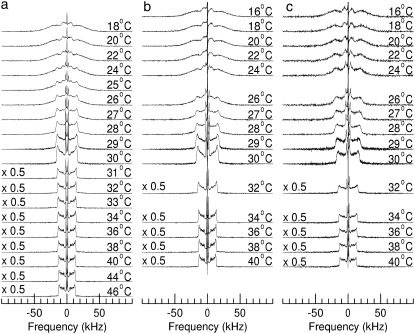
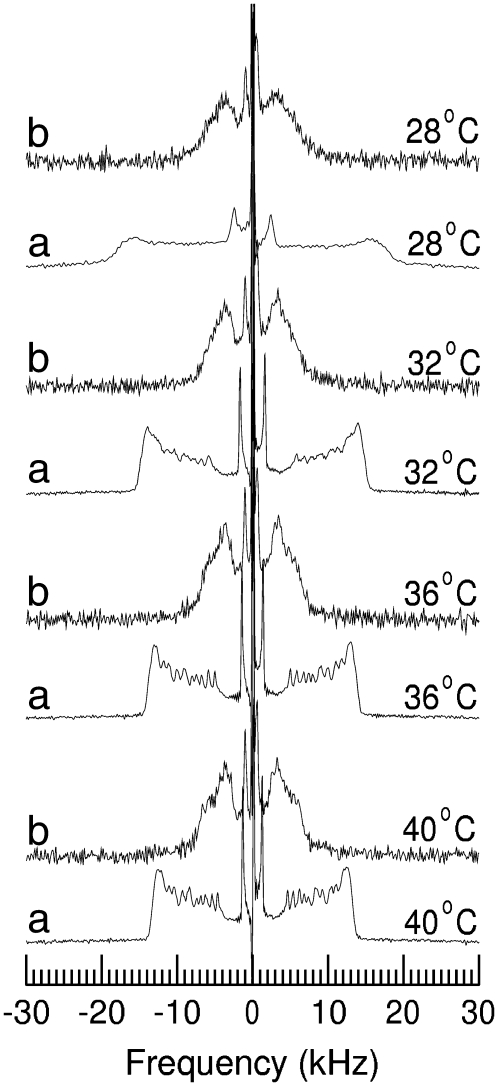
Fig. 1 shows 2H-NMR spectra at selected temperatures obtained from bilayers composed of DPPC-d62/egg PG (7:3, w/w) without peptide (Fig. 1 a), DPPC-d62/egg PG (7:3, w/w) plus 10% by weight of palmitoylated N-terminal SP-C peptide (Fig. 1 b) and DPPC-d62/egg PG (7:3, w/w) plus 10% by weight of nonpalmitoylated N-terminal SP-C peptide (Fig. 1 c). The spectra in the three panels of Fig. 1 illustrate how the properties of each bilayer sample change as it undergoes the transition from liquid crystalline phase, at the highest temperatures, to gel phase at the lowest temperatures. At the highest temperatures for each series, the spectra in Fig. 1 are superpositions of Pake doublets that reflect the fast, axially symmetric CD bond reorientation characteristic of the liquid-crystalline phase. The splitting of the 90° edges in a given doublet is proportional to the orientational order parameter for that bond,
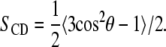 |
(1) |
In Eq. 1, θ is the angle between the CD bond and the symmetry axis, typically the bilayer normal, for the bond motion. The average is over motions of the CD bond that modulate the orientation-dependent quadrupole interaction with correlation times much shorter than the characteristic timescale (∼10−5 s) of the deuterium NMR measurement (45). For perdeuterated lipid acyl chains in the liquid-crystalline phase, SCD is lowest near the bilayer center where the amplitude of chain motion is high and increases in a characteristic way to a plateau value near the headgroup end of the chain where motion is relatively restricted. The resulting orientational order parameter profile gives rise to the characteristic distribution of splittings in the liquid-crystalline phase spectra for phospholipids with perdeuterated saturated chains. At low temperatures, the spectra are typical of the gel phase and reflect reorientation characterized by reduced axial symmetry on the timescale of the measurement.
FIGURE 1.
2H-NMR spectra at selected temperatures of bilayers containing (a) DPPC-d62/egg-PG (7:3 w/w), (b) DPPC-d62/egg-PG (7:3 w/w) plus 10 wt % pSP-C(palm) palmitoylated N-terminal SP-C peptide, or (c) DPPC-d62/egg-PG (7:3 w/w) plus 10 wt % pSP-C(free) nonpalmitoylated N-terminal SP-C peptide. Bilayers are hydrated in a buffer of 150 mM NaCl and 5 mM Tris at pH 7.
For the sample containing no peptide (Fig. 1 a), the spectra above 29°C indicate a single liquid-crystalline phase that implies that DPPC-d62 and egg-PG in this mixture remain homogeneously mixed to below the main transition temperature for DPPC-d62 (∼37°C). Between 29°C and 22°C, the spectra are superpositions of both liquid-crystalline and gel spectral components indicating coexistence of domains with different compositions through the range of temperatures over which the sample undergoes its transition from liquid-crystalline to gel. The superposition is most apparent between 26°C and 24°C where prominent spectral edges corresponding to “plateau” deuterons in the liquid-crystalline phase coexist with the broad wings of the gel phase and where the sharp doublet with a splitting of ∼4.4 kHz, arising from acyl chain methyl deuterons in the liquid-crystalline phase is superimposed on the feature with a width of ∼14 kHz arising from acyl chain methyl deuterons in the gel phase.
Fig. 1 b shows spectra obtained from DPPC-d62 in the sample containing the palmitoylated SP-C N-terminal peptide. The distributions of doublet splittings in the corresponding liquid-crystalline phase spectra indicate that the peptide has little effect on lipid chain orientational order. Spectra for this sample between 29°C and 24°C are also indicative of two-phase coexistence. The 24°C spectrum in Fig. 1 b contains a smaller liquid-crystalline component than the corresponding spectrum in Fig. 1 a suggesting that the temperature range for two phase coexistence in the DPPC-d62/egg-PG bilayers is slightly reduced by the presence of the palmitoylated peptide. Overall, though, the perturbation of DPPC-d62/egg-PG phase behavior by this peptide is small.
Fig. 1 c shows spectra obtained from DPPC-d62 in the sample containing the nonpalmitoylated SP-C N-terminal peptide. The range of quadrupole splittings over the whole temperature range examined is similar to that seen for the lipid only and lipid plus palmitoylated peptide samples indicating that perturbation of DPPC-d62 chain order by the nonpalmitoylated peptide is also small. The liquid-crystalline phase spectra in Fig. 1 c do reflect some broadening, relative to corresponding spectra for the other two samples, which may reflect changes in the correlation times of slower motions that can affect transverse relaxation without changing orientational order. The spectra in Fig. 1 c also differ slightly from those of the other samples through the range of temperatures over which the sample undergoes its main transition. Although the splittings are similar for the three samples at a given temperature, the spectra in Fig. 1 c, particularly at 27°C and 26°C, are not as clearly separable into distinct liquid crystalline and gel spectral components as the corresponding spectra in Fig. 1, a and b. This may indicate that the sizes of any coexisting domains are small enough to allow a given lipid to sample all motional environments over the ∼10−5 s time-scale characteristic of the 2H-NMR experiment.
For a spectrum denoted by f(ω), the first spectral moment,
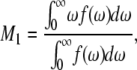 |
(2) |
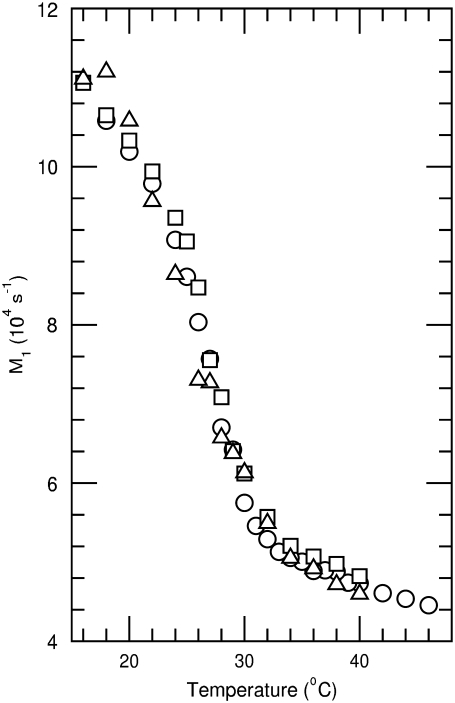
is proportional to the average, over all deuterated chain segments, of the orientational order parameter. M1 typically jumps discontinuously at the liquid crystal to gel transition of a single component phospholipid bilayer. The temperature dependence of M1 is thus indicative of bilayer phase behavior. Changes in M1 with sample composition can provide evidence for perturbation of chain order by protein or peptides within the gel or liquid-crystalline phase. Fig. 2 shows the temperature dependence of M1 as calculated from the spectra of DPPC-d62/egg-PG and DPPC-d62/egg-PG plus 10% by weight of either palmitoylated or nonpalmitoylated peptide. For temperatures above 30°C the addition of the peptides corresponding to the N-terminal segment of SP-C modifies chain order only slightly. Within the range of temperatures corresponding to two-phase coexistence, the palmitoylated peptide raises average DPPC-d62 chain order slightly whereas the nonpalmitoylated peptide appears to reduce it slightly. At the lowest temperatures studied, both peptides appear to raise average DPPC-d62 chain order slightly. Although it is clear that neither peptide substantially perturbs average DPPC-d62 chain order at the concentrations studied here, the palmitoylated peptide did seem to promote a small but significant increase in average chain order within the transition range of temperatures where the bilayer system may be particularly susceptible to perturbation. This suggests that the palmitoylated peptide may have a slight tendency to promote increased chain order. If so, palmitoylation may facilitate interaction of the N-terminal segment of SP-C with ordered regions as they become organized in membranes on cooling below the main phase transition temperature. A qualitatively similar behavior has been observed when studying the interaction of synthetic peptides corresponding to the N-terminal segment of SP-C with phospholipid membranes by electron spin resonance (46).
FIGURE 2.
Temperature dependence of the first spectral moment (M1) for 2H-NMR spectra obtained from bilayers containing DPPC-d62/egg-PG (7:3 w/w) (circle), DPPC-d62/egg-PG (7:3 w/w) plus 10 wt % pSP-C(palm) palmitoylated N-terminal SP-C peptide (square), or DPPC-d62/egg-PG (7:3 w/w) plus 10 wt % pSP-C(free) nonpalmitoylated N-terminal SP-C peptide (triangle). Bilayers are hydrated in a buffer of 150 mM NaCl and 5 mM Tris at pH 7. Estimated uncertainty in M1 is ±3%.
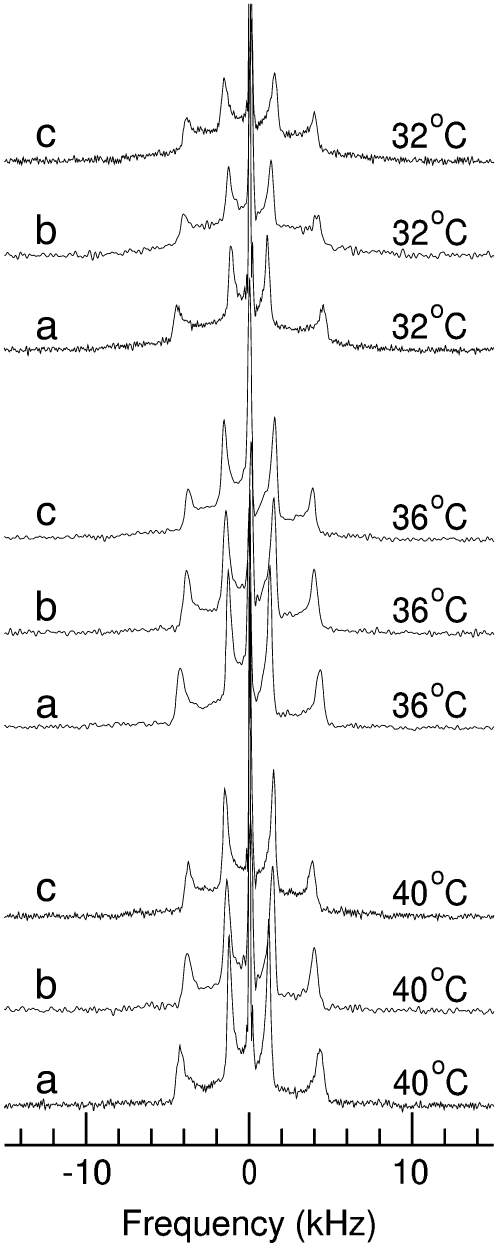
Interactions between the N-terminal segment of SP-C and the headgroup region of phospholipid bilayers were studied by observing the effect of palmitoylated and nonpalmitoylated SP-C peptides on bilayers containing DPPC deuterated in the α and β positions of the choline group (DPPC-d4). 2H-NMR spectroscopy of membranes made of headgroup deuterated phosphatidylcholine has proven to be useful for studying the influence of external perturbations, such as electric charges, hydrostatic pressure or the interaction of proteins on the headgroup orientation of the phospholipid molecules (40,47,48). The interaction between the phosphatidylcholine P−-N+ dipole and external perturbing surface charges causes the headgroup to tilt toward or away from the bilayer surface depending on the sign of the net surface charge. The headgroup tilts toward or away from the bilayer surface as its positively-charged quaternary nitrogen is either attracted to or repelled by opposite or like surface charge respectively (47,49–51). It was shown previously that the interaction of pulmonary surfactant protein SP-C with the DPPC headgroup is primarily electrostatic in nature. SP-C has three positively charged side chains. The response of headgroup conformation to SP-C concentration was consistent with an interaction between the lipid headgroup dipole and the net positive charge associated with the protein (40). Fig. 3 shows spectra at selected temperatures obtained from DPPC-d4/egg-PG (7:3) bilayers in the absence or in the presence of either palmitoylated or nonpalmitoylated SP-C peptide. For each temperature, spectrum a is for DPPC-d4/egg PG (7:3, w/w) without peptide, spectrum b is for DPPC-d4/egg PG (7:3, w/w) plus 10% by weight of palmitoylated N-terminal SP-C peptide and spectrum c is for DPPC-d4/egg PG (7:3, w/w) plus 10% by weight of nonpalmitoylated N-terminal SP-C peptide. The central spike visible in each spectrum likely arises from natural abundance deuterium present in the buffer. Its prominence reflects the relatively weak signal from the small number of choline deuterons included in these samples. The outer and inner doublets in these 2H-NMR spectra arise from pairs of deuterons on the α and β carbons of the choline group, respectively.
FIGURE 3.
2H-NMR spectra at the indicated temperatures obtained from bilayers containing (a) DPPC-d4/egg-PG (7:3 w/w), (b) DPPC-d4/egg-PG (7:3 w/w) plus 10 wt % pSP-C(palm) palmitoylated N-terminal SP-C peptide, or (c) DPPC-d4/egg-PG (7:3 w/w) plus 10 wt % pSP-C(free) nonpalmitoylated N-terminal SP-C peptide. Bilayers are hydrated in a buffer of 150 mM NaCl and 5 mM Tris at pH 7.
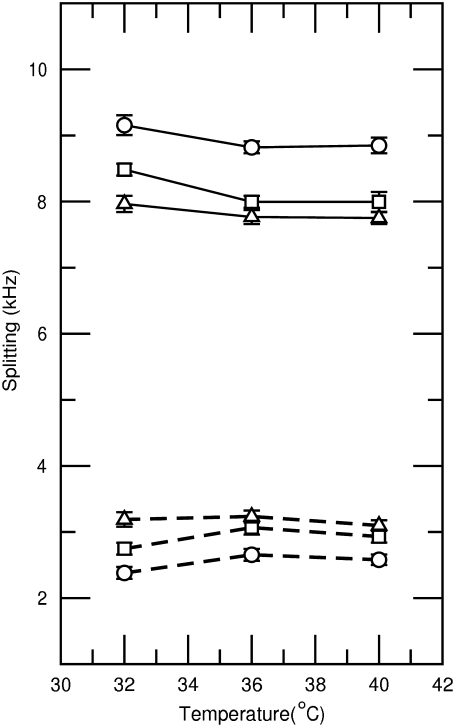
The prominent edges of the Pake doublets reflect signal from lipids reorienting about bilayer normals perpendicular to the applied field. Fig. 4 shows the splittings of these 90° edges for the choline α and β deuterons in each of the three samples. The assignment of the splittings is based on previous observations of DPPC-d4 alone and the known effect of anionic amphiphiles on the choline α and β deuteron quadrupole splitting. For DPPC-d4 alone, the α and β splittings are both between 5 kHz and 6 kHz just above the main transition (40). Negative surface charge tends to raise the choline α deuteron splitting and reduce the choline β deuteron splitting, presumably as a result of an electrostatically-induced change in average orientation of the phosphocholine electric dipole (47,52). Identification of the larger splitting with the choline α deuterons is supported by noting that the 8.85 kHz splitting observed at 40°C for the sample containing only DPPC-d4/egg-PG 7:3 (w/w) is similar to the splitting for α-CD2-POPC in the presence of 20 mol % POPG reported by Macdonald and Seelig (52).
FIGURE 4.
Temperature dependence of quadrupole splittings for choline α deuterons (solid lines) and choline β deuterons (dashed lines) obtained from bilayers containing DPPC-d4/egg-PG (7:3 w/w) (circle), DPPC-d4/egg-PG (7:3 w/w) plus 10 wt % pSP-C(palm) palmitoylated N-terminal SP-C peptide (square), or DPPC-d4/egg-PG (7:3 w/w) plus 10 wt % pSP-C(free) nonpalmitoylated N-terminal SP-C peptide (triangle).
In previous studies involving DPPC-d4 alone (40) or DPPC-d4/egg-PG 7:3 in the presence of calcium (49), the choline α-deuteron splitting in the liquid crystalline phase was largely independent of temperature whereas the choline β-deuteron splitting increased as the temperature was lowered toward the transition. This behavior was attributed to a competition between the effects of increasing amplitude of motion with increasing temperature and an overall change in average lipid headgroup orientation due to changes in lipid packing with increasing temperature. In contrast, the β deuteron splittings observed in this work depend only weakly on temperature above 32°C. The difference likely reflects the effect of anionic egg-PG being present in the bilayer without any compensating effect from calcium.
Fig. 4 shows that addition of the N-terminal SP-C segments to the bilayers causes counterdirectional shifts of the choline α and β deuteron splittings that are larger than those due to temperature over the range studied. The observed shift of α splittings to lower frequency and β splittings to higher frequency on addition to peptide implies a tilting of the phosphocholine dipole away from the bilayer surface (toward the bilayer normal) as would be expected for addition of positive charge to the bilayer interface.
The observations described above indicate that the palmitoylated and nonpalmitoylated SP-C N-terminal peptide segments have small effects on chain orientational order and phase behavior of DPPC/egg-PG bilayers at the concentrations studied. To investigate the motions of palmitic chains covalently attached to the N-terminal segment of surfactant protein SP-C we synthesized and characterized the behavior of a peptide sterified with fully deuterated palmitic chains. Fig. 5 shows the 2H-NMR spectra of this peptide once reconstituted in DPPC/egg-PG (7:3 w/w) membranes at a peptide/lipid ratio of 10% by weight. Spectra obtained from equivalent samples of DPPC-d62/egg-PG containing 10% of pSP-C(palm) peptide, at corresponding temperatures, are shown for comparison. For each temperature, spectrum a is obtained from the deuterated-lipid sample and spectrum b is obtained from the sample containing peptide with deuterated palmitate chains. As described above, the spectra from deuterons in the DPPC-d62/egg-PG bilayers at temperatures above the onset of the transition are characteristic of fast, axially symmetric reorientation for all samples observed. The distributions of splittings reflect the orientational order parameter profile typically observed for saturated chain lipids in the liquid-crystalline phase (45,53,54). In contrast, the 2H-NMR spectra obtained from the sample containing peptide with covalently-attached deuterated palmitate chains are very different. The lower signal/noise ratios in the spectra obtained from deuterated palmitic chains on the peptide are due to the smaller fraction of deuterated chains in this sample. Despite the lower signal/noise ratio, it is still possible to draw a meaningful comparison between the motions of the lipid and peptide palmitate chains on the basis of the spectra in Fig. 5. The narrow doublet at the center of each peptide palmitate spectrum indicates that the methyl group on each peptide palmitate chain is undergoing fast axially symmetric reorientation about the bilayer normal. The splitting is slightly lower than for the methyl doublet on the corresponding DPPC-d62 spectra, which indicates that the peptide palmitate chains have less orientational order than the lipid chains. The average splitting for the peptide palmitate deuteron doublets is less than that of the lipid chain deuterons, which indicates that the average orientational order along the peptide palmitate chain is also less than that along the lipid chain in the corresponding mixture. Finally, the distribution of splittings for the peptide palmitate chain is significantly different from that for the lipid palmitate chain. The 2H-NMR spectrum of a fully deuterated saturated lipid acyl chain typically shows a prominent edge at the largest splitting. This arises from the superposition of multiple doublets with very similar splittings and is characteristic of a plateau, in the orientational order parameter profile, corresponding to the most motionally-constrained chain segments near the headgroup end of the chain. For the peptide palmitate chain, the intensity of the nonmethyl portion of the spectrum peaks at about half of the maximum observed splitting. Although this shape might suggest some underlying departure from axially-symmetric reorientation, the methyl doublets are indicative of axially-symmetric reorientation and it is likely that the shape of the nonmethyl portion of the spectrum reflects, at least in part, a distribution of splittings that is significantly different from typical lipid acyl chain orientational order parameter profile.
FIGURE 5.
2H-NMR spectra at the indicated temperatures obtained from (a) bilayers of DPPC-d62/PG (7:3 w/w) containing 10% peptide SP-C(palm) and (b) bilayers of DPPC/PG (7:3, w/w) containing 10% peptide pSP-C(2H-palm), sterified with deuterated palmitic chains.
As the temperature is reduced from 40°C to 28°C, the distinct edges seen at maximum splitting in the spectrum of the deuterated peptide palmitate chain become less prominent and the spectrum becomes increasingly rounded. This is consistent with changes seen in the lipid acyl chain spectrum as the sample approaches the transition and suggests that the peptide palmitate chains are sensitive to the phase state of the surrounding lipid bilayer.
Aside from the shape of the nonmethyl portion of the peptide palmitate spectrum, the most striking difference from the lipid palmitate chains observed in a sample of the same composition is the much lower degree of average orientational order. This implies that acyl chain packing in the neighborhood of the peptide palmitate chains is much less dense than in the rest of the bilayer interior. This could reflect local perturbation of lipid headgroup spacing by the portion of the peptide interacting with the bilayer in the neighborhood of the peptide palmitate chains. It has been proposed that the N-terminal segment of SP-C forms an amphipathic β-hairpin, with the middle part of the turn occupied by hydrophobic residues whereas both ends contain polar, positively charged amino acids. This allows the association of the N-terminal segment with the polar/nonpolar interface offered by the membrane surface, either in the absence or presence of palmitoylation (41,46). The association of the amphipathic hairpin with the bilayer surface probably gives rise to packing defects and provides further space to the thioester-linked palmitic chains inside the membrane. This could account for the additional conformational freedom compared with that sensed by the acyl chains on the phospholipid molecules, especially with respect to the chain segments close to the polar headgroups. The small size of the studied peptides may also contribute to the higher conformational freedom and different orientational dynamics of the peptide palmitate chains relative to those of the phospholipids in a given sample.
DISCUSSION
The function of palmitoylation in SP-C, other than a structural role in stabilizing the α-helical conformation of the protein, is not clear. The acyl chains are not strictly required to promote the association of SP-C with membranes, because in the absence of the fatty acyl chains SP-C remains membrane-associated via its transmembrane domain (32,34). Palmitic chains are not even required to promote association of the N-terminal segment of SP-C with surfactant bilayers and films, as synthetic peptides designed from this sequence associate and perturb bilayers and monolayers even in the absence of cysteine-linked acylation (41,42). An intracellular role for the palmitic chains to modulate trafficking of SP-C is not very likely either, because the substitution of cysteines by alanines or serines, resulting in the absence of the palmitoyl chains, had no apparent influence on targeting of proSP-C (55,56). However, the palmitoyl chains of SP-C seem to play an important role in surface activity. It has been observed that the lack of covalently bound palmitoyl groups reduces the mechanical stability of SP-C-containing surface films and the re-spreading, on expansion, of phospholipids from surface reservoirs, back into the air-liquid interface (30,32–34). This indicates that palmitoylation is required for optimal surface activity of SP-C. The role of palmitoylation on the activity of SP-C has been investigated by using infrared spectroscopy to study the behavior of palmitoylated and nonpalmitoylated versions of peptides from the N-terminal segment of SP-C in lipid/protein monolayers subjected to compression/expansion dynamics (43). Palmitoylation was important for the peptides to maintain association with phospholipids films once compressed to the highest pressures. These results have been the basis for a model that proposes that SP-C, mainly via its palmitoylated N-terminal segment, would be required to maintain the association of surfactant complexes with the interfacial film at the most compressed states, those reached at the end of expiration (12,57–59). A recent study has determined that palmitoylation favors association of the N-terminal SP-C segment with ordered interdigitated-like regions in DPPC or DPPG bilayers (46). Interdigitated-like phospholipid organization has been proposed to occur in membrane regions in the P′β ripple phase, typical of DPPC bilayers at 37°C (60).
Deuterium NMR is a valuable tool for studying interactions of various lipid and protein components in membrane environments (45,61) and has also been applied to models of lung surfactant (40,49,62,63). The results presented in this study are consistent with a role of palmitic chains to favor intercalation of SP-C N-terminal segment within ordered lipid regions. Palmitoylated peptide increases average orientational of the acyl chains of bilayers particularly at temperatures for which domains of gel and liquid-crystalline phase lipid coexist. An alternative possibility could be that the peptide withdraws PG from the gel phase domains through, perhaps, selective electrostatic interactions, contributing to increase the order in the gel phase, which could be then further enriched in DPPC. The intrinsic disorder of the peptide-attached acyl chains could also contribute to a preferential interaction of unsaturated phospholipid chains with the palmitoylated peptide. However, the palmitoylated peptide is also more efficient than the nonpalmitoylated peptide in promoting ordering of gel phase in pure DPPC bilayers, as recently shown by electron spin resonance spectroscopy (46), which cannot therefore depend so much on peptide-promoted demixing of phospholipid species. It is conceivable that the order of the peptide chains is limited by the way they are anchored to the peptide sequence. If so, and if the resulting lateral area of the peptide chains is larger than the lateral area that the peptide occupies among the lipid headgroups, then there might still be a slight higher tendency of palmitoylated peptide to attract disordered lipids and to increase further average chain order in gel-like domains. An effect like this might still be most apparent around the transition where it is easiest to develop nonrandom mixing of the components.
A very rough estimate of the effect of the peptide charge on the PC headgroup can be obtained by considering the extent to which the added peptide reverses the effect of the egg-PG already present in these bilayers. At 40°C, the choline α deuteron splitting for DPPC-d4 is ∼5.8 kHz (40). For a mixture of DPPC-d4/egg-PG at a ratio of 7:3 by weight, this splitting increases to ∼8.9 kHz. Addition of 10% peptide by weight reduces this splitting to 8.0 kHz for the palmitoylated peptide and 7.8 kHz for the nonpalmitoylated peptide. This represents the removal of about a third of the change in splitting due to the presence of egg-PG alone. Response of the choline α deuteron splitting to PG concentration is not linear (52) but this comparison suggests that the effects, per mass, of these peptides and egg-PG on PC headgroup orientations are roughly similar in magnitude and opposite in direction. It is interesting to note that using the average molecular weight for egg-PG and assuming a charge of −1 for PG and +3 for the peptide, the magnitude of the charge per unit mass for the palmitoylated peptide is ∼15% higher than that of egg-PG. The magnitude of the charge per unit mass of the nonpalmitoylated peptide is ∼50% higher than that of egg-PG and the nonpalmitoylated peptide is indeed slightly more effective than the palmitoylated peptide in reversing the effect of the egg-PG on the choline deuteron splittings. Although the nonlinearity of the response of choline deuteron splittings to bilayer surface charge precludes a more detailed analysis at this time, it does seem that the response of the lipid headgroup deuteron splittings is largely accounted for by electrostatic considerations. This observation also implies that charged residues in SP-C can have a small effect on average lipid headgroup orientation and, presumably, a larger effect on local lipid headgroup orientation. Interestingly, both palmitoylated and nonpalmitoylated peptides cause similar perturbations of DPPC headgroup orientation suggesting that, whatever the role of palmitoylation, it does not significantly alter the largely electrostatic interaction of the peptide with DPPC headgroups.
The most interesting and novel aspect of this work is probably the analysis of the mobility of the palmitoyl groups covalently bound to the N-terminal segment. To our knowledge, this is the first time the specific behavior of protein-attached acyl chains has been studied to this extent in a membrane context. The results presented in this study suggest that these acyl chains possess a very different configurational dynamics from that of the phospholipid acyl chains in the same membranes. The acyl chains of the phospholipid molecules are subjected to a marked gradient of orientational order in the bilayer, from the highly constrained motion of chain segments close to the headgroups to the much larger amplitude reorientation of acyl segments near the termini methyl group in the center of the bilayers (45,53,54). Such gradients are responsible for the typical spectra obtained from phospholipids bearing fully deuterated chains under conditions of typical bilayer packing. The spectra obtained from the deuterated acyl chains on the protein segments studied here indicate that the deuterated peptide palmitate chains are subjected to very different motional constraints than the chains attached to the lipids. It is probable that the acyl segments close to the carbonyl group in the peptide context are less constrained than in the phospholipid context for a given bilayer. Still, the peptide acyl chains also sense changes in motional properties of the lipid environment as the system approaches the main bilayer phase transition on cooling. To what extent the change in order, mobility, and dynamics associated with phase transition might be “transmitted” to the peptide structure through the peptide acyl chains has still to be explored.
Several items of evidence connect acylation of proteins to their association with ordered phospholipid structures. Acylation has been proposed to promote association of several proteins with ordered raft-like domains in cell membranes (64–67). Acylation of peptides seems to induce preferential perturbations on the ripple Pβ′ phase of DPPC bilayers (68). Furthermore, in the particular case of SP-C, a study of palmitoylated and nonpalmitoylated peptides indicated that palmitoylation is required to maintain association of the peptide segments with highly ordered compressed states of phospholipid interfacial films (43). In all these examples, it has been proposed that protein-attached acyl tails have the potential to intercalate between phospholipid chains in membrane ordered arrays, participating in their tight packing and permitting a closer association of protein motifs. We show in this study, however, that mobility and dynamics of protein-attached acyl segments differ substantially from the average properties of the chains in the host phospholipid, arguing against an entropic factor promoting the coupling between palmitic chains in lipids and proteins. Additional data are required to determine how much of the acyl chain dynamics is defined by the bearing protein sequence, and how much of the singular dynamics of the protein-attached chains is important to define relevant structure-function determinants of palmitoylated proteins.
Acknowledgments
The authors are grateful to Dr. Kevin Keough for facilities used in sample preparation during the NMR phase of this work.
This research has been supported by grants from the Spanish Ministry of Science (BIO2006-03130, CONSOLIDER-INGENIO 2010 CSD2007-00010), and Community of Madrid (S0505/MAT/0283) to J.P.-G. and from the Natural Sciences and Engineering Research Council (Canada) to M.R.M.
Editor: Lukas K. Tamm.
References
- 1.Daniels, C. B., and S. Orgeig. 2003. Pulmonary surfactant: the key to the evolution of air breathing. News Physiol. Sci. 18:151–157. [DOI] [PubMed] [Google Scholar]
- 2.deMello, D. E. 2004. Pulmonary pathology. Semin. Neonatol. 9:311–329. [DOI] [PubMed] [Google Scholar]
- 3.Whitsett, J. A., S. E. Wert, and Y. Xu. 2005. Genetic disorders of surfactant homeostasis. Biol. Neonate. 87:283–287. [DOI] [PubMed] [Google Scholar]
- 4.Wright, J. R. 1997. Immunomodulatory functions of surfactant. Physiol. Rev. 77:931–962. [DOI] [PubMed] [Google Scholar]
- 5.Goerke, J. 1998. Pulmonary surfactant functions and molecular composition. Biochim. Biophys. Acta. 1408:79–89. [DOI] [PubMed] [Google Scholar]
- 6.Veldhuizen, J. A., and H. P. Haagsman. 2000. Role of pulmonary surfactant component in surface film formation and dynamics. Biochim. Biophys. Acta. 1467:255–270. [DOI] [PubMed] [Google Scholar]
- 7.Whitsett, J. A., and T. E. Weaver. 2002. Hydrophobic surfactant proteins in lung function and disease. N. Engl. J. Med. 347:2141–2148. [DOI] [PubMed] [Google Scholar]
- 8.Chaby, R. 2004. Lipopolysaccharide-binding molecules: transporters, blockers and sensors. Cell. Mol. Life Sci. 61:1697–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crouch, E. C. 1998. Structure, biologic properties and expression of surfactant protein D (SP-D). Biochim. Biophys. Acta. 1408:278–289. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Gil, J. 2002. Molecular interactions in pulmonary surfactant films. Biol. Neonate. 81(Suppl 1):6–15. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Gil, J., and K. M. Keough. 1998. Interfacial properties of surfactant proteins. Biochim. Biophys. Acta. 1408:203–217. [DOI] [PubMed] [Google Scholar]
- 12.Serrano, A. G., and J. Pérez-Gil. 2006. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem. Phys. Lipids. 141:108–115. [DOI] [PubMed] [Google Scholar]
- 13.Lukovic, D., I. Plasencia, F. J. Taberner, J. Salgado, J. J. Calvete, J. Perez-Gil, and I. Mingarro. 2006. Production and characterization of recombinant forms of human pulmonary surfactant protein C (SP-C): structure and surface activity. Biochim. Biophys. Acta. 1758:509–518. [DOI] [PubMed] [Google Scholar]
- 14.Oosterlaken-Dijksterhuis, M. A., H. P. Haagsman, L. M. van Golde, and R. A. Demel. 1991. Characterization of lipid insertion into monomolecular layers mediated by lung surfactant proteins SP-B and SP-C. Biochemistry. 30:10965–10971. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Gil, J., J. Tucker, G. Simatos, and K. M. Keough. 1992. Interfacial adsorption of simple lipid mixtures combined with hydrophobic surfactant protein from pig lung. Biochem. Cell Biol. 70:332–338. [DOI] [PubMed] [Google Scholar]
- 16.Palmblad, M., M. Gustafsson, T. Curstedt, J. Johansson, and S. Schurch. 2001. Surface activity and film formation from the surface associated material of artificial surfactant preparations. Biochim. Biophys. Acta. 1510:106–117. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuizen, E. J., R. V. Diemel, G. Putz, L. M. van Golde, J. J. Batenburg, and H. P. Haagsman. 2001. Effect of the hydrophobic surfactant proteins on the surface activity of spread films in the captive bubble surfactometer. Chem. Phys. Lipids. 110:47–55. [DOI] [PubMed] [Google Scholar]
- 18.Glasser, S. W., M. S. Burhans, T. R. Korfhagen, C. L. Na, P. D. Sly, G. F. Ross, M. Ikegami, and J. A. Whitsett. 2001. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc. Natl. Acad. Sci. USA. 98:6366–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz, A., C. Casals, and J. Perez-Gil. 1995. Conformational flexibility of pulmonary surfactant proteins SP-B and SP-C, studied in aqueous organic solvents. Biochim. Biophys. Acta. 1255:68–76. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Gil, J., A. Cruz, and C. Casals. 1993. Solubility of hydrophobic surfactant proteins in organic solvent/water mixtures. Structural studies on SP-B and SP-C in aqueous organic solvents and lipids. Biochim. Biophys. Acta. 1168:261–270. [DOI] [PubMed] [Google Scholar]
- 21.Vandenbussche, G., A. Clercx, T. Curstedt, J. Johansson, H. Jornvall, and J.-M. Ruysschaert. 1992. Structure and orientation of the surfactant associated protein C in a lipid bilayer. Eur. J. Biochem. 203:201–209. [DOI] [PubMed] [Google Scholar]
- 22.Johansson, J., T. Szyperski, T. Curstedt, and K. Wuthrich. 1994. The NMR structure of the pulmonary surfactant-associated polypeptide SP-C in an apolar solvent contains a valyl-rich alpha-helix. Biochemistry. 33:6015–6023. [DOI] [PubMed] [Google Scholar]
- 23.Johansson, J., G. Nilson, R. Stromberg, B. Robertson, H. Jörnvall, and T. Curstedt. 1995. Secondary structure and biophysical activity of synthetic analogues of the pulmonary surfactant polypeptide SP-C. Biochem. J. 307:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plasencia, I., L. Rivas, C. Casals, K. M. Keough, and J. Pérez-Gil. 2001. Intrinsic structural differences in the N-terminal segment of pulmonary surfactant protein SP-C from different species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129:129–139. [DOI] [PubMed] [Google Scholar]
- 25.Dunphy, J. T., and M. E. Linder. 1998. Signaling functions of protein palmitoylation. Biochim. Biophys. Acta. 1436:245–261. [DOI] [PubMed] [Google Scholar]
- 26.Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1451:1–16. [DOI] [PubMed] [Google Scholar]
- 27.Bijlmakers, M. J., and M. Marsh. 2003. The on-off story of protein palmitoylation. Trends Cell Biol. 13:32–42. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson, M., W. J. Griffiths, E. Furusjo, and J. Johansson. 2001. The palmitoyl groups of lung surfactant protein C reduce unfolding into a fibrillogenic intermediate. J. Mol. Biol. 310:937–950. [DOI] [PubMed] [Google Scholar]
- 29.Schürch, S., F. H. Green, and H. Bachofen. 1998. Formation and structure of surface films: captive bubble surfactometry. Biochim. Biophys. Acta. 1408:180–202. [DOI] [PubMed] [Google Scholar]
- 30.Gustafsson, M., M. Palmblad, T. Curstedt, J. Johansson, and S. Schürch. 2000. Palmitoylation of a pulmonary surfactant protein C analogue affects the surface associated lipid reservoir and film stability. Biochim. Biophys. Acta. 1466:169–178. [DOI] [PubMed] [Google Scholar]
- 31.Johansson, J. 2001. Membrane properties and amyloid fibril formation of lung surfactant proteins C. Biochem. Soc. Trans. 29:601–606. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Z., O. Gurel, J. E. Baatz, and R. H. Notter. 1996. Acylation of pulmonary surfactant protein-C is required for its optimal surface active interactions with phospholipids. J. Biol. Chem. 271:19104–19109. [DOI] [PubMed] [Google Scholar]
- 33.Flach, C. R., A. Gericke, K. M. W. Keough, and R. Mendelsohn. 1999. Palmitoylation of lung surfactant protein SP-C alters surface thermodynamics, but not protein secondary structure or orientation in 1,2-dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta. 1416:11–20. [DOI] [PubMed] [Google Scholar]
- 34.Qanbar, R., S. Cheng, F. Possmayer, and S. Schurch. 1996. Role of the palmitoylation of surfactant associated protein C in surfactant film formation and stability. Am. J. Physiol. Lung Cell. Mol. Physiol. 271:L572–L580. [DOI] [PubMed] [Google Scholar]
- 35.Shiffer, K., S. Hawgood, H. P. Haagsman, B. Benson, J. A. Clements, and J. Goerke. 1993. Lung surfactant proteins, SP-B and SP-C, alter the thermodynamic properties of phospholipid membranes: a differential calorimetry study. Biochemistry. 32:590–597. [DOI] [PubMed] [Google Scholar]
- 36.Simatos, G. A., K. B. Forward, M. R. Morrow, and K. M. W. Keough. 1990. Interaction between perdeuterated dimyristoylphosphatidylcholine and low molecular weight pulmonary surfactant protein SP-C. Biochemistry. 29:5807–5814. [DOI] [PubMed] [Google Scholar]
- 37.Pastrana-Rios, B., S. Taneva, K. M. Keough, A. J. Mautone, and R. Mendelsohn. 1995. External reflection absorption infrared spectroscopy study of lung surfactant proteins SP-B and SP-C in phospholipid monolayers at the air/water interface. Biophys. J. 69:2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Gil, J., C. Casals, and D. Marsh. 1995. Interactions of hydrophobic lung surfactant proteins SP-B and SP-C with dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylglycerol bilayers studied by electron spin resonance spectroscopy. Biochemistry. 34:3964–3971. [DOI] [PubMed] [Google Scholar]
- 39.Dico, A. S., S. Taneva, M. R. Morrow, and K. M. Keough. 1997. Effect of calcium on phospholipid interaction with pulmonary surfactant protein C. Biophys. J. 73:2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrow, M. R., S. Taneva, G. A. Simatos, L. A. Allwood, and K. M. W. Keough. 1993. 2H-NMR studies of the effect of pulmonary surfactant SP-C on the 1,2-dipalmitoyl-sn-glycero-3-phosphocholine headgroup: a model for transbilayer peptides in surfactant and biological membranes. Biochemistry. 32:11338–11344. [DOI] [PubMed] [Google Scholar]
- 41.Plasencia, I., L. Rivas, K. M. Keough, D. Marsh, and J. Pérez-Gil. 2004. The N-terminal segment of pulmonary surfactant lipopeptide SP-C has intrinsic propensity to interact with and perturb phospholipid bilayers. Biochem. J. 377:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plasencia, I., K. M. Keough, and J. Perez-Gil. 2005. Interaction of the N-terminal segment of pulmonary surfactant protein SP-C with interfacial phospholipid films. Biochim. Biophys. Acta. 1713:118–128. [DOI] [PubMed] [Google Scholar]
- 43.Bi, X., C. R. Flach, J. Pérez-Gil, I. Plasencia, D. Andreu, E. Oliveira, and R. Mendelsohn. 2002. Secondary structure and lipid interactions of the N-terminal segment of pulmonary surfactant SP-C in Langmuir films: IR reflection-adsorption spectroscopy and surface pressure studies. Biochemistry. 41:8385–8395. [DOI] [PubMed] [Google Scholar]
- 44.Bartlett, G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466–468. [PubMed] [Google Scholar]
- 45.Davis, J. H. 1983. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim. Biophys. Acta. 737:117–171. [DOI] [PubMed] [Google Scholar]
- 46.Plasencia, I., F. Baumgart, D. Andreu, D. Marsh, and J. Perez-Gil. 2008. Effect of acylation on the lipid-protein interactions of the N-terminal segment of pulmonary surfactant protein SP-C. Biochim. Biophys. Acta. 1778:1274–1282. [DOI] [PubMed] [Google Scholar]
- 47.Seelig, J., P. M. Macdonald, and P. G. Scherer. 1987. Phospholipid headgroups as sensors of electric charge in membranes. Biochemistry. 26:7535–7541. [DOI] [PubMed] [Google Scholar]
- 48.Bloom, M., and I. C. P. Smith. 1985. Manifestations of lipid-protein interactions in deuterium NMR. In Progress in Protein-Lipid Interactions. Elsevier, Amsterdam. 61–76.
- 49.Morrow, M. R., N. Abu-Libdeh, J. Stewart, and K. M. W. Keough. 2003. Interaction of pulmonary surfactant protein SP-A with DPPC/egg-PG bilayers. Biophys. J. 85:2397–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scherer, P. G., and J. Seelig. 1989. Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry. 28:7720–7728. [DOI] [PubMed] [Google Scholar]
- 51.MacDonald, P. M., J. Leisen, and F. M. Marassi. 1991. Response of phosphatidylcholine in the gel and liquid-crystalline states to membrane surface charges. Biochemistry. 30:3558–3566. [DOI] [PubMed] [Google Scholar]
- 52.Macdonald, P. M., and J. Seelig. 1987. Calcium binding to mixed phosphatidylglycerol-phosphatidylcholine bilayers as studied by deuterium nuclear magnetic resonance. Biochemistry. 26:1231–1240. [DOI] [PubMed] [Google Scholar]
- 53.Davis, J. H. 1979. Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys. J. 27:339–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seelig, J. 1977. Deuterium magnetic resonance: theory and application to lipid membranes. Q. Rev. Biophys. 10:353–418. [DOI] [PubMed] [Google Scholar]
- 55.Conkright, J. J., J. P. Bridges, C. L. Na, W. F. Voorhout, S. W. Trapnell, and T. E. Weaver. 2001. Secretion of surfactant protein C (SP-C), an integral membrane protein requires the N-terminal propeptide. J. Biol. Chem. 276:14658–14664. [DOI] [PubMed] [Google Scholar]
- 56.Brinke, A. T., J. J. Batenburg, B. M. Gadella, H. P. Haagsman, A. B. Vaandrager, and L. M. G. van Golde. 2001. The juxtamembrane lysine and arginine residues of surfactant protein C precursor influence palmitoylation via effects on trafficking. Am. J. Respir. Cell Mol. Biol. 25:156–163. [DOI] [PubMed] [Google Scholar]
- 57.Kramer, A., A. Wintergalen, M. Sieber, H. J. Galla, M. Amrein, and R. Guckenberger. 2000. Distribution of the surfactant-associated protein C within a lung surfactant model film investigated by near-field optical microscopy. Biophys. J. 78:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malcharek, S., A. Hinz, L. Hilterhaus, and H. J. Galla. 2005. Multilayer structures in lipid monolayer films containing surfactant protein C: effects of cholesterol and POPE. Biophys. J. 88:2638–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, L., P. Cai, H. J. Galla, H. He, C. R. Flach, and R. Mendelsohn. 2005. Monolayer-multilayer transitions in a lung surfactant model: IR reflection-absorption spectroscopy and atomic force microscopy. Eur. Biophys. J. 34:243–254. [DOI] [PubMed] [Google Scholar]
- 60.de Vries, A. H., S. Yefimov, A. E. Mark, and S. J. Marrink. 2005. Molecular structure of the lecithin ripple phase. Proc. Natl. Acad. Sci. USA. 102:5392–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis, J. H. 1991. 2H-NMR spectroscopy in partially ordered systems. In Isotopes in the Physical and Biomedical Science, vol. 2. E. Buncel and J. R. Jones, editors. Elsevier, Amsterdam. 99–157.
- 62.Morrow, M. R., J. Perez-Gil, G. Simatos, C. Boland, J. Stewart, D. Absolom, V. Sarin, and K. M. W. Keough. 1993. Pulmonary surfactant-associated protein SP-B has little effect on acyl chains in dipalmitoylphosphatidylcholine dispersions. Biochemistry. 32:4397–4402. [DOI] [PubMed] [Google Scholar]
- 63.Nag, K., K. M. W. Keough, and M. R. Morrow. 2006. Probing perturbation of bovine lung surfactant extracts by albumin using DSC and 2H-NMR. Biophys. J. 90:3632–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Higuchi, M., K. M. Izumi, and E. Kieff. 2001. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc. Natl. Acad. Sci. USA. 98:4675–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moffett, S., D. A. Brown, and M. E. Linder. 2000. Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 275:2191–2198. [DOI] [PubMed] [Google Scholar]
- 66.Shogomori, H., A. T. Hammond, A. G. Ostermeyer-Fay, D. J. Barr, G. W. Feigenson, E. London, and D. A. Brown. 2005. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J. Biol. Chem. 280:18931–18942. [DOI] [PubMed] [Google Scholar]
- 67.Tanimura, N., S. Saitoh, S. Kawano, A. Kosugi, and K. Miyake. 2006. Palmitoylation of LAT contributes to its subcellular localization and stability. Biochem. Biophys. Res. Commun. 341:1177–1183. [DOI] [PubMed] [Google Scholar]
- 68.Pedersen, T. B., T. Kaasgaard, M. O. Jensen, S. Frokjaer, O. G. Mouritsen, and K. Jorgensen. 2005. Phase behavior and nanoscale structure of phospholipid membranes incorporated with acylated C14-peptides. Biophys. J. 89:2494–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yousefi-Salakdeh, E., J. Johansson, and R. Stromberg, 1999. A method for S- and O-palmitoylation of peptides: synthesis of pulmonary surfactant protein-C models. Biochem. J. 343:557–562. [PMC free article] [PubMed] [Google Scholar]