Abstract
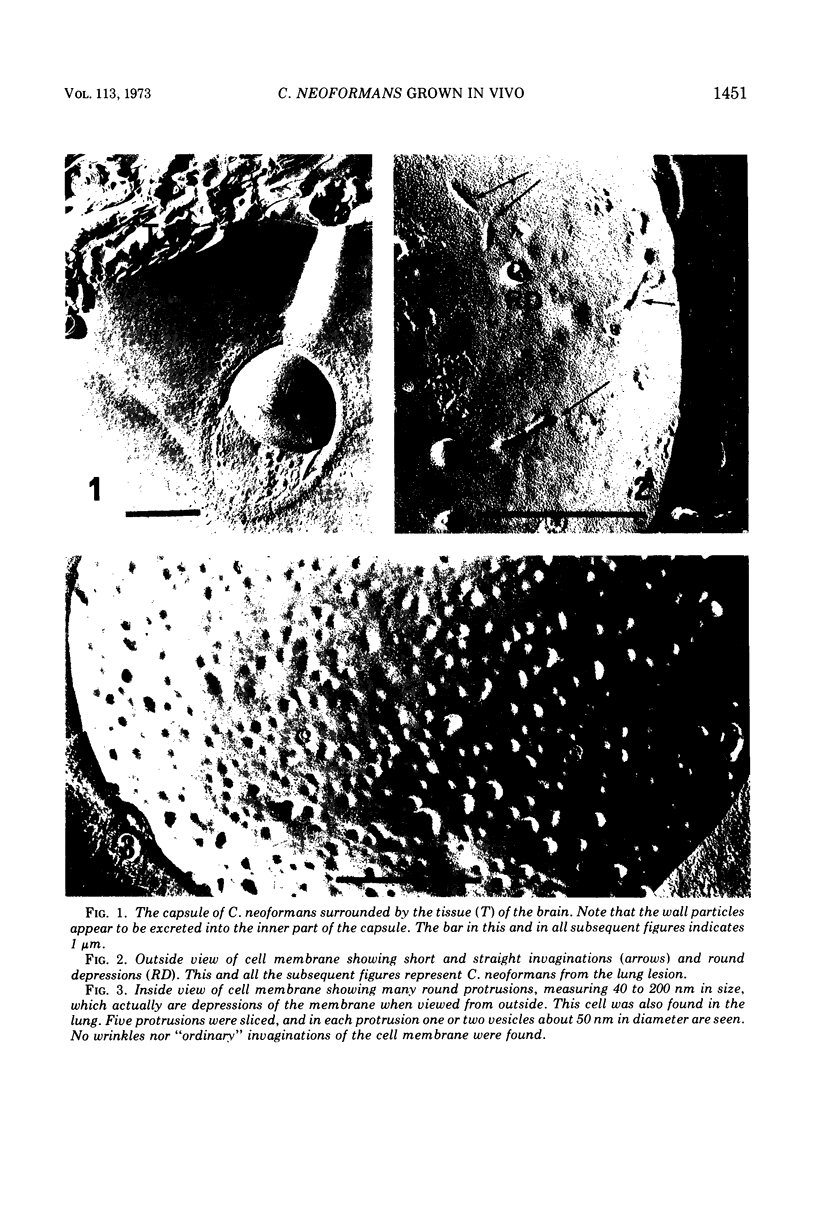
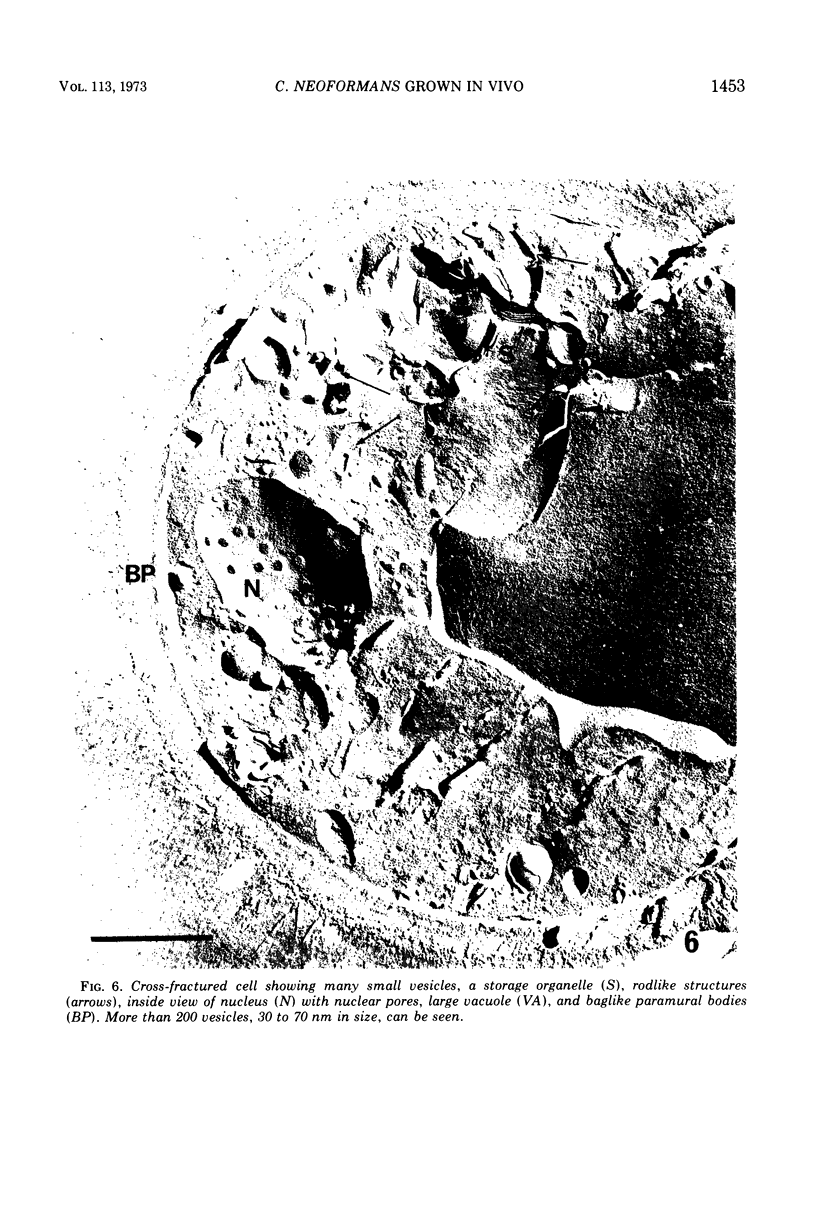
Cryptococcus neoformans grown in the parasitic state was observed by the freeze-etching technique and was compared with that grown on culture media. Unlike other yeasts, this organism grown in vivo is very often devoid of the “ordinary” invaginations. The membrane of the cell grown in vivo was almost free from concavity and convexity except for many round depressions which represent the surface view of paramural bodies. Some of the paramural bodies were found to be multivesicular systems. Most were spherical invaginations containing a single vesicle or its ghost remaining after secretion of the vesicles. In clear contrast to the cell grown in vitro, the in vivo cell contained a great number of vesicles in the cytoplasm. These seemed to show high-secretion activity in C. neoformans grown in the parasitic state. On transfer from in vitro to in vivo, this organism enlarged the cell wall, capsule, and cell body. The appearance of a large vacuole, accumulation of storage organelles, and the existence of rodlike structures, seemingly lipid deposits, were also noted in the cytoplasm of the cell grown in vivo. the meaning of these results as well as the mode of capsular production are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINING P. R., KENNEDY E. R. CHARACTERISTICS OF A STRAIN OF STAPHYLOCOCCUS AUREUS GROWN IN VIVO AND IN VITRO. J Bacteriol. 1963 Apr;85:732–741. doi: 10.1128/jb.85.4.732-741.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH H., SEGAL W. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J Bacteriol. 1956 Aug;72(2):132–141. doi: 10.1128/jb.72.2.132-141.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADMUS M. C., LAGODA A. A., ANDERSON R. F. Production of a new polysaccharide with Cryptococcus laurentii var. flavescens. Appl Microbiol. 1962 Mar;10:153–156. doi: 10.1128/am.10.2.153-156.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R., Gordon M. A., Lapa E. W., Ghiorse W. C. Micromorphology of Cryptococcus neoformans. J Bacteriol. 1967 Sep;94(3):766–777. doi: 10.1128/jb.94.3.766-777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELLENBECK S. M. Aerobic respiratory metabolism of Staphylococcus aureus from an infected animal. J Bacteriol. 1962 Mar;83:450–455. doi: 10.1128/jb.83.3.450-455.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y. F. Preliminary observations on the phosphatases of Cryptococcus neoformans: a histochemical study. Chin Med J. 1959 Aug;79:138–142. [PubMed] [Google Scholar]
- Matile P., Jost M., Moor H. Intrazelluläre Lokalisation proteolytischer Enzyme von Neurospora crassa. Z Zellforsch Mikrosk Anat. 1965 Oct 12;68(2):205–216. [PubMed] [Google Scholar]
- Northcote D. H. Fine structure of cytoplasm in relation to synthesis and secretion in plant cells. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):21–30. doi: 10.1098/rspb.1969.0033. [DOI] [PubMed] [Google Scholar]
- Steele B., Boman H. G. Capsular material and morphology of some ampicillin sensitive and resistant strains of Escherichia coli K12. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(1):59–74. doi: 10.1111/j.1699-0463.1970.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Takeo K., Uesaka I., Uehira K., Nishiura M. Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. J Bacteriol. 1973 Mar;113(3):1442–1448. doi: 10.1128/jb.113.3.1442-1448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDerWoude W. J., Morré D. J., Bracker C. E. Isolation and characterization of secretory vesicles in germinated pollen of Lilium longiflorum. J Cell Sci. 1971 Mar;8(2):331–351. doi: 10.1242/jcs.8.2.331. [DOI] [PubMed] [Google Scholar]