Abstract
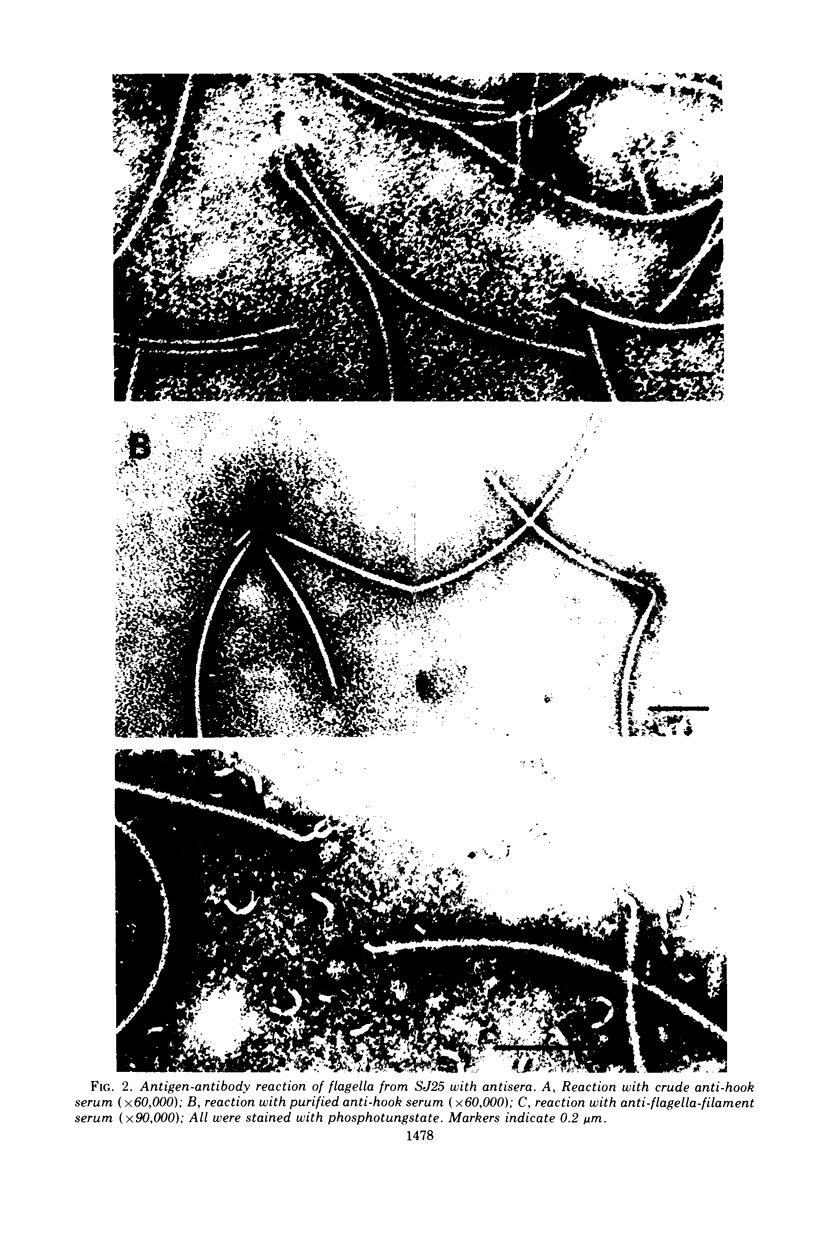
Bacterial hooks were partially purified from flagella isolated from Salmonella SJ25, by treatment with heat to depolymerize flagellar filaments and with n-butanol and calcium chloride to remove membranes. Antihook serum was obtained from a rabbit inoculated with a preparation of hooks. The serum contained antibodies directed against the flagellar filament and cell membrane. These antibodies could be removed from the serum by absorption with purified flagellar filaments and cells of a nonflagellated mutant strain. It was shown by electron microscopy that anti-SJ25-hook antibody reacts with hooks from a number of strains of Salmonella which differed from SJ25 in H and O antigens, flagellar shape, and motility. Hooks possessed by various strains of Salmonella have a common antigenicity. In addition, anti-SJ25-hook cross-reacted with hooks from Escherichia coli W3110 but did not react at all which those from strains of Serratia, Proteus, Aerobacter, and Klebsiella. It is well known that bacteria stop moving upon addition of antiflagella serum to the medium. However, the addition of purified antihook was found to have little effect on motility. At physiological ionic strength and pH, flagellin (Salmonella) can polymerize into flagellar filaments only in the presence of seeds. It was shown that a crude preparation of hooks was able to initiate in vitro polymerization of flagellin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASAKURA S., EGUCHI G., IINO T. RECONSTITUTION OF BACTERIAL FLAGELLA IN VITRO. J Mol Biol. 1964 Oct;10:42–56. doi: 10.1016/s0022-2836(64)80026-7. [DOI] [PubMed] [Google Scholar]
- Abram D., Mitchen J. R., Koffler H., Vatter A. E. Differentiation within the bacterial flagellum and isolation of the proximal hook. J Bacteriol. 1970 Jan;101(1):250–261. doi: 10.1128/jb.101.1.250-261.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura S., Eguchi G., Iino T. Unidirectional growth of Salmonella flagella in vitro. J Mol Biol. 1968 Jul 14;35(1):227–236. doi: 10.1016/s0022-2836(68)80050-6. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Fine structure and isolation of the hook-basal body complex of flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmitt K., Simon M. I. Purification and partial characterization of Bacillus subtilis Flagellar hooks. J Bacteriol. 1971 Oct;108(1):282–286. doi: 10.1128/jb.108.1.282-286.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmitt K., Simon M. Antigenic nature of bacterial flagellar hook structures. Infect Immun. 1970 Feb;1(2):212–213. doi: 10.1128/iai.1.2.212-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmitt K., Simon M. Purification and thermal stability of intact Bacillus subtilis flagella. J Bacteriol. 1971 Jan;105(1):369–375. doi: 10.1128/jb.105.1.369-375.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Tokuyasu K., Simon M. I. Bacterial flagella: polarity of elongation. Science. 1970 Jul 10;169(3941):190–192. doi: 10.1126/science.169.3941.190. [DOI] [PubMed] [Google Scholar]
- Enomoto M. Genetic studies of paralyzed mutant in Salmonella. I. Genetic fine structure of the mot loci in Salmonella typhimurium. Genetics. 1966 Sep;54(3):715–726. doi: 10.1093/genetics/54.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Rownd R., Baron L. S. GENETIC HOMOLOGY BETWEEN ESCHERICHIA COLI K-12 AND SALMONELLA. J Bacteriol. 1962 Dec;84(6):1303–1312. doi: 10.1128/jb.84.6.1303-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T., Mitani M. A mutant of Salmonella possessing straight flagella. J Gen Microbiol. 1967 Oct;49(1):81–88. doi: 10.1099/00221287-49-1-81. [DOI] [PubMed] [Google Scholar]
- Iino T., Mitani M. Flagella-shape mutants in Salmonella. J Gen Microbiol. 1966 Jul;44(1):27–40. doi: 10.1099/00221287-44-1-27. [DOI] [PubMed] [Google Scholar]
- Iino T. Polarity of flagellar growth in salmonella. J Gen Microbiol. 1969 May;56(2):227–239. doi: 10.1099/00221287-56-2-227. [DOI] [PubMed] [Google Scholar]
- Iino T., Suzuki H., Yamaguchi S. Reconstruction of Salmonella flagella attached to cell bodies. Nat New Biol. 1972 Jun 21;237(77):238–240. doi: 10.1038/newbio237238a0. [DOI] [PubMed] [Google Scholar]
- Lawn A. M. Simple immunological labelling method for electron microscopy and its application to the study of filamentous appendages of bacteria. Nature. 1967 Jun 10;214(5093):1151–1152. doi: 10.1038/2141151a0. [DOI] [PubMed] [Google Scholar]
- Ota T., Kimura M. On the constancy of the evolutionary rate of cistrons. J Mol Evol. 1971;1(1):18–25. doi: 10.1007/BF01659391. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Iino T., Kuroiwa T. Possession of flagellar hooks by some non-flagellate mutants of Salmonella abortusequi. J Gen Microbiol. 1972 Apr;70(2):299–303. doi: 10.1099/00221287-70-2-299. [DOI] [PubMed] [Google Scholar]







