SUMMARY
The endoplasmic reticulum (ER) stress response, also known as the unfolded protein response (UPR), has been implicated in the normal physiology of the immune defense and in several disorders including diabetes, cancer, and neurodegenerative disease. Here we show that the apoptotic receptor CED-1 and a network of PQN/ABU proteins involved in a non-canonical UPR response are required for proper defense to pathogen infection in Caenorhabditis elegans. A full-genome microarray analysis indicates that CED-1 functions to activate the expression of pqn/abu genes. We also show that ced-1 and pqn/abu genes are required for survival of C. elegans exposed to live S. enterica and that overexpression of pqn/abu genes confers protection to pathogen-mediated killing. The results indicate that unfolded protein response genes, regulated in a CED-1-dependent manner, are involved in the C. elegans immune response to live bacteria.
50-word summary
Microbial infections are controlled by a range of immune effectors whose upregulation upon pathogen exposure may overwhelm the protein folding capacity of the endoplasmic reticulum. Haskins et al. show that the increased demand on protein folding in the endoplasmic reticulum during bacterial infections requires unfolded protein response genes that are transcriptionally regulated by the apoptotic receptor CED-1.
Keywords: CED-1, unfolded protein response, programmed cell death, C. elegans, innate immunity
INTRODUCTION
The first line of defense against pathogens is the phylogenetically ancient innate immune system. Activation of the innate immune system upon pathogen recognition triggers intracellular signals that result in a rapid and definitive microbicidal response to invading microorganisms (Akira et al., 2006). Another key aspect of the metazoan response to pathogen infection is the activation of a primitive apoptotic genetic program (Yuan, 2006). Interestingly, the microbicidal and apoptotic processes may be highly related since the acute endoplasmic reticulum (ER) stress induced by the immune response leads to an unfolded protein response (UPR) or apoptosis when ER function cannot be restored (Hoyer-Hansen and Jaattela, 2007).
The nematode Caenorhabditis elegans, which lives in the soil where it is in contact with soil-borne microbes, has evolved mechanisms to recognize different pathogens and to respond accordingly. C. elegans defense against pathogen infections requires interacting pathways that control stress response, aging, and immunity (Garsin et al., 2003; Kim et al., 2002; Singh and Aballay, 2006b). These pathways regulate the expression of a wide variety of genes including those encoding conserved immune effectors such as antimicrobial peptides, lectins, and lysozymes (Kerry et al., 2006; Mallo et al., 2002; O’Rourke et al., 2006; Shapira et al., 2006; Troemel et al., 2006). The C. elegans response to pathogen infection also involves an apoptotic pathway (Aballay and Ausubel, 2001; Aballay et al., 2003). Using a set of C. elegans mutants in which apoptosis is blocked, it was shown that infection by the human pathogen Salmonella enterica results in the activation of germline cell death which is dependent on the well-characterized CED-9/CED-4/CED-3 pathway, homologous to the BCL2/APAF-1/CASPASE pathway in mammals. Moreover, ced-3(lf) and ced-4(lf) mutants were found to be hypersensitive to S. enterica-mediated killing, suggesting that the apoptotic pathway may be involved in a C. elegans defense response to pathogen attack (Aballay and Ausubel, 2001). In addition, taking advantage of both host and pathogen mutants, it was shown that S. enterica lipopolysaccharide acts as a pathogen-associated molecular pattern that triggers programmed cell death in C. elegans (Aballay et al., 2003). Similar to the homologous pathway in mammals, the pathogen-induced CED-3 pathway in C. elegans appears to lie downstream of a PMK-1/P38 MAPK signaling pathway (Aballay et al., 2003).
In addition to the CED pathway that controls apoptosis, two other converging CED pathways (Chung et al., 2000; Ellis et al., 1991; Gumienny et al., 2001; Henson, 2005; Kinchen et al., 2005) are known to control the process of the engulfment of dying cells and to promote apoptosis (Hoeppner et al., 2001; Reddien et al., 2001). With the exception of CED-1 and CED-7, the remaining components of the engulfment pathways appear to act intracellularly (Gumienny et al., 2001; Liu and Hengartner, 1998; Reddien and Horvitz, 2000; Wu and Horvitz, 1998; Wu et al., 2001; Yu et al., 2006; Zhou et al., 2001a). CED-7, an ABC transporter and homologue of ABCA1, is essential for the recognition of cell corpses by CED-1 (Zhou et al., 2001b). CED-1, a phagocytic receptor that recognizes cell corpses and initiates their engulfment (Zhou et al., 2001b), is a single-pass transmembrane receptor that contains various extracellular EGF-repeats and an intracellular candidate signaling domain.
Here we examine the potential role of CED-1 in C. elegans immunity. We found that loss-of-function ced-1 mutants are immunocompromised animals and are rapidly killed by live bacteria. Full-genome expression analyses demonstrated that CED-1 upregulates a family of genes encoding proteins with prion-like glutamine/asparagine (Q/N)-rich domains which are known to be activated by ER stress and thought to aid in the unfolded protein response (Urano et al., 2002). When expression of these genes was abrogated, the animals exhibited wild-type lifespan when exposed to dead bacteria but showed reduced lifespan when exposed to live bacteria. These studies indicate that CED-1 is required for the transcriptional activation of an unfolded protein response pathway required for proper response to bacterial infections.
RESULTS
ced-1 Loss-of-function Mutants are Immunocompromised Animals and are Rapidly Killed by Live Bacteria
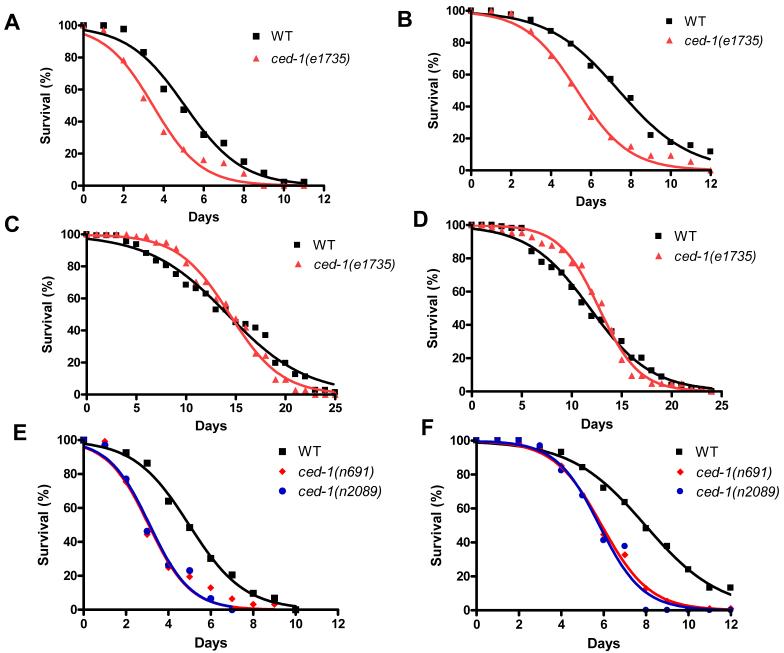
To study the role of CED-1 in C. elegans defense response, we first examined whether ced-1(e1735) mutants were susceptible to S. enterica-mediated killing. As shown in Figure 1A, ced-1(e1735) loss-of-function mutants (Hedgecock et al., 1983; Zhou et al., 2001b) died more quickly than wild-type animals when feeding on S. enterica strain 1344. The time for 50% of the nematodes to die (TD50) when fed at 25°C on live S. enterica was 5.09 ± 0.17 days for wild-type animals compared to 3.47 ± 0.31 days for ced-1(e1735) mutants, which represents a reduction of 32%. ced-1(e1735) animals also exhibited a 27% reduced lifespan when grown on live E. coli strain OP50 (Figure 1B). The short lifespan exhibited by ced-1(e1735) animals fed live E. coli is consistent with the observations that proliferating E. coli is a cause of death in C. elegans (Garigan et al., 2002), E. coli grown on rich media kills C. elegans (Garsin et al., 2001), and immunocompromised animals are killed and persistently colonized by E. coli (Kerry et al., 2006; Singh and Aballay, 2006b; Tenor and Aballay, 2008). To ensure that the difference in mortality between ced-1(e1735) and wild-type animals was not caused by a reduction in the overall health of the mutant, we exposed ced-1(e1735) and wild-type animals to both heat-killed S. enterica and heat-killed E. coli. When ced-1(e1735) mutants were grown on dead S. enterica or dead E. coli, they exhibited a lifespan comparable to that of wild-type animals (Figures 1C and 1D). Thus, ced-1(e1735) mutants are killed by live but not dead bacteria, indicating that they are immunocompromised.
Figure 1.
ced-1 Loss-of-function Mutants are Immunocompromised Animals Killed by Live Bacteria
(A) Wild-type and ced-1(e1735) animals were exposed to live S. enterica: ced-1(e1735) P < 0.0001.
(B) Wild-type and ced-1(e1735) animals were exposed to live E. coli: ced-1(e1735) P < 0.0001.
(C) Wild-type and ced-1(e1735) animals were exposed to heat-killed S. enterica: ced-1(e1735) P > 0.1.
(D) Wild-type and ced-1(e1735) animals were exposed to heat-killed E. coli: ced-1(e1735) P > 0.1.
(E) Wild-type, ced-1(n691) and ced-1(n2089) animals were exposed to live S. enterica: ced-1(n691) P < 0.0001, ced-1(n2089) P < 0.0001.
(F) Wild-type, ced-1(n691), and ced-1(n2089) animals were exposed to live E. coli: ced-1(n691) P < 0.0001, ced-1(n2089) P < 0.0001.
For each condition 110-140 animals were used, with the exception of ced-1(n2089) where 35 animals were used. P values are relative to wild-type animals.
We confirmed that CED-1 is required for C. elegans survival on live bacteria by exposing two additional ced-1 mutants to S. enterica and E. coli and comparing their lifespan to that of wild-type animals (Figures 1E and 1F). The enhanced susceptibility to live S. enterica and live E. coli of ced-1(n691) and ced-1(n2089) mutants, which carry frameshift and missense mutations respectively (Zhou et al., 2001b), confirms that CED-1 is required for the defense response to live bacteria. Furthermore, these results make it unlikely that the enhanced susceptibility of ced-1 animals is caused by secondary mutations or the effect of a particular allele on a process unrelated to CED-1 function.
CED-1 Regulates pqn/abu Unfolded Protein Response Genes
To determine the mechanism underlying CED-1-mediated defense to live bacteria, we utilized Affymetrix GeneChip C. elegans Genome Arrays and hierarchical clustering to find clusters of genes commonly upregulated or downregulated in ced-1(e1735) mutants relative to wild-type animals grown on live E. coli. Hierarchical clustering identified a family of 10 pqn/abu genes in a 17 gene cluster of transcripts which were similarly downregulated in ced-1(e1735) mutants (Table 1).
Table 1. Cluster of C. elegans CED-1 regulated genes.
| ORF Name | Gene Name | Expression in Wild-type (WT) | Expression in ced-1 (lf) Mutant (C) | Fold Difference WT/C |
|---|---|---|---|---|
| D2096.6 | D2096.6 | 1492.22 ± 149.65 | 81.67 ± 35.23 | 18.27 |
| ZK1067.7 | pqn-95 | 4087.87 ± 316.15 | 172.50 ± 62.85 | 23.70 |
| F35A5.3 | abu-10 | 1248.88 ± 371.91 | 62.96 ± 3.86 | 21.44 |
| AC3.3/AC3.4 | abu-1/pqn-2 | 1655.47 ± 331.13 | 60.20 ± 16.15 | 27.49 |
| W02A2.3 | pqn-74 | 2374.52 ± 483.42 | 89.93 ± 17.52 | 26.40 |
| C03A7.8/C03A7.14 | abu-7/abu-8 | 4137.28 ± 1144.27 | 101.25 ± 41.58 | 40.86 |
| C03A7.7 | abu-6 | 3501.07 ± 974.25 | 87.78 ± 44.75 | 39.89 |
| F41E6.11 | F41E6.11 | 1118.84 ± 168.85 | 71.95 ± 1.48 | 15.55 |
| W08E12.4/W08E12.5 | W08E12.4/W08E12.5 | 2934.21 ± 313.82 | 238.26 ± 245.54 | 12.32 |
| Y47D3B.6 | Y47D3B.6 | 2602.44 ± 411.67 | 107.46 ± 18.78 | 24.22 |
| R09B5.5 | pqn-54 | 1145.01 ± 316.23 | 41.05 ± 1.96 | 27.89 |
| T01D1.6 | abu-11 | 1372.46 ± 273.74 | 65.21 ± 9.69 | 21.05 |
| T05B4.3 | phat-4 | 1654.16 ± 140.24 | 68.66 ± 0.14 | 24.10 |
| F20B10.3 | F20B10.3 | 632.81 ± 132.49 | 46.21 ± 11.39 | 13.69 |
| ZK662.2 | ZK662.2 | 2173.22 ± 170.18 | 123.65 ± 46.22 | 17.58 |
| C03A7.14 | abu-8 | 4242.83 ± 1060.59 | 89.41 ± 36.81 | 47.45 |
| C03A7.4 | pqn-5 | 4765.04 ± 906.42 | 85.26 ± 43.12 | 55.89 |
Shown are the mean ± error of expression levels in wild-type (n = 3) and ced-1(e1735) (n = 2) animals. Eggs were placed in S basal to hatch overnight, causing growth arrest in L1. Synchronized L1 wild-type and ced-1(e1735) animals were grown on E. coli at 25°C for 26 hours and RNA was isolated. Expression data were normalized and cluster of CED-1 regulated genes was performed as described in Experimental Procedures.
The pqn (prion-like glutamine[Q]/asparagine[N]-rich domain-bearing protein) genes constitute a 79-member family characterized by prion-like glutamine/asparagine-rich amino acid sequences. Eleven genes in the pqn family have been further classified as abu (activated in blocked unfolded protein response) (Urano et al., 2002). The abu genes were found to be upregulated upon ER stress in an xbp-1 mutant, which is defective in the canonical UPR (Urano et al., 2002). In addition, the abu genes are believed to encode UPR proteins, either functioning in a pathway parallel to the canonical UPR or in the ER associated degradation of misfolded proteins (Urano et al., 2002).
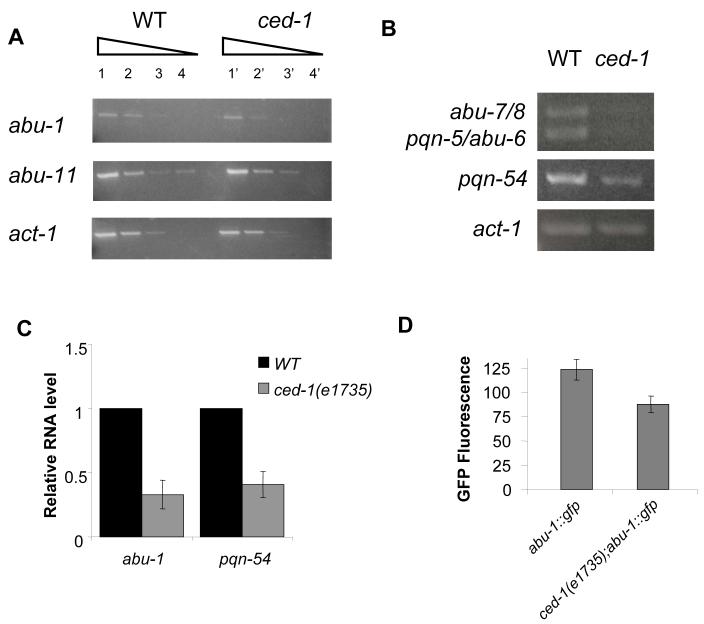
Further studies revealed that nine of the pqn/abu genes residing in the CED-1-regulated cluster are also grouped together in mountain 29 of the C. elegans three-dimensional topographical expression map (Kim et al., 2001). Since this gene expression map correlates gene regulation among different growth conditions, developmental stages, and mutant backgrounds, pqn/abu genes in mountain 29 have a high probability to be regulated by CED-1. In addition, we confirmed the microarray results showing that pqn/abu genes are downregulated in ced-1(e1735) mutants by performing reverse transcription polymerase chain reaction (RT-PCR) analysis on abu-1, abu-6, abu-7, abu-8, abu-11, pqn-5, and pqn-54, all of which are likely to be co-regulated as they are part of mountain 29 (Figures 2A and 2B). We also performed quantitative RT-PCR (qRT-PCR) to provide another independent confirmation that pqn/abu genes are downregulated in ced-1(e1735) animals. Figure 2C shows that abu-1 and pqn-54 are downregulated 3.3 and 2.6 fold, respectively, in ced-1(e1735) mutants. Even though the degree of misregulation of abu/pqn genes observed by RT-PCR and qRT-PCR (Figures 2A, 2B, and 2C) is lower than that seen in the microarrays, the results confirm the CED-1 requirement for proper pqn/abu gene expression. The requirement of CED-1 for proper abu gene expression was further confirmed by comparing the GFP intensities of abu-1::gfp(ZcEx8) and ced-1(e1735);abu-1::gfp(ZcEx8) animals (Figure 2D). The results shown in Figure 2 confirm that CED-1 is required for the proper expression of pqn/abu genes and suggest that these UPR genes may be required for C. elegans survival on live bacteria. The transcriptional profiling of wild-type and ced-1(e1735) animals grown on live S. enterica also indicated that pqn/abu genes are upregulated by CED-1 (Table S1), suggesting that pqn/abu genes may be involved in CED-1-mediated protection against potentially pathogenic bacteria.
Figure 2.
CED-1 Regulates pqn/abu Unfolded Protein Response Genes
(A) Wild-type (lanes 1-4) and ced-1(e1735) (lanes 1′-4′) cDNAs were stepwise 10-fold serially diluted. PCR was performed using gene specific primers and expression levels of act-1, a housekeeping gene, was used to confirm cDNA equalization.
(B) Wild-type and ced-1(e1735) cDNAs were 10-fold serially diluted and the 1:1,000 dilutions were used. PCR was performed using gene specific primers and expression levels of act-1, a housekeeping gene, was used to confirm cDNA equalization. abu-7 and abu-8 mRNA are 93.4% identical and abu-6 and abu-7 mRNA are 98.3% identical; although one primer set was used to amplify all four transcripts, the two groups could be differentiated by size. (A-B) L1 stage animals fed E. coli were grown to L4 stage. RNA was then isolated, RT-PCR was performed, and PCR products were run on a gel and stained with ethidium bromide. RT-PCR was performed in duplicate from independent RNA isolations, and similar results were achieved.
(C) Quantitative reverse transcription-PCR analysis of abu-1 and pqn-54 expression in ced-1(e1735) relative to wild-type nematodes grown on E. coli to L4 stage. Data were analyzed by relative quantitation using the comparative cycle threshold method and normalization to actin. One sample Student’s exact t-test indicates that differences between wild type and ced-1(e1735) are significantly different (P<0.05); n=3; bars correspond to mean±s.d.
(D) GFP expression in a standard defined area encompassing the entire pharynx of L4 stage abu-1::gfp(zcEx8) animals and ced-1(e1735);abu-1::gfp(zcEx8) animals was analyzed using max green channel intensity calculated by ImageJ 1.37v freeware. Student’s exact t-test indicates that differences between abu-1::gfp(zcEx8) and ced-1(e1735);abu-1::gfp(zcEx8) are significantly different (P<0.05); n=15; bars correspond to mean±s.d.
pqn/abu Genes Expressed in a CED-1-dependent Manner are Required for C. elegans Immunity to Live Bacteria
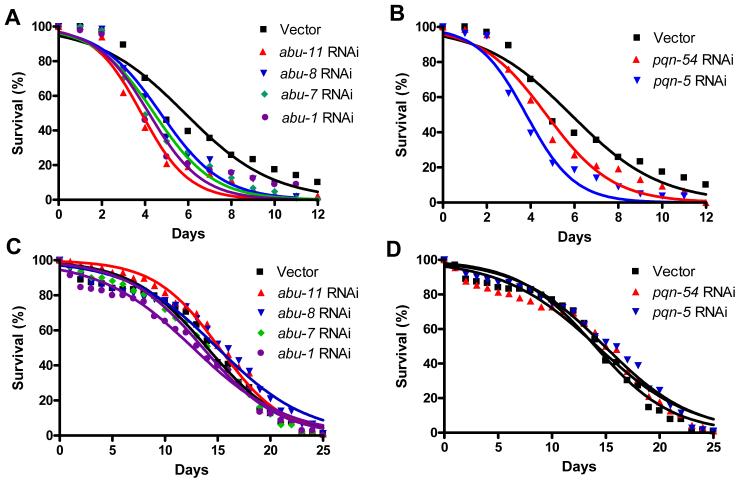
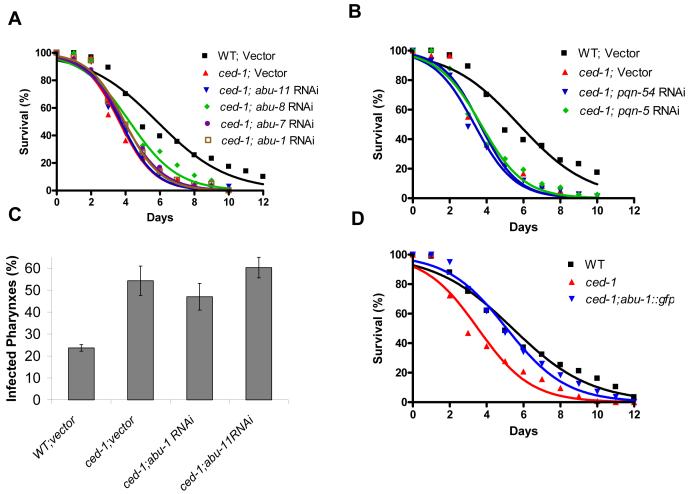
To test the hypothesis that pqn/abu genes function in the C. elegans immunity to pathogenic bacteria, we first compared S. enterica killing of wild-type nematodes to that of nematodes in which abu-1/7/8/11 and pqn-5/54 gene expression was abrogated by RNAi. As shown in Figures 3A and 3B, pqn/abu RNAi increased nematode susceptibility to S. enterica-mediated killing. Importantly, RNAi reduction of pqn/abu gene expression did not affect nematode lifespan on killed S. enterica (Figures 3C and 3D); similar results were obtained using live and killed E. coli (Figure S1). In mutant animals in ced-1 or in any of the genes involved in apoptotic corpse clearance, several dying cells are not engulfed and remain as cell corpses in the gonads. As shown in Figure S2, no significant differences were found in the number of apoptotic corpses among control, abu-1 RNAi, and abu-11 RNAi animals after 24 hours exposure to either E. coli or S. enterica. Taken together, these results indicate that these pqn/abu genes are required for proper C. elegans immunity to pathogenic bacteria without affecting ced-1 functions related to apoptotic corpse removal.
Figure 3.
pqn/abu Genes Expressed in a CED-1-dependent Manner are Required for C. elegans Immunity
(A) Wild-type animals grown on dsRNA for vector control or dsRNA for abu genes were exposed to live S. enterica: abu-11 RNAi P < 0.0001, abu-8 RNAi P = 0.0032, abu-7 RNAi P < 0.0001, abu-1 RNAi P = 0.0459.
(B) Wild-type animals grown on dsRNA for vector control or dsRNA for pqn genes were exposed to live S. enterica: pqn-54 RNAi P = 0.0056, pqn-5 RNAi P < 0.0001.
(C) Wild-type animals grown on dsRNA for vector control or dsRNA for abu genes were exposed to heat-killed S. enterica: abu-11 RNAi P > 0.1, abu-8 RNAi P > 0.1, abu-7 RNAi P > 0.1, abu-1 RNAi P > 0.1.
(D) Wild-type animals grown on dsRNA for vector control or dsRNA for pqn candidate genes were exposed to heat-killed S. enterica: pqn-54 RNAi P > 0.1, pqn-5 RNAi P > 0.1.
For each condition, 90-140 animals were used. P values are relative to wild-type animals fed dsRNA for vector control.
Given the high sequence similarity among pqn/abu genes, the occurrence of cross-RNAi was likely. To identify potential off-target cross-reactions, the BLAT algorithm was used (Kent, 2002). In C. elegans, cross-RNAi is known to occur when a target mRNA shares at least 95% identity, over a span of 40 or more nucleotides, to the dsRNA encoded by the RNAi construct (Rual et al., 2007). Indeed, the abu-1, abu-7, abu-8, pqn-5 and pqn-54 RNAi constructs appear to simultaneously target as many as 17 other pqn/abu genes (Table S2). Our analysis revealed no potential off-target cross-reactions for the abu-11 RNAi construct (Table S2). However, based on the sequence similarity among pqn/abu genes, we cannot rule out the possibility that abu-11 RNAi caused cross-RNAi effects which our BLAT analysis was not capable of predicting. On the other hand, since several PQN and ABU proteins may interact (Li et al., 2004), it is possible that they function in a cooperative network that requires each member to be properly expressed, such that perturbing expression of a single member causes the function of the cooperative network to be impaired.
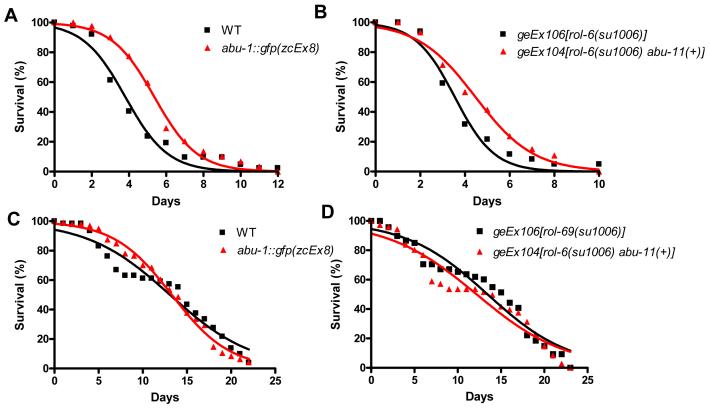
If a network of PQN/ABU proteins, involved in the UPR, is required for proper immunity to live bacteria and downregulation of a single member of the network can impair function, then the overexpression of a single member may enhance the function of the network. To test this, we next investigated whether the overexpression of ABU proteins confers resistance to pathogenic bacteria. Specifically, we compared S. enterica-mediated killing of control animals and animals overexpressing ABU-1 or ABU-11. Consistent with the idea that UPR proteins protect C. elegans from bacterial infection, animals overexpressing ABU-1 or ABU-11 were significantly more resistant to live S. enterica than control animals (Figures 4A and 4B). However, when fed heat-killed S. enterica, all transgenic animals overexpressing ABU proteins exhibited a lifespan comparable to that of control animals (Figures 4 C and 4D). Taken together, these results indicate that the PQN/ABU proteins are required for C. elegans immunity to live bacteria and that they may be important components of the CED-1-mediated immune response.
Figure 4.
Overexpression of abu-1 or abu-11 Extends C. elegans Lifespan on Live Bacteria
(A) Wild-type and abu-1::gfp(zcEx8) animals were exposed to live S. enterica: abu-1::gfp(zcEx8) P = 0.0005.
(B) geEx106[rol-6(su1006)] and geEx104[rol-6(su1006) abu-11(+)] animals were exposed to live S. enterica: geEx104[rol-6(su1006) abu-11(+)] P = 0.0373.
(C) Wild-type and abu-1::gfp(zcEx8) animals were exposed to heat-killed S. enterica: abu-1::gfp(zcEx8) P > 0.1.
(D) geEx106[rol-6(su1006)] and geEx104[rol-6(su1006) abu-11(+)] animals were exposed to heat-killed S. enterica: geEx104[rol-6(su1006) abu-11(+)] P > 0.1.
For each condition, 60-105 animals were used. P values are relative to control animals.
CED-1 and a Network of UPR Proteins are Part of a Pathway that Prevents S. enterica Invasion of Pharyngeal Tissue
The results described above implicate CED-1 in the regulation of the expression of genes encoding PQN/ABU proteins required for proper immune response to live bacteria. Interestingly, various pqn/abu genes have a reported strong expression in the pharynx (Urano et al., 2002), which constitutes one of the first physiological barriers against pathogens in C. elegans. In addition, it has recently been reported that the pharyngeal tissue can be invaded by S. enterica (Tenor and Aballay, 2008). More specifically, animals lacking TOL-1-mediated immunity exhibited a significant pharyngeal invasion, which is not observed in other immunocompromised animals such as pmk-1(km25) and dbl-1(nk3) mutants (Tenor and Aballay, 2008). Thus, we sought to determine whether CED-1 and the network of PQN/ABU proteins described here also play a role in preventing S. enterica invasion of the pharynx.
We examined the profile of bacterial accumulation in the pharyngeal tissue by feeding nematodes S. enterica expressing green fluorescent protein (GFP) and following the accumulation of bacteria by direct observation under the fluorescence microscope as described (Aballay et al., 2000; Tenor and Aballay, 2008). As shown in Figure 5, ced-1(e1735) nematodes exhibited significantly more pharyngeal invasion than that observed in wild-type nematodes (Figure 5G and comparison of Figures 5B and 5D), and intact S. enterica cells are observed in the pharyngeal tissue where abu-1 is known to be expressed (Urano et al., 2002) (Figures 5E, 5F, S3). By 48 hours, over 50% of the ced-1(e1735) nematodes exhibited infected pharynxes (Figure 5G). The S. enterica invasion does not appear to be a consequence of pharyngeal defects in ced-1(e1735) animals, since the pumping rates of ced-1(e1735) and wild-type animals are not perceptibly different and there is no visible S. enterica invasion during early infection (data not shown). Consistent with the idea that a lack of CED-1 does not affect the general physiology of the pharynx,the expression levels of pharyngeal genes in ced-1(e1735) animals are not significantly different from expression levels observed in wild-type animals (Table S3). In addition, the pharyngeal invasion of ced-1(e1735) animals is comparable to that of abu-1 RNAi and abu-11 RNAi animals, which greatly contrasts to the limited pharyngeal invasion observed in wild-type nematodes grown on control RNAi plates (Figure 5G). The increased pharyngeal invasion of S. enterica observed in ced-1(e1735) and abu RNAi nematodes is distinct since it is observed in tol-1(nr2033) mutants but not in other immunocompromised animals such as pmk-1(km25) and dbl-1(nk3) mutants (Tenor and Aballay, 2008). In addition, no other ced engulfment mutant displays increased S. enterica pharyngeal invasion compared to wild-type (Figure S4), suggesting that the engulfment function of CED-1 is independent of the immune function. Consistent with this idea, we have not observed CED-1-mediated phagocytosis of S. enterica (Aballay, unpublished observation).
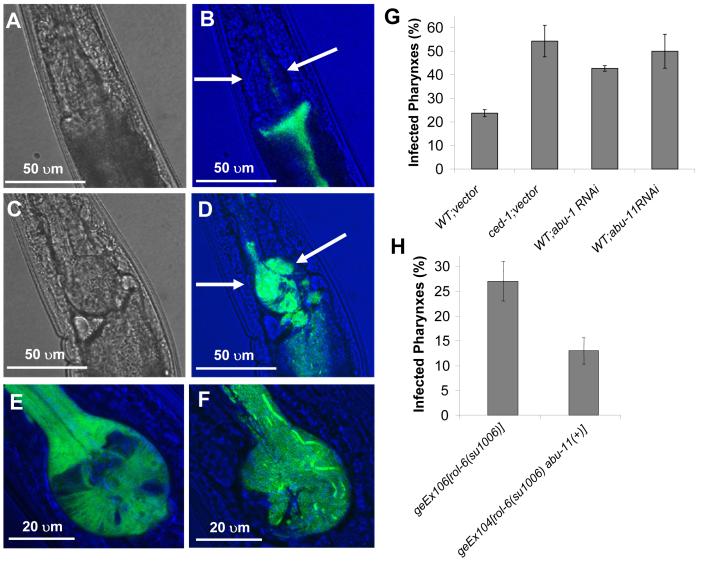
Figure 5.
pqn/abu Genes Expressed in a CED-1-dependent Manner are Required for C. elegans Defense to Pharyngeal Invasion by S. enterica
(A-D) Confocal images show the pharynx of wild-type (A-B) and ced-1(e1735) (C-D) animals infected for 48 hours with S. enterica expressing GFP. In the merged images (B and D), the terminal bulb of the pharynx is indicated with arrows.
(E) Confocal image of the terminal bulb of an abu-1::gfp(zcEx8) animal showing pharyngeal expression of ABU-1::GFP.
(F) Confocal image of the infected terminal bulb of a ced-1(e1735) animal fed S. enterica expressing GFP for 48 hours.
(G) The percentage of nematodes with infected pharynxes when fed S. enterica expressing GFP for 48 hours was determined for wild-type and ced-1(e1735) animals exposed to dsRNA for vector control and wild-type animals exposed to dsRNA targeting abu-1 and abu-11.
(H) The percentage of nematodes with infected pharynxes when fed S. enterica expressing GFP for 48 hours was determined for geEx106[rol-6(su1006)] and geEx104[rol-6(su1006) abu-11(+)] animals.
Bars correspond to mean±s.d.
Our results suggest that decreased expression of UPR proteins in ced-1(e1735) nematodes facilitates S. enterica invasion of the pharyngeal tissue. To further investigate the potential role of UPR proteins in mediating protection to S. enterica invasion, we studied whether ABU-1 and ABU-11 overexpression can provide protection against S. enterica invasion. We found that, relative to control nematodes, pharyngeal invasion is lower in nematodes overexpressing ABU-11 (Figure 5H). These results suggest that UPR proteins are crucial for protection against S. enterica invasion of the pharyngeal tissue.
The increased susceptibility of pqn/abu RNAi animals, together with the microarray and RT-PCR data, indicate that PQN/ABU proteins are required for the CED-1-mediated protection to bacteria. To confirm that PQN/ABU proteins are part of a CED-1-dependent immunity, we performed S. enterica killing assays using RNAi to abrogate the expression of pqn/abu genes in a ced-1(e1735) background. As would be expected if PQN/ABU proteins and CED-1 are part of the same pathway, we observed no additive effect of pqn/abu RNAi ablation on ced-1(e1735) mutants (Figures 6A and 6B). Furthermore, we did not observe any increased pharyngeal invasion when abu-1 and abu-11 expression was abrogated by RNAi in a ced-1(e1735) background (Figure 6C); abu-1 overexpression rescues the enhanced susceptibility to S. enterica of ced-1(e1735) mutants (Figure 6D). Taken together, these results support the hypothesis that UPR proteins are part of a CED-1-dependent immunity to live bacteria.
Figure 6.
CED-1 and PQN/ABU Unfolded Protein Response Proteins are Part of a Pathway Required for Innate Immunity to S. enterica
(A) Wild-type animals grown on dsRNA for vector control and ced-1(e1735) animals grown on dsRNA for vector control or dsRNA for abu genes were exposed to live S. enterica: wild type;vector P < 0.0001, ced-1;abu-11 RNAi P > 0.1, ced-1;abu-8 RNAi P > 0.1, ced-1;abu-7 RNAi P > 0.1, ced-1;abu-1 RNAi P > 0.1. 74-140 animals were used. P values are relative to ced-1(e1735) animals fed dsRNA for vector control.
(B) Wild-type animals grown on dsRNA for vector control and ced-1(e1735) animals grown on dsRNA for vector control or dsRNA for pqn genes were exposed to live S. enterica: wild-type; vector P < 0.0001, ced-1;pqn-54 RNAi P > 0.1, ced-1;pqn-5 RNAi P > 0.1. 74-140 animals were used. P values are relative to ced-1(e1735) animals fed dsRNA for vector control.
(C) The percentage of nematodes with infected pharynxes when fed S. enterica expressing GFP for 48 hours was determined for wild-type and ced-1(e1735) animals grown on dsRNA for control and ced-1(e1735) animals grown dsRNA targeting abu-1 and abu-11 candidate genes. Bars correspond to mean ± standard deviation.
(D) Wild-type, ced-1(e1735), and ced-1(e1735);abu-1::gfp(zcEx8) animals were exposed to live S. enterica: wild-type P < 0.0001, ced-1(e1735);abu-1::gfp(zcEx8) P > 0.1. 174-179 animals were used. P values are relative to ced-1(e1735) animals.
DISCUSSION
In this study, we have shown that CED-1 is required for C. elegans survival in the presence of live bacteria. Animals lacking CED-1-mediated responses were rapidly killed by the human pathogen S. enterica and by E. coli which, even though it is the food source of nematodes in the laboratory, has been shown to kill immunocompromised animals (Kerry et al., 2006; Singh and Aballay, 2006b; Tenor and Aballay, 2008). However, the survival of ced-1(lf) animals grown on dead S. enterica and dead E. coli was comparable to that of wild-type animals, indicating that a CED-1-mediated mechanism is required for immune response to live but not dead bacteria. Importantly, whole-genome microarray analyses demonstrated that CED-1 regulates transcription of pqn/abu genes, which are part of a non-canonical UPR response in C. elegans (Urano et al., 2002) and are also required for the resveratrol-mediated extension of lifespan on live bacteria (Viswanathan et al., 2005). Furthermore, we found that pqn/abu genes are required for survival of C. elegans exposed to live S. enterica and that their overexpression confers protection against S. enterica-mediated killing and invasion of the pharyngeal tissue. The results indicate that the unfolded protein response, regulated in a CED-1-dependent manner, is critical for a successful immune response to bacteria in C. elegans.
As in mammals, peristalsis, low pH, and antimicrobial substances prevent microbial colonization of the C. elegans intestine. Typically, C. elegans animals are propagated in the laboratory by feeding them E. coli. E. coli is effectively disrupted by the C. elegans pharyngeal grinder and almost no intact bacterial cells can be found in the intestinal lumen. Once in the gut, however, pathogenic bacteria can overcome innate immune responses to proliferate and kill C. elegans. In the case of animals deficient in immune responses, even ordinarily benign E. coli can proliferate in the intestine and eventually kill the animals (Kerry et al., 2006; Singh and Aballay, 2006b; Tenor and Aballay, 2008). In fully immunocompetent animals, bacterial infections are controlled by a range of immune effectors that are upregulated upon pathogen exposure (Alper et al., 2007; Kerry et al., 2006; Mallo et al., 2002; Shapira et al., 2006; Troemel et al., 2006; Wong et al., 2007). Presumably, this upregulation of immune-related proteins requires a system of chaperones that help maintain protein homeostasis during bacterial infections (Singh and Aballay, 2006a; Singh and Aballay, 2006b). Here we show that, in addition to the chaperone system, the increased demand on protein folding in the ER during bacterial infections must be successfully alleviated by the UPR for a complete defense response to be mounted.
In all eukaryotic cells, UPR signaling confers protection to ER stress by expanding the amount of ER in the cell, enhancing the degradation of misfolded proteins, and reducing the synthesis of new proteins (Kaufman, 2002; Lin et al., 2007; Mori, 2000; Ron, 2002). However, when the UPR cannot maintain protein homeostasis due to excessive or long-term ER stress, cells typically die by apoptosis (Nakagawa et al., 2000; Nishitoh et al., 2002). Interestingly, the somatic cells of C. elegans have a fixed lineage or population of cells which do not undergo apoptosis after development. Therefore, the animals must use a non-apoptotic mechanism to deal with the stresses that pathogen infection causes to the somatic cells. Indeed, S. enterica infection has been shown to elicit apoptosis only in the cells of the C. elegans germline (Aballay and Ausubel, 2001; Aballay et al., 2003). This increased germline apoptosis is regulated through the CED-9/CED-4/CED-3 pathway, and mutants in this pathway that are deficient in apoptosis exhibit increased susceptibility to S. enterica-mediated killing (Aballay and Ausubel, 2001). Thus, it is intriguing that an apoptotic receptor such as CED-1 upregulates UPR proteins that prevent apoptosis during ER stress. However, several lines of evidence indicate that the regulation of pqn/abu expression by CED-1 is part of a non-apoptotic function of this receptor. A few years ago, two seminal papers demonstrated that, in addition to its critical role in the engulfment of dying cells, CED-1 functions in the engulfing cells to ensure the apoptotic death of cells undergoing CED-3-mediated apoptosis (Hoeppner et al., 2001; Reddien et al., 2001). These studies highlight a pro-apoptotic function of CED-1 that is only seen in ced-3 backgrounds and therefore should not play any role in S. enterica-elicited apoptosis in the germline of wild-type animals. On the other hand, the increased number of corpses in the germline of ced-1(lf) animals (Gumienny et al., 1999) cannot account for the increased susceptibility to S. enterica as it has been demonstrated that extra corpses does not affect C. elegans survival (Aballay and Ausubel, 2001). In addition, unlike the ced-9/ced-4/ced-3 mutants deficient in apoptosis, ced-1(lf) mutants are hypersusceptible to not just live S. enterica but also live E. coli, which does not elicit apoptosis in the germline. Thus, the observation that ced-1(lf) animals are susceptible to live E. coli indicates that the CED-1-mediated defense is separate from the effects of the CED-9/CED-4/CED-3 pathway in the germline. Our results, however, suggest the possibility of a crosstalk between UPR signaling and CED signaling that will require further investigation to be elucidated.
Not much is known about the regulation of pqn/abu genes. Interestingly, one of the known co-regulators of pqn/abu genes is the NAD+-dependent histone deacetylase SIR-2.1, which represses transcription of various members of this gene family (Viswanathan et al., 2005). Since ABU-1 or ABU-11 overexpression confers protection to S. enterica-mediated killing (Figures 4A and 4B) and ABU-1 overexpression reduces S. enterica invasion of the pharynx (Figure 5H), sir-2.1(lf) mutants would be expected to be more resistant to S. enterica-mediated killing. However, sir-2.1(lf) animals do not exhibit increased resistance to live S. enterica, and there is a trend toward increased susceptibility to S. enterica in sir-2.1(lf) animals (P = 0.09) (Figure S5). This result is consistent with the observation that sir-2.1(lf) animals do not exhibit an extended lifespan when grown in the presence of live E. coli (Viswanathan et al., 2005) and with the fact that sir-2.1 promotes daf-16 activity, which is required for proper innate immunity to S. enterica (Singh and Aballay, 2006b). Thus, the potential benefits of pqn/abu overexpression in sir-2.1(lf) animals appear to be compensated by a reduced daf-16 activity.
The UPR mediated by pqn/abu gene products is independent of the canonical UPR mediated by the conserved HAC1-like transcription factor XBP-1 (Urano et al., 2002), making it unlikely that XBP-1 is involved in the CED-1-mediated upregulation of pqn/abu genes. Consistent with the idea that CED-1 specifically upregulates the expression of pqn/abu genes without affecting other genes involved in UPR, we did not observe overlap between the genes regulated by XBP-1 or IRE-1 (Shen et al., 2005) and the CED-1-regulated genes (data not shown). Given the importance of the CED-1-dependent UPR in immunity to live bacteria, it would be interesting to study the role of the canonical UPR pathway in immunity.
In summary, our results provide evidence that the stress caused by bacterial infections is likely met by the CED-1-mediated upregulation of pqn/abu genes involved in the unfolded protein response. Our findings indicating that overexpression of pqn/abu genes enhances C. elegans survival on live bacteria and protects the animals from S. enterica-mediated killing and invasion of the pharyngeal tissue, suggest a mechanism that can potentially be exploited to alleviate bacterial infections.
EXPERIMENTAL PROCEDURES
Bacterial and Nematode Strains
The Escherichia coli OP50 (Brenner, 1974) and Salmonella enterica serovar Typhimurium 1344 (Wray and Sojka, 1978) strains were used. C. elegans N2 Bristol, ced-1(e1735), ced-1(n691), and ced-1(n2089) strains were obtained from the Caenorhabditis elegans Genetics Center. The abu-1::gfp(ZcEx8) strain (Urano et al., 2002) was generously provided by the Ron laboratory. The abu-11 extrachromosomal array line geEx104[(rol-6su1006)abu-11(+)] and the strain geEx106[(rol-6su1006)] (Viswanathan et al., 2005) were generously provided by the Guarente laboratory. The abu-1::gfp(ZcEx8) transgenics were outcrossed four times to our N2 strain, which was used as control. geEx106[(rol-6su1006)] was used as a control for geEx104[(rol-6su1006)abu-11(+)].
Growth Conditions
Nematodes were maintained on nematode growth medium (NGM, minimal medium containing NaCl, agar, peptone, cholesterol, CaCl2, MgSO4, and potassium phosphate (Brenner, 1974)) containing a lawn of Escherichia coli OP50 at 20 °C. Synchronous populations were acquired by placing gravid adults on NGM plates containing Escherichia coli OP50 for 5 hours at 20 °C. The gravid adults were removed, leaving the eggs to hatch and develop into 1 day old hermaphroditic adults at 20 °C for use in the different assays.
C. elegans Killing Assay
For all bacterial strains, individual bacterial colonies were inoculated into LB and grown overnight on a rotary wheel at 37 °C. 20 μl of culture were plated onto a 3.5 cm plate containing modified NGM (3.5% peptone instead of 2.5%). One day old adult hermaphroditic nematodes were transferred to lawns of the various bacteria and transferred daily to a fresh lawn until progeny were no longer produced. All experiments were performed at 25 °C. Animals were considered dead upon failure to respond to touch and animals missing from the agar plate were censored on day of loss.
C. elegans Lifespan Assay on Killed Bacteria
Synchronized young adult animals were collected using M9 solution and were washed in antibiotic for 3 hours. Animals grown on E. coli OP50 were washed in M9 with 100 μg/ml ampicillin and animals grown on E. coli HT115(DE3) were washed in M9 with 50 μg/ml kanamycin.
Bacteria was grown as previously described, concentrated 1:10, and then heat-killed at 100 °C for 1 hour. Bacterial death was confirmed by failure to grow on LB plates at 37 °C overnight. 100 μl of the concentrated, killed bacteria was plated onto a 3.5 cm plate containing modified NGM with appropriate antibiotic. Animals grown on E. coli OP50 were put on modified NGM with 100 μg/ml ampicillin and animals grown on E. coli HT115(DE3) were put on modified NGM with 50 μg/ml kanamycin. Killing assays were performed as described above.
Pharyngeal Invasion Assay
For pharyngeal invasion microscopy, 20 μl of S. enterica expressing GFP grown in 3 ml LB with kanamycin 50 μg/ml were plated onto a 3.5 cm plate of modified NGM containing kanamycin 50 μg/ml. The plates were incubated at 37°C overnight. One day old adult nematodes were transferred to S. enterica expressing GFP strain smo22 (Vazquez-Torres et al., 1999) and incubated for two days with daily transfers to a new lawn of pathogen. After 48 hours, the nematodes were moved to a lawn of E. coli OP50. This enables the identification of nematodes with infected pharynxes and excludes the fluorescence from the background lawns. Nematodes were monitored for infected pharynxes using a Leica MZ FLIII fluorescence stereomicroscope. An infected pharynx was defined as the presence of GFP in the terminal bulb visible at 10x magnification. All experiments were performed at 25°C.
Confocal Microscopy
Nematodes were anesthetized in 1% sodium aside on an agar pad (2% agarose) and examined using a Leica TCS SL confocal microscope with Leica Confocal software version 2.61 Build 1537 (Leica Microsystems Heidelberg GMbH). Confocal images were imported into Adobe Photoshop for processing, including size adjustments, layering and brightness contrast.
RNAi
We used the RNA interference technique to generate loss-of-function RNAi phenotypes by feeding nematodes with E. coli expressing double-stranded RNA that is homologous to a target gene (Fraser et al., 2000; Timmons and Fire, 1998). The E. coli strain HT115(DE3) harboring the appropriate vectors was grown in LB broth containing 100 μg/ml ampicillin and 10 μg/ml tetracycline at 37 °C overnight. Bacteria were plated onto NGM plates containing 100 μg/ml ampicillin and 10 mM isopropyl β-D-thiogalactoside and were allowed to grow overnight at 37 °C.
L4 nematodes were placed on RNAi-expressing lawns of bacteria and allowed to grow to gravid adults. These gravid adults laying eggs were then transferred to new RNAi-expressing lawns of bacteria for 5 hours and then removed. The eggs were allowed to develop into young adults at 20°C on plates containing E. coli strain HT115 harboring a vector control or the appropriate RNAi vectors. Animals were then transferred to plates containing E. coli OP50 or S. enterica 1344, according to the experimental conditions described for each specific assay. Bacteria strains expressing double-stranded RNA to inactivate the C. elegans genes were obtained from Wellcome/Cancer Research (Cambridge, U.K) and Open Biosystems (Huntsville, AL). The identity of clones was confirmed by sequencing.
RNA Isolation, Reverse-Transcriptase PCR, and Quantitative Real-Time PCR
N2 and ced-1(e1735) strains were grown to gravid adults and treated with alkaline hypochlorite (Emmons et al., 1979) to isolate eggs. Eggs were placed in S basal to hatch overnight, causing growth arrest in L1. Synchronized nematodes were then grown on NGM plates with Escherichia coli OP50 or Salmonella enterica SL1344 at 25 °C for 26 hours. Nematodes were harvested and freeze-thawed 3 times in liquid nitrogen before total RNA was extracted using TRIzol reagent (Invitrogen).
For reverse transcriptase PCR, poly(A)+ RNA was isolated using the oligotex mRNA kit (Qiagen). We used the oligo-dT method from the SuperScript First-Strand Synthesis System (Invitrogen) with 50 nanograms of poly(A)+ RNA isolated as described above to generate a cDNA template. PCR was performed by amplifying from the cDNA using primers listed in Table S4. cDNA samples were 10-fold serially diluted four times. 30 cycles were used for PCR of act-1 control primers. 40 cycles were used for PCR of candidate genes.
qRT-PCR was conducted using the Applied Biosystems Taqman One-Step Real-time PCR protocol using SYBR Green fluorescence (Applied Biosystems) on an Applied Biosystems StepOnePlus Real-Time PCR System. Independent RNA preparations were measured at least twice and normalized to the housekeeping genes act-1, act-3, and act-4 (pan-actin). Gene expression in ced-1(e1735) was compared to wild type using the comparative Ct method and normalization to actin was used.
Microarray Analyses
Nematodes were grown and infected essentially as described above. Briefly, N2 and ced-1(e1735) strains were grown to gravid adults and eggs were isolated and placed in S basal to hatch overnight. Synchronized nematodes were then grown on NGM plates with Escherichia coli OP50 or Salmonella enterica SL1344 at 25 °C for 26 hours. Total RNA was obtained as described above. Affymetrix DNA microarray processing was prepared according to the manufacturer’s instructions (http://www.affymetrix.com/support/technical/manual/expression_manual.affx), and targets were hybridized to the C. elegans GeneChip (Affymetrix, Santa Clara, CA). The microarray data was subjected to the Robust Multichip Averaging (RMA) Algorithm using Partek Software (Partek, Inc., St. Charles, Missouri). GeneSpring Software 9.0 (Agilent Technologies) was used to perform hierarchical cluster analysis.
Cell Corpse Assay
To quantify the number of apoptotic germ cells, animals were stained with SYTO 12 (Molecular Probes) as previously described (Gumienny et. al. 1999). In brief, worms were incubated in 50 μM SYTO 12 for 3-4 h at room temperature and then seeded on bacterial lawns to reduce the amount of stained bacteria in the gut. After 20-30 min, more than 20 animals were mounted in a drop of M9 salt solution containing 30 mM NaN3 and observed by using a Leica MZ FLIII fluorescence stereomicroscope. Only animals that were brightly and equally stained were scored.
GFP Fluorescence Analysis
For GFP fluorescence microscopy, 20 μl of E. coli OP50 was plated on modified NGM plates and grown at 37° overnight. Eggs were isolated from N2 and ced-1(e1735) strains by alkaline hypochlorite treatment (Emmons et al., 1979) and were grown on the OP50 plates at 20° until the animals reached the L4 stage (approximately 36 hours).
Animals were anesthetized using a M9 salt solution containing 30 mM NaN3 and visualized using a Leica MZ FLIII fluorescence stereomicroscope. Images were taken of more than 15 worms per condition and max green channel fluorescence was analyzed with ImageJ 1.37v freeware. Intensities were averaged and a t-test was performed using GraphPad Prism 4.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Caenorhabditis Genetics Center (University of Minnesota) for providing all mutant strains used in this study. We also thank the Ron and Guarente laboratories for generously providing the abu-1 and abu-11 transgenic strains, respectively. Jennifer Tenor provided technical advice and performed the initial pharyngeal invasion assays. Holly K. Dressman provided excellent technical advice on microarray data analysis. A.A. is funded by The Whitehead Scholars Program, NIH SERCEB (U54 AI057157), and NIH GM070977.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aballay A, Ausubel FM. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc Natl Acad Sci U S A. 2001;98:2735–2739. doi: 10.1073/pnas.041613098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aballay A, Drenkard E, Hilbun LR, Ausubel FM. Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol. 2003;13:47–52. doi: 10.1016/s0960-9822(02)01396-9. [DOI] [PubMed] [Google Scholar]
- Aballay A, Yorgey P, Ausubel FM. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol. 2000;10:1539–1542. doi: 10.1016/s0960-9822(00)00830-7. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alper S, McBride SJ, Lackford B, Freedman JH, Schwartz DA. Specificity and complexity of the C. elegans innate immune response. Mol Cell Biol. 2007 doi: 10.1128/MCB.02070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Gumienny TL, Hengartner MO, Driscoll M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol. 2000;2:931–937. doi: 10.1038/35046585. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Jacobson DM, Horvitz HR. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics. 1991;129:79–94. doi: 10.1093/genetics/129.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons SW, Klass MR, Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979;76:1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Sifri CD, Mylonakis E, Qin X, Singh KV, Murray BE, Calderwood SB, Ausubel FM. A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A. 2001;98:10892–10897. doi: 10.1073/pnas.191378698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, Ausubel FM. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Sulston JE, Thomson JN. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 1983;220:1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- Henson PM. Engulfment: ingestion and migration with Rac, Rho and TRIO. Curr Biol. 2005;15:R29–30. doi: 10.1016/j.cub.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 2001;412:202–206. doi: 10.1038/35084103. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry S, Tekippe M, Gaddis NC, Aballay A. GATA transcription factor required for immunity to bacterial and fungal pathogens. PLoS ONE. 2006;1:e77. doi: 10.1371/journal.pone.0000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, Ausubel FM. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- Kim SK, Lund J, Kiraly M, Duke K, Jiang M, Stuart JM, Eizinger A, Wylie BN, Davidson GS. A gene expression map for Caenorhabditis elegans. Science. 2001;293:2087–2092. doi: 10.1126/science.1061603. [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R, Hengartner MO. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- Li S, Armstrong CM, Bertin N, Ge H, Milstein S, Boxem M, Vidalain PO, Han JD, Chesneau A, Hao T, et al. A map of the interactome network of the metazoan C. elegans. Science. 2004;303:540–543. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QA, Hengartner MO. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell. 1998;93:961–972. doi: 10.1016/s0092-8674(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, Kohara Y, Ewbank JJ. Inducible antibacterial defense system in C. elegans. Curr Biol. 2002;12:1209–1214. doi: 10.1016/s0960-9822(02)00928-4. [DOI] [PubMed] [Google Scholar]
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke D, Baban D, Demidova M, Mott R, Hodgkin J. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 2006;16:1005–1016. doi: 10.1101/gr.50823006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature. 2001;412:198–202. doi: 10.1038/35084096. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual JF, Klitgord N, Achaz G. Novel insights into RNAi off-target effects using C. elegans paralogs. BMC Genomics. 2007;8:106. doi: 10.1186/1471-2164-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M, Hamlin BJ, Rong J, Chen K, Ronen M, Tan MW. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci U S A. 2006;103:14086–14091. doi: 10.1073/pnas.0603424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Ellis RE, Sakaki K, Kaufman RJ. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 2005;1:e37. doi: 10.1371/journal.pgen.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Aballay A. Heat shock and genetic activation of HSF-1 enhance immunity to bacteria. Cell Cycle. 2006a;5:2443–2446. doi: 10.4161/cc.5.21.3434. [DOI] [PubMed] [Google Scholar]
- Singh V, Aballay A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci U S A. 2006b;103:13092–13097. doi: 10.1073/pnas.0604050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenor J, Aballay A. A conserved Toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep. 2008;9:103–109. doi: 10.1038/sj.embor.7401104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2 doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Calfon M, Yoneda T, Yun C, Kiraly M, Clark SG, Ron D. A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol. 2002;158:639–646. doi: 10.1083/jcb.200203086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Wong D, Bazopoulou D, Pujol N, Tavernarakis N, Ewbank JJ. Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol. 2007;8:R194. doi: 10.1186/gb-2007-8-9-r194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray C, Sojka WJ. Experimental Salmonella typhimurium infection in calves. Res Vet Sci. 1978;25:139–143. [PubMed] [Google Scholar]
- Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell. 2001;1:491–502. doi: 10.1016/s1534-5807(01)00056-9. [DOI] [PubMed] [Google Scholar]
- Yu X, Odera S, Chuang CH, Lu N, Zhou Z. C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev Cell. 2006;10:743–757. doi: 10.1016/j.devcel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Yuan J. Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol Cell. 2006;23:1–12. doi: 10.1016/j.molcel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Caron E, Hartwieg E, Hall A, Horvitz HR. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell. 2001a;1:477–489. doi: 10.1016/s1534-5807(01)00058-2. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001b;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.