Abstract
Orthopoxviruses are among the largest and most complex of the animal viruses. In response to the recent emergence of monkeypox in Africa and the threat of smallpox bioterrorism, two orthopoxviruses with different pathogenic potentials, human monkeypox virus and vaccinia virus, were proteomically compared with the goal of identifying proteins required for pathogenesis. Orthopoxviruses were grown in HeLa cells to two different viral forms (intracellular mature virus and extracellular enveloped virus), purified by sucrose gradient ultracentrifugation, denatured using RapiGest™ surfactant, and digested with trypsin. Unfractionated samples and strong cation exchange HPLC fractions were analyzed by high-resolution reversed-phase nano-LC-MS/MS, and analyses of the MS/MS spectra using SEQUEST® and X! Tandem resulted in the confident identification of hundreds of monkeypox, vaccinia, and copurified host-cell proteins. The unfractionated samples were additionally analyzed by LC-MS on an LTQ-Orbitrap™, and the accurate mass and elution time tag approach was used to perform quantitative comparisons. Possible pathophysiological roles of differentially abundant Orthopoxvirus proteins are discussed. Data, processed results, and protocols are available at http://www.proteomicsresource.org/.
Keywords: monkeypox, vaccinia, proteomics, mass spectrometry, SEQUEST®, X! Tandem
INTRODUCTION
The recent emergence of monkeypox, a lethal zoonosis endemic to regions of Africa, is the most serious threat by an Orthopoxvirus since the eradication of smallpox in 1977 1-3. At the genetic level, monkeypox virus (MPV) is 85% identical to Variola major 4-6, the causative agent of smallpox, and the clinical manifestations of monkeypox and smallpox are essentially indistinguishable. As a result, MPV has been classified as a Category A select bioterrorism agent by the Centers for Disease Control and Prevention. Fortunately, the human-to-human transmissibility of monkeypox appears to be poor 7. Nevertheless, there are currently no treatments for monkeypox that have been robustly demonstrated to be effective, and global eradication of MPV is infeasible because, unlike variola, MPV is maintained within a reservoir of wild animals. In addition, while vaccination with vaccinia virus (VV) is broadly effective against orthopoxviruses, using mass vaccination to prevent sporadic outbreaks is impractical due to the resulting mortality and morbidity (estimated to be 1 death and 20 serious complications per million vaccinations 8, 9), because of the financial costs, and because some people are unable to be safely vaccinated (e.g., immunocompromised individuals) 10. Therefore, because of the threat posed by monkeypox (e.g., the U.S. outbreak in 2003 1), and because orthopoxviruses could be used as bioterrorism agents, there is a need to develop therapeutics against orthopoxviruses.
MPV, VV, and variola are all members of the genus Orthopoxvirus, which itself is a member of the family Poxviridae of double-stranded DNA viruses. Orthopoxvirus genomes are ∼200,000 base pairs in length and encode ∼200 proteins. At ∼200 nm in diameter and ∼300 nm in length (almost the size of a small bacterium), orthopoxviruses are among the largest and most complex of the animal viruses. Orthopoxvirus assembly involves the acquisition and shedding of multiple lipid bilayers, and during this complex process, four distinct forms of virions are produced 11: intracellular mature virus (IMV), intracellular enveloped virus (IEV), cell-associated enveloped virus (CEV), and extracellular enveloped virus (EEV). Importantly, each form has unique infectivity, immune evasion, and weaponization properties.
The morphogenesis of VV, a prototypical Orthopoxvirus, has been studied extensively 12. Vaccinia IMV form within specific regions of the host-cell called “virus factories”, and are the most abundant form of infectious virus. Some IMV become wrapped in a double layer of lipid bilayers (most likely derived from the trans-Golgi network or from endosomes) and form IEV. IEV are subsequently localized to the host-cell plasma membrane where fusion results in the loss of one of the two aforementioned lipid bilayers, forming CEV. CEV are, by definition, attached to the outer host-cell surface; if they detach from the cell surface, they are called EEV. Thus, the major structural difference between IMV and EEV is that IMV lack the outermost EEV membrane. Because this membrane is known to be fragile 13, IMV particles are thought to be the form responsible for inter-host viral transmission. In contrast, EEV are known to be important for intra-host viral dissemination 14.
Recently, the results of three bottom-up proteomic studies of vaccinia IMV were reported 15-17. All three investigations employed liquid chromatography coupled with (tandem) mass spectrometry (LC-MS(/MS)). Bottom-up proteomics has greatly benefited from recent advances in high-throughput LC-MS(/MS), and comparative proteomics has been used to tentatively identify proteins required for virulence in studies of a variety of closely-related yet differentially pathogenic microorganisms 18-20. Key to such investigations is the identification of protein abundances that correlate with pathogenicity. Putative virulence factors identified by comparative proteomics require validation by orthogonal experimentation (e.g., pathogenicity assays of mutants). A discovery of a novel virulence factor can aid in the identification of pathogenic mechanisms, diagnostic markers, and therapeutic targets. For example, some immunomodulatory orthopoxvirus proteins are potential novel biotherapeutics 21, 22.
In this study, LC-MS(/MS) was used to compare purified MPV to VV. VV was selected because it is potentially differentially pathogenic, because it has been extensively studied, and because it is used as a smallpox vaccine. We additionally compared the proteomes of IMV and EEV particles. As a result, hundreds of virion-associated proteins (i.e., proteins bound to or contained within virions) were identified and quantified, and possible pathophysiological roles of differentially abundant Orthopoxvirus proteins are discussed.
MATERIALS AND METHODS
Reagents
Water was purified using a NANOpure® system (≥ 18 MΩ×cm, Barnstead International, Dubuque, IA). Reagents were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise specified, and included acetic acid, acetonitrile (Fisher Scientific, Pittsburgh, PA), ammonium bicarbonate, RapiGest™ surfactant (Waters Corp., Milford, MA), sequencing grade modified trypsin (Promega Corp., Madison WI), and trifluoroacetic acid. Protein and peptide concentrations were determined using bicinchoninic acid (BCA) protein assay reagents (Pierce, Rockford, IL).
Virion Preparation
Human MPV (Zaire strain v95-I-005) was kindly provided by Dr. Inger Damon (Centers for Disease Control and Prevention, Atlanta, GA). This strain was originally isolated from a human victim who died of monkeypox. VV (Western Reserve strain) was acquired from the American Type Culture Collection (catalog number VR-1354 (a plaque purified VV isolate), ATCC, Manassas, VA).
Monkeypox and vaccinia IMV and EEV particles were grown in HeLa cells and purified by sucrose gradient ultracentrifugation using a protocol similar to one reported previously 23 (see Supplemental Methods for details). Orthopoxvirus are capable of infecting many different types of cells, and the composition of their proteomes may be influenced by the host-cell type. HeLa cells were selected to be the host-cells used in this investigation so that the resulting virions could be used as base models of Orthopoxviruses from human infections. Plaque assays were performed using BSC40 (monkey kidney) cells (ATCC, Manassas, VA) to titer the viral preparations. Protein masses were determined by BCA assays. To assess purity of each preparation, 100 viral particles were sectioned, photographed by transmission electron microscopy, and determined to be IMV or EEV (see Supplemental Methods for details), and the preparations were found to be > 98% pure. Note that some of the viral purification attempts failed because of the complexity of the purification protocol (e.g., the initial VV EEV preparation had a negligible protein mass). Also, refinements of the protocol were made over the course of the study to increase the purity and yield of the samples (e.g., some of the preparations were scaled-up versions of earlier ones).
Aliquots of purified virions were analyzed by sodium dodecyl sulfate - polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analyses. Representative IMV and EEV pairs of virion preparations were mixed with SDS-PAGE loading buffer, incubated at 100°C for 10 min, run on 10% polyacrylamide gels, and silver stained or electrotransferred to nitrocellulose membranes. The membranes were then probed with a polyclonal antibody generated against a vaccinia EEV-specific envelope glycoprotein known to be absent from IMV 12 (anti-VV (Western Reserve strain) A33R, cat# NR-628, BEI Resources, http://www.beiresources.org/, Manassas, VA). The sequence of VV A33R was found to be 96.11% identical to that of MPV A35R using a sequence homology analysis (described below). Protein bands were detected by chemiluminescence generated by horseradish peroxidase.
Trypsin Digestion, SCX HPLC, and nano-LC-MS(/MS)
Sucrose-gradient purified IMV and EEV preparations were centrifuged to pellet the virus at 20,000 rpm (avg. RCF = 18,000 × g) for 20 min at 4°C in an Optima™ TL ultracentrifuge using a TLA 55 rotor. The supernatants were discarded, and the pellets were dissolved in 300 μL of 100 mM NH4HCO3 pH 8.4 by sonication for 30 sec. The samples were centrifuged to pellet the virus a second time at 20,000 rpm (avg. RCF = 18,000 × g) for 20 min at 4°C in an Optima™ TL ultracentrifuge using a TLA 55 rotor. The supernatants were discarded and the pellets were dissolved in 300 μL of 100 mM NH4HCO3 pH 8.4, 0.1% (w/v) RapiGest™ surfactant using repeated pipetting. The sample tubes were placed in boiling water for 5 min and then cooled on ice. Protein concentrations were determined using BCA protein assays, trypsin was added to the samples at a ratio of 1 μg trypsin to 50 μg sample protein, and the samples were digested for 60 min at 37°C. Each sample was then acidified by adding 1 μL of 2% trifluoroacetic acid, checking the resulting pH, and repeating this process until the sample was approximately pH 2. The samples were incubated at 37°C for 60 min to hydrolyze the RapiGest™ surfactant and cause its hydrophilic head-group and its hydrophobic tail-group to disassociate. The samples were frozen in liquid nitrogen and then thawed at room temperature to assist the precipitation of the RapiGest™ tail-group. The samples were microcentrifuged at 13,000 rpm (16,000 × g) for 10 min to pellet the RapiGest™ tail-group, the supernatants were transferred to fresh microcentrifuge tubes, and the pellets were discarded. Each sample was neutralized by adding 1 μL of 20% ammonium hydroxide, checking the resulting pH, and repeating this process until the sample was approximately pH 7. Peptide masses were determined by BCA assays. Some of the samples were concentrated in a SpeedVac (Thermo Electron Corp., Waltham, MA), but none were allowed to concentrate to dryness. Plaque assays using BSC40 (monkey kidney) cells (ATCC, Manassas, VA) were used to confirm that the samples were no longer infectious. The samples were stored at -80°C.
Portions of the peptide samples were fractionated by strong cation exchange (SCX) HPLC as described previously 24. Unfractionated and SCX fractionated samples were then analyzed by reversed-phase nanocapillary HPLC - nanoelectrospray ionization - LTQ™ ion trap tandem mass spectrometry as described previously 24. Separate LTQ-Orbitrap™ nano-LC-MS analyses of the unfractionated samples were performed in parallel (see Supplemental Methods for details). Groups of Orthopoxvirus samples were analyzed by LC-MS as they became available (four groups over a period of seven months).
MS/MS Data Analysis
MS/MS spectra were analyzed by using both SEQUEST® (TurboSEQUEST® (cluster) v.27 (rev. 12), Thermo Electron Corp.) and X! Tandem (v. 2006.09.15.1) 25 to search the spectra against two dual-organism (MPV-human and VV-human) concatenated protein FASTA data files. The MPV (Zaire strain) and VV (Western Reserve strain) FASTA data files of proteins translated from genetic code contained 191 and 218 protein sequences, respectively, and were provided by the Poxvirus Bioinformatics Resource Center (http://www.poxvirus.org/, July 4, 2005, University of Alabama at Birmingham) 26. The original MPV and VV genomic sequences are available at the National Center for Biotechnology Information (Bethesda, MD, http://www.ncbi.nlm.nih.gov/) as accession numbers NC_003310 and NC_006998, respectively. MPV and VV protein homologues were identified using BLAST (Basic Local Alignment Search Tool) 27 and were required to have ≥ 50% sequence identity. The human FASTA data file of proteins translated from genetic code was provided by the International Protein Index (49,161 protein sequences, http://www.ebi.ac.uk/IPI/, April 4, 2005, European Bioinformatics Institute, Cambridge, UK) 28. The SEQUEST® analyses used a standard parameter file with peptide_mass_tolerance = 3, fragment_ion_tolerance = 0, and no amino acid modifications. Also, these analyses searched for all possible peptide termini (i.e., not limited to only tryptic termini). The X! Tandem analyses used the default settings with the exception that the second round of searching included four potential (dynamic) modifications (Met oxidation, amino-terminal acetylation, Gln NH3 loss, and Glu H2O loss).
For each SEQUEST® analysis of an MS/MS spectrum (at a given parent ion charge state), only the peptide identification with the highest XCorr value (i.e., the “top ranked hit”) was retained. In addition, the SEQUEST® peptide identifications were required to satisfy Washburn-Yates criteria 29. Specifically, ΔCn ≥ 0.1 was required and, for each parent ion charge state, XCorr was required to be ≥ 1.9 (+1), 2.2 (+2), 3.75 (≥ +3). For the X! Tandem peptide identifications, the expectation values were required to be ≤ 0.01. For each peptide identification, a discriminant score was calculated from the SEQUEST® or X! Tandem scores, from the difference between the predicted 30 and observed 31 normalized elution time (NET) values, and from other factors 32. In addition to the above data filters, all of the peptide identifications were required to have a discriminant score of ≥ 0.7. Additionally, peptide identifications that corresponded to both a viral and a host-cell protein were discarded, as were peptide identifications that corresponded to two different MPV proteins or to two different VV proteins (with the exception of proteins encoded within the inverted terminal repetitions of the MPV and VV genomes). The estimated percentage of false-positive peptide identifications was determined using the scrambled protein database approach 33 and was < 1% for both the SEQUEST® and X! Tandem data. Filter-passing peptide identifications of ≥ 2 different (i.e., chemically distinct) peptides were required for each protein identification. The data were additionally analyzed using ProteinProphet 34, primarily to organize host-cell protein splice isoforms into protein groups. The estimated percentage of false-positive protein identifications (determined using the scrambled search results) was < 1%. Spectrum counting (i.e., tallying of filter-passing peptide identifications) was used as a rough measure of protein abundance 35. The Proteomics Research Information Storage and Management system 36 was used to run SEQUEST® and X! Tandem in an automated fashion, to run software that calculated predicted and observed NET values, and to manage the proteomics data in general.
LC-MS Data Analysis
Orbitrap™ spectra were analyzed using the accurate mass and elution time (AMT) tag approach 18-20. Briefly, the theoretical mass and the observed NET of each peptide identified by LC-MS/MS were used to construct a reference database of AMT tags. Features from the LC-MS analyses (i.e., m/z peaks deconvolved of isotopic and charge state effects and then correlated by mass and NET) were matched to AMT tags to identify peptides 37. LC-MS mass 38 and NET 39 recalibration was performed using algorithms described previously. A peptide identification probability score was calculated for each match of an LC-MS feature to an AMT tag 40, and the percentage of false-positive peptide identifications was estimated to be 1.4% using an approach described previously 41. The LC-MS peptide peak area (NET vs. peak height) was used as a measure of peptide abundance. Protein abundances values were calculated by averaging the corresponding peptide abundances, and were subjected to hierarchical cluster analyses using Genesis v1.7.2 42 with average linkage correlations determined by Kendall’s Tau function.
RESULTS
Identification of MPV and VV Proteins by LC-MS/MS
Ten virion samples were prepared from cultured HeLa cells: four monkeypox IMV, three monkeypox EEV, two vaccinia IMV, and one vaccinia EEV. The HeLa cell pellets contained “cell associated virions” (i.e., primarily IMV, but also some IEV and CEV), and the cell culture supernatants contained “extracellular virions” (i.e., primarily EEV, but also some IMV from HeLa cell breakage), and the IMV and EEV were enriched using multiple rounds of sucrose gradient ultracentrifugation. To confirm that the IMV and EEV purifications were successful, Western blot analyses of representative preparations were performed using a polyclonal antibody against an EEV-specific envelope glycoprotein (Figure 1). In addition, representative virion preparations were imaged by transmission electron microscopy (Figure 2).
Figure 1. Western Blot Analysis of Monkeypox IMV and EEV for an EEV-specific Protein.
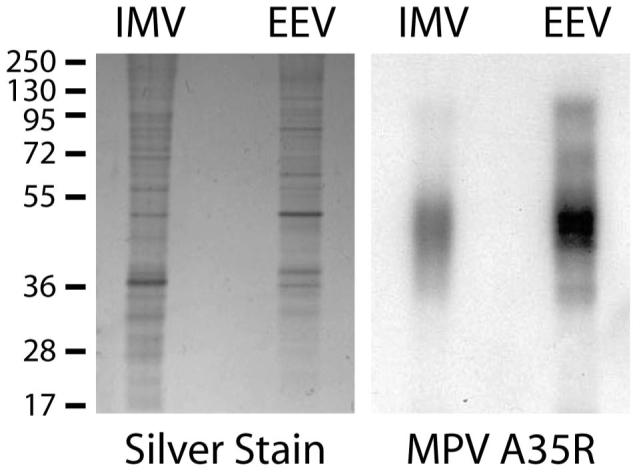
Gradient purified monkeypox IMV and EEV particles were analyzed for the presence of MPV A35R protein, a homolog of the vaccinia EEV-specific 12 envelope glycoprotein A33R (MPV A35R shares 96.1% identity with VV A33R). Intact MPV particles were denatured in SDS-PAGE loading buffer and run in duplicate on 10% polyacrylamide gels. One of the gels was silver stained (left), and the replicate gel underwent Western blot analysis using a polyclonal antibody that recognizes VV A33R (right). MPV A35R is predicted to encode a 20 kDa protein, but was detected as a diffuse band at ∼45 kDa possibly because it is a glycoprotein or because it comigrated with other viral proteins 54. The numbers (left) indicate molecular masses in kDa.
Figure 2. Electron Micrographs of Monkeypox IMV and EEV Particles.
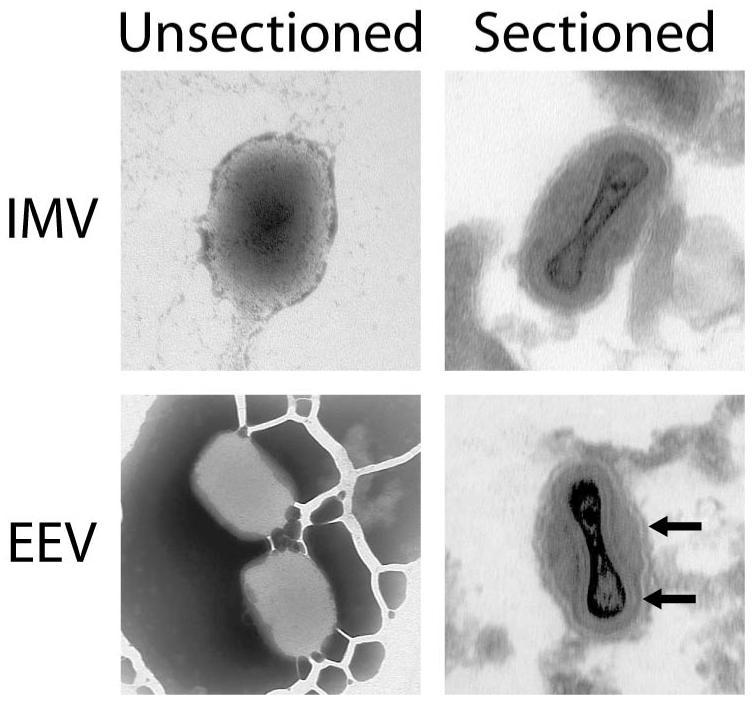
Viral particles from a representative monkeypox IMV and EEV pair of preparations were imaged by transmission electron microscopy to confirm that both purifications were successful. Unsectioned Virions: IMV and EEV particles displayed differential staining, possibly because the stain could not traverse the outermost EEV membrane. Sectioned Virions: The outermost EEV membrane was visible in the micrograph of the sectioned EEV virions, and was absent in the corresponding IMV image. The arrows denote the outermost EEV membrane and the next-to-outermost membrane (i.e., the internal “IMV membrane” of the EEV virion).
Orthopoxvirus proteins were denatured with RapiGest™, digested with trypsin, and analyzed by LC-MS/MS. Parallel SEQUEST® and X! Tandem analyses produced thousands of high-confidence peptide identifications. Most of the SEQUEST® and X! Tandem peptide identifications overlapped (Table 1), which is consistent with the results of a comprehensive comparison of the two search engines 43. Overall, 164 viral and 2,975 host-cell proteins were identified as virion-associated by LC-MS/MS. Selected viral protein (i.e., proteins encoded within the viral genome) data were included in Table 2, and comprehensive datasets were included as Supplemental Tables 2, 3. However, due to the high sensitivity of the methodology, many of these protein identifications were probably of low-abundance, virion-unassociated contaminants. Because this investigation focused on MPV, more MPV samples than VV samples were prepared and analyzed by LC-MS(/MS) (seven MPV vs. three VV), and this is probably one of the reasons why more MPV peptides were identified than VV peptides (Table 1). Nevertheless, when the VV protein identifications were compared to those detected by three prior investigations 15-17, there was only a single previously detected VV protein that was not detected by this study. This protein (VV ORF 074) was detected by two of the three prior investigations 15, 16.
Table 1.
Summary of Peptide and Protein Identifications
| Monkeypox Virus: | LC-MS/MS |
LC-MS |
|||
|---|---|---|---|---|---|
| Unique Peptide Identifications by: | Global | SCX | Total | Global | |
| SEQUEST®, but not X! Tandem | unmodified | 1,313 | 3,332 | 3,495 | 157 (4%) |
| SEQUEST® & X! Tandem | unmodified | 6,749 | 12,062 | 13,970 | 7,154 (51%) |
| X! Tandem, but not SEQUEST® { | unmodified Modified* |
1,363 855 |
2,883 2,672 |
3,187 2,925 |
92 (3%) 657 (22%) |
| Total: | 10,280 | 20,949 | 23,577 | 8,060 (34%) | |
| Protein Identifications: | 1,843 | 2,705 | 2,718 | 1,677 (62%) | |
| Vaccinia Virus: | LC-MS/MS |
LC-MS |
|||
|---|---|---|---|---|---|
| Unique Peptide Identifications by: | Global | SCX | Total | Global | |
| SEQUEST®, but not X! Tandem | unmodified | 859 | 1,871 | 2,266 | 96 (4%) |
| SEQUEST®& X! Tandem | unmodified | 3,124 | 7,876 | 9,355 | 3,053 (33%) |
| X! Tandem, but not SEQUEST® { | unmodified modified* |
540 439 |
1,524 1,852 |
1,683 2,080 |
20 (1%) 353 (17%) |
| Total: | 4,962 | 13,123 | 15,384 | 3,522 (23%) | |
| Protein Identifications: | 962 | 1,814 | 1,847 | 747 (40%) | |
Met oxidation, amino-terminal acetylation, Gln NH3 loss, or Glu H2O loss
Table 2.
Selected Viral Proteins Identified by LC-MS/MS
| Monkeypox Virus |
Vaccinia Virus |
||||
|---|---|---|---|---|---|
| ORF | Peptides | IDs | ORF | Peptides | IDs |
| 002 (J2L) | 3 | 29 | 002 | 0 | 0 |
| 002 (J2L) | 3 | 29 | 004 (C22L) | 2 | 20 |
| tumor necrosis factor receptor homolog, host defense modulator | |||||
| 003 (J3L) | 3 | 5 | 005 | 0 | 0 |
| 003 (J3L) | 3 | 5 | 006 | 0 | 0 |
| 003 (J3L) | 3 | 5 | 007 | 0 | 0 |
| 003 (J3L) | 3 | 5 | 008 (C19L) | 0 | 0 |
| unknown function (contains ankyrin repeats) | |||||
| 007 (D4L) | 0 | 0 | 010 (C10L) | 6 | 7 |
| unknown function | |||||
| 010 (D7L) | 7 | 22 | 014 | 0 | 0 |
| 010 (D7L) | 7 | 22 | 015 | 0 | 0 |
| 010 (D7L) | 7 | 22 | 016 | 0 | 0 |
| 010 (D7L) | 7 | 22 | 017 | 0 | 0 |
| unknown function (contains ankyrin repeats) | |||||
| 017 (D14L) | 0 | 0 | 025 (C3L) | 2 | 12 |
| secreted, complement-binding host defense modulator | |||||
| 022 (D19L) | 0 | 0 | 027 (C1L) | 5 | 16 |
| unknown function | |||||
| 027 (C1L) | 3 | 4 | 032 (K1L) | 8 | 27 |
| NF-κB inhibitor, host defense modulator | |||||
| 055 (F6R) | 8 | 77 | 063 (E7R) | 3 | 7 |
| unknown function | |||||
| 060 (Q1L) | 2 | 3 | 068 (O1L) | 15 | 29 |
| unknown function | |||||
| 130 (A20L) | 3 | 10 | 139 (A19L) | 0 | 0 |
| unknown function | |||||
| NA | 0 | 0 | 163 (A39R) | 5 | 6 |
| semaphorin homolog, host defense modulator | |||||
| NA | 0 | 0 | 165 (A40R) | 4 | 17 |
| unknown function (contains a lectin-like domain) | |||||
| 162 (B1R) | 0 | 0 | 180 (A55R) | 6 | 11 |
| unknown function (contains BTB, kelch domains) | |||||
| 165 (B4R) | 22 | 70 | 184 (B2R) | 6 | 10 |
| 165 (B4R) | 22 | 70 | 185 | 1 | 1 |
| unknown function | |||||
| 169 (B8R) | 10 | 70 | 189 (B7R) | 3 | 6 |
| unknown function | |||||
| 171 (B10R) | 4 | 6 | 191 (B9R) | 0 | 0 |
| unknown function | |||||
| 182 (B21R) | 43 | 211 | NA | 0 | 0 |
| unknown function (contains a cadherin-like domain) | |||||
Viral proteins were quantified by LC-MS (peak areas) and LC-MS/MS (spectrum counts), and then a selection of proteins were tentatively identified as MPV-specific, VV-specific, MPV and VV virion-associated, or as contaminants based on a number of criteria including their known physiological roles, their overall abundances in the samples, and their abundances across the virion preparation replicates. Only the MPV-specific and VV-specific viral proteins that were differentially detected by LC-MS/MS were included in this table (for the comprehensive list, see Supplemental Table 2). “Peptides” refers to the number of peptides that were identified by LC-MS/MS, and “IDs” refers to the total number of LC-MS/MS peptide identifications (i.e., including replicate identifications of the same peptide).
Not surprisingly, the majority of the peptide identifications corresponded to the major viral proteins. For example, MPV peptides derived from the major viral core proteins 4a and 4b (MPV ORFs 121 and 114, respectively) were observed 4,500 and 4,316 times, respectively. To identify non-annotated viral proteins, MS/MS spectra were searched against MPV and VV protein FASTA files constructed from translations of DNA sequences that were flanked by stop codons (i.e., stop-to-stop translations). Both the MPV and VV stop-to-stop FASTA files were supplemented with the human IPI protein sequences, and the searches were performed using SEQUEST®. However, none of these searches resulted in a confident identification of an unannotated Orthopoxvirus protein.
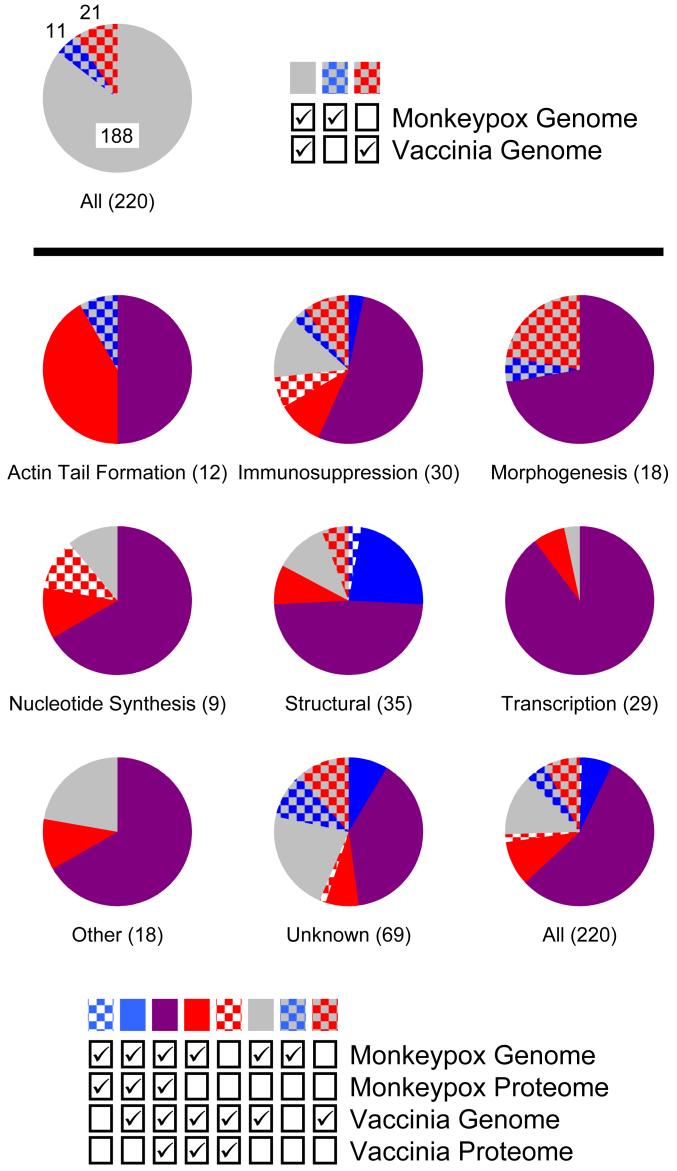
While the MPV and VV proteomes overlapped significantly, 8 MPV-specific and 22 VV-specific viral proteins were identified (Figure 3). Some of the MPV-specific proteins were fragmented in VV. While some of these proteins were unambiguously differentially detected (e.g., MPV ORFs 010 and 182, and VV ORFs 163 and 165 (see Table 2 for more)), many of the others were only marginally detected at all (i.e., from only two different peptides) and therefore may have been virally-unassociated contaminants. The MPV-specific proteins consisted of three structural proteins and five proteins having unknown functions, and some of these may account in part for the more severe pathology of monkeypox.
Figure 3. Comparison of the Viral Proteomes in Context of the Two Genomes.
Each viral protein was assigned one of eight functional annotations (Actin Tail Formation, Immunosuppression, Morphogenesis, Nucleotide Synthesis, Other, Structural, Transcription, or Unknown). Additionally, each protein was classified as being present or absent from each viral genome and proteome (note that to be present in the proteome it had to be present in the corresponding genome). A majority of the proteins (188/220) were encoded by both genomes (i.e., were homologs between MPV and VV), but 32 were encoded by only one of the two genomes (top pie chart) (the MPV and VV genomes encode 199 and 209 predicted proteins, respectively). For each of the eight functional annotations (Actin Tail Formation, Immunosuppression, etc.) and for all of the proteins combined, the percentage of each protein classification (i.e., absent or present in each genome and proteome) is displayed as a pie chart (bottom nine pie charts). The total number of proteins assigned to each functional annotation is indicated in parenthesis.
Quantification of Virion-Associated Proteins by LC-MS
To perform quantitative comparisons, unfractionated MPV and VV samples were analyzed by LC-MS on an LTQ-Orbitrap™, and the resulting spectra were analyzed using the AMT tag approach. The average mass error of the quantified peptides was 0.41 ppm, and the average NET error was 0.56%, indicating that these were extremely high-confidence identifications. Of the proteins identified by LC-MS/MS, 53% were quantified by LC-MS (Table 1). Therefore, compared with the LC-LTQ-Orbitrap™-MS analyses of the unfractionated samples, an improved depth of coverage was obtained from the LC-LTQ™-MS/MS analyses of the SCX fractions.
It was noted that the vast majority of the quantified, unmodified peptides were originally identified by both SEQUEST® and X! Tandem (Table 1), and therefore that these were high-confidence identifications. Also, a significantly larger percentage of viral proteins were quantified (77% or 126/164), which was expected because previous studies of the VV proteome confirmed that they have relatively high abundances compared to incorporated host-cell proteins 15-17. In addition to using the LC-MS data to determine viral protein abundances, these data were also used to tentatively distinguish virion-associated host-cell proteins from low-abundance, host-cell protein contaminants, and highly abundant host-cell proteins were further differentiated by their known physiological roles (Table 3).
Table 3.
Selected Host-Cell Proteins
| Putative Virion-Associated Proteins (Role) | Putative Contaminants (Role) |
|---|---|
| Actin* (Structural) | Acyl-CoA Dehydrogenase (Metabolism) |
| Basigin (Structural) | Aldolase (Metabolism) |
| Cofilin* (Structural) | Enolase (Metabolism) |
| Drebrin (Structural) | Glyceraldehyde-3-P Dehydrogenase (Metabolism) |
| Fascin (Structural) | Heat Shock Proteins* (Stress Response) |
| Filamin (Structural) | Histone Proteins (Chromatin Structure) |
| Lamin (Structural) | Initiation, Elongation Factors* (Translation) |
| Moesin (Structural) | Keratin* (Structural) |
| Prohibitin* (Signaling) | Lactate Dehydrogenase (Metabolism) |
| Radixin (Structural) | Peroxiredoxin* (Antioxidation) |
| Transgelin* (Structural) | Phosphoglycerate Kinase* (Metabolism) |
| Tubulin* (Structural) | Pyruvate Kinase* (Metabolism) |
| Vimentin* (Structural) | Ribosomal Proteins* (Translation) |
Detected previously in vaccinia IMV17. Host-cell proteins were quantified by LC-MS (peak areas) and LC-MS/MS (spectrum counts), and then a selection of abundant proteins were tentatively identified as virion-associated or as contaminants based on a number of criteria including their known physiological roles, their overall abundances in the samples, their abundances across the virion preparation replicates, and their typical abundances in mammalian cells.
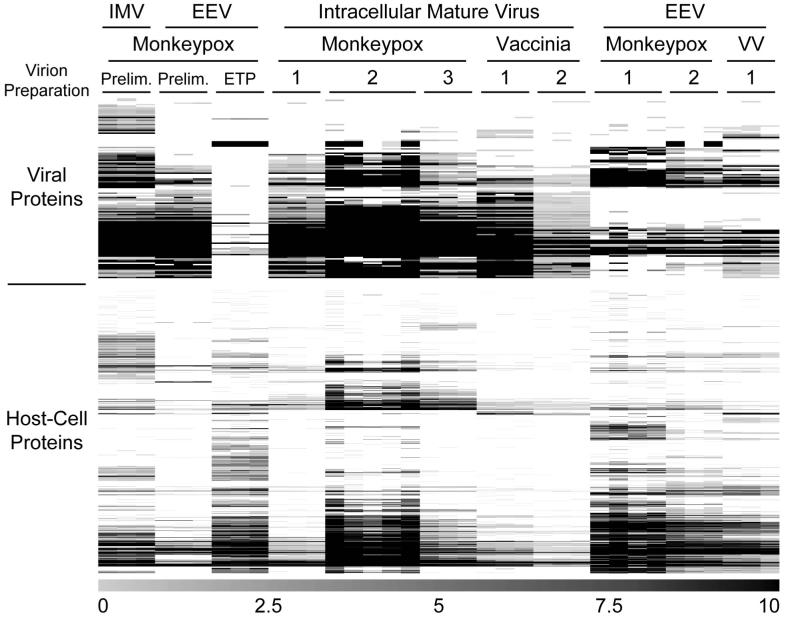
To visualize the LC-MS results, the data were subjected to hierarchical cluster analyses and displayed as protein expression maps (Figure 4). Clustering the columns (i.e., the experiments) in addition to the rows (i.e., the proteins) showed that the LC-MS analyses were more reproducible than the virion sample preparations (Supplemental Figure 1). In part, this finding reflects the refinement of the virus purification protocol through the various preparations. For example, the preliminary MPV preparation did not include the final 10% sucrose cushion step. This additional step resulted in noticeably different viral and host-cell protein abundances. Also, some of the preparations were scaled-up versions of earlier ones, and it’s possible that more low-abundance proteins were detected in these as a result.
Figure 4. Quantitative Comparison of Orthopoxvirus Particles by LC-MS.
Monkeypox and vaccinia IMV particles were purified from host-cell lysates and EEV particles from the corresponding culture media. Each sample was digested with trypsin and analyzed by LC-MS in triplicate (or more), and the resulting data were analyzed using hierarchical cluster analyses. Columns correspond to individual LC-MS analyses, rows correspond to individual proteins, protein abundances are indicated by the grayscale bar (bottom), and missing protein abundance values are colored white. Data from the two “Prelim.” (i.e., “preliminary”) MPV preparations were not representative of the subsequent results, but were included in the figure for completeness (these initial preparations were probably not purified as successfully as the subsequent ones). The monkeypox EEV ETP (i.e., “early time point”) sample was prepared from the culture medium of MPV-infected cells recovered 10 h post-infection (i.e., prior to significant EEV production), and was processed using the EEV sample preparation protocol to serve as a negative control.
A monkeypox EEV “early time point” sample was prepared as a negative-control experiment from the culture medium of MPV-infected HeLa cells recovered 10 h post-infection (i.e., prior to significant EEV production). This sample was processed using the EEV preparation protocol, and LC-MS analysis resulted in very few viral protein identifications, as expected. In contrast, many host-cell proteins were identified. This finding was also anticipated because this sample was concentrated in a SpeedVac to a peptide concentration suitable for LC-MS (0.6 μg/μL determined by a BCA assay), and as a result peptides derived from host-cell protein contaminants were enriched.
Comparison of IMV and EEV Particles
To compare different forms of infectious MPV and VV, IMV particles were isolated from disrupted host-cells and EEV particles were isolated from the corresponding culture media. These samples were digested with trypsin, analyzed by LC-MS, and the resulting data were analyzed using hierarchical clustering (Figure 4). The preliminary monkeypox EEV preparation more closely resembled the IMV preparations than the other EEV preparations (Supplemental Figure 1). The three subsequent EEV samples (two MPV and one VV) contained significantly different viral protein compositions compared with the IMV samples. Eighty-five viral proteins were common to a majority of the IMV samples, 48 were common to a majority of the EEV samples (ignoring the unrepresentative initial monkeypox EEV sample), and 45 were common to a majority of both types of samples. While it is known that the intracellular mechanisms that produce EEV from IMV significantly alter the viral protein composition of these particles 12, it is likely that a combination of factors caused the observed protein composition differences. For example, because the IMV particles were purified from host-cell lysates, it is possible that these samples were contaminated with intracellular viral proteins that were not really IMV-associated. In addition, the EEV preparations generally had lower viral titers compared with the IMV samples, and it is possible that fewer EEV viral proteins were detected as a result. Also, some of the proteins detected in the IMV samples may have been posttranslationally modified in the EEV samples, and therefore not detected.
MPV ORFs 009, 039, and 145 were absent in a majority of the IMV samples, but present in a majority of the EEV samples (ignoring the unrepresentative initial monkeypox EEV sample). MPV ORF 009 encodes a homolog of a secreted, interleukin-18-binding protein from cowpox virus 44. MPV ORF 039 (also known as MPV C13L, a homolog of VV (Copenhagen strain) F7L) encodes a hypothetical 74 amino acid protein of unknown function (i.e., an investigation of this protein or of a homolog has not been reported). VV A33R, an MPV ORF 145 homolog, encodes a membrane phosphoglycoprotein involved in CEV-cell adherence and actin tail formation and is known to be absent in vaccinia IMV and present in vaccinia EEV 12 (this was the protein probed against in Figure 1). A number of MPV-specific and VV-specific proteins were also identified and are discussed below.
DISCUSSION
The primary objective of this investigation was to use qualitative and quantitative proteomics to compare MPV to a potentially differentially pathogenic and well-characterized Orthopoxvirus, VV. The other major objective was to proteomically compare IMV and EEV particles.
The original source of VV is unknown, but among the multiple possibilities is that it might be an attenuated variant of cowpox virus 45. It has been hypothesized that this attenuation resulted from a small number of virulence genes (i.e., genes required for pathogenesis in animals, but not for viral production in cultured cells) that were mutated, fragmented, deleted, or otherwise made ineffective. Therefore, proteins present in MPV and absent in VV are possible virulence factors, and while many such proteins were tentatively identified by a comparative genomic analysis 5, only protein-level studies can reveal which genes are actually expressed as proteins and which are differentially abundant. Seven MPV proteins (MPV ORFs 010, 055, 130, 165, 169, 171, and 182) were absent or had relatively low abundances in VV. MPV ORF 182 encodes a putative structural protein that was detected by both LC-MS and LC-MS/MS and has no homologue in VV. Two others (ORFs 010 and 165) correspond to genes that are fragmented in VV, and both of these are discussed further below. Of the four remaining genes, ORF 169 encodes a homolog of a tentative VV protein that might be involved in virulence 46, and the three others have unknown functions.
Orthopoxviruses are known to have many genes that are functional in some species but fragmented in others 47. Fragmented genes represent > 15% of the total number of genes in some Orthopoxvirus genomes, and they are especially difficult to annotate correctly. Our BLAST comparison of the annotated proteomes of MPV and VV revealed a single fragmented MPV gene (corresponding to VV ORF 026), and seven fragmented VV genes (corresponding to MPV ORFs 002, 003, 008, 010, 031, 165, and 178), four of which were detected in MPV in this study (ORFs 002, 003, 010, and 165). These genes have putative immunosuppressive (ORF 002), structural (ORFs 003 and 010), and unknown (ORF 165) functions based on their homology to other proteins. ORF 002 encodes a homolog of a secreted tumor necrosis factor receptor (TNFR) from cowpox virus 48. ORFs 003 and 010 encode proteins that contain ankyrin-like regions 49, and ankyrin repeats are known to form protein-binding domains in a wide variety of proteins 50. Nevertheless, the functions of both of these proteins are unknown.
Putative immunoregulatory proteins found to be differentially abundant in the MPV and VV samples could provide some of the most important insights as to which of the viral genes are responsible for the pathogenic variance between these closely related viruses. As mentioned above, the TNFR homolog MPV J2L (ORF 002) was detected, while the VV homolog (ORFs 002-004) is fragmented. Another VV TNFR homolog, A53R (ORF 179), was not detected in the VV samples, and regardless, this ORF is truncated in VV (Western Reserve strain) and is non-functional 51. These observations suggest a potential role of the MPV TNFR homolog in the increased virulence of MPV compared to VV.
Another immunoregulatory protein that was differentially detected was the secreted viral complement binding protein MPV D14L (ORF 017) and VV C3L (ORF 025). Complement is an important aspect of the innate immune response to invading pathogens, including viruses, and can result in virus inactivation via neutralization, opsonization, viral particle lysis, or phagocytosis 52. Complement regulatory proteins have been found to be encoded by several herpesviruses and poxviruses, and MPV D14L has been speculated to be a major factor in the pathogenesis of Central African strains of MPV (e.g., some Zaire strains) because this gene is completely absent from less virulent strains of MPV (e.g., some USA and West African strains) 7. Interestingly, our proteomic analysis revealed that this protein was not detected in any of the MPV samples, but that its VV homolog was (Table 2). Further, the VV complement binding protein was more abundant in the EEV sample compared to the IMV samples (Supplemental Table 4). Although the MPV complement binding protein probably does play some role in MPV pathogenesis, its absence suggests that its ability to inhibit complement-mediated virus neutralization might actually be hampered compared to VV or to other orthopoxviruses.
Investigating protein abundance variations between different forms of infectious orthopoxviruses (e.g., IMV vs. EEV) could additionally help with the identification of factors that are important to pathogenic properties of each type of virion. The IMV and EEV protein abundance data revealed numerous proteins that were differentially abundant. For example, numerous transcription-associated proteins (e.g., MPV ORFs 050, 094, and 098) were identified as predominantly IMV-specific in both VV and MPV. Although these protein abundance differences might be attributed to contamination (i.e., due to purification of IMV from whole cell lysates versus EEV from cell culture supernatants), it might also indicate that some of these proteins are somehow excluded from virions as they progress from IMV to EEV. Variations in the abundances of proteins that may be involved in pathogenesis were also observed. For example, MPV F3L (ORF 052) was more abundant in monkeypox IMV than EEV. The VV homolog (E3L) is an inhibitor of protein kinase R, which is known to be activated in response to viral infection 53. The higher abundance of F3L in monkeypox IMV suggests a potential variation between IMV and EEV that affects the pathogenic properties of these different forms of infectious virus.
CONCLUSION
Overall, nine MPV-specific and eight VV-specific proteins were identified including four MPV proteins that correspond to fragmented VV ORFs (Table 2). Investigating the pathophysiological roles of proteins observed to be differentially abundant within differentially pathogenic orthopoxviruses increases our understanding of these viruses. Hopefully, this additional knowledge will aid in the discovery of therapeutic targets and in the development of more efficacious vaccines.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of Michael Webb, Inger Damon, Angela Norbeck, Samuel Purvine, Kenneth Auberry, Ashoka Polpitiya, Christina Sorensen, Stephen Callister, Navdeep Jaitly, Vladislav Petyuk, Marina Gritsenko, Liang Shi, Penny Colton, Karin Rodland, Julie Gephart, and Harold Udseth for their assistance. The following reagent was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Polyclonal Anti-Vaccinia Virus (WR) A33R Protein, (antiserum, Rabbit), NR-628. Portions of this work were supported by the National Institute of Allergy and Infectious Diseases (NIH/DHHS through interagency agreement Y1-AI-4894-01) and the NIH National Center for Research Resources (RR18522). Significant portions of this research were performed in the Environmental Molecular Sciences Laboratory, a U.S. Department of Energy (DOE) national scientific user facility located at the Pacific Northwest National Laboratory (PNNL) in Richland, Washington. PNNL is a multiprogram national laboratory operated by Battelle Memorial Institute for the DOE under Contract No. DE-AC05-76RLO-1830.
ABBREVIATIONS
- AMT
accurate mass and elution time
- ATCC
American Type Culture Collection
- BCA
bicinchoninic acid
- CEV
cell-associated enveloped virus
- EEV
extracellular enveloped virus
- IEV
intracellular enveloped virus
- IMV
intracellular mature virus
- LC-MS(/MS)
liquid chromatography coupled with (tandem) mass spectrometry
- MPV
monkeypox virus
- NET
normalized elution time
- SCX
strong cation exchange
- SDS-PAGE
sodium dodecyl sulfate - polyacrylamide gel electrophoresis
- TNFR
tumor necrosis factor receptor
- VV
vaccinia virus
References
- 1.Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis-Jones S. Zoonotic poxvirus infections in humans. Curr Opin Infect Dis. 2004;17(2):81–9. doi: 10.1097/00001432-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 3.McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3(3):201–13. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shchelkunov SN, Totmenin AV, Babkin IV, Safronov PF, Ryazankina OI, Petrov NA, Gutorov VV, Uvarova EA, Mikheev MV, Sisler JR, Esposito JJ, Jahrling PB, Moss B, Sandakhchiev LS. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shchelkunov SN, Totmenin AV, Safronov PF, Mikheev MV, Gutorov VV, Ryazankina OI, Petrov NA, Babkin IV, Uvarova EA, Sandakhchiev LS, Sisler JR, Esposito JJ, Damon IK, Jahrling PB, Moss B. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–94. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shchelkunov SN, Totmenin AV, Safronov PF, Gutorov VV, Ryazankina OI, Petrov NA, Babkin IV, Uvarova EA, Mikheev MV, Sisler JR, Esposito JJ, Jahrling PB, Moss B, Sandakhchiev LS. Multiple genetic differences between the monkeypox and variola viruses. Dokl Biochem Biophys. 2002;384:143–7. doi: 10.1023/a:1016016013042. [DOI] [PubMed] [Google Scholar]
- 7.Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, Davidson W, Galloway R, Khristova ML, Reynolds MG, Zhao H, Carroll DS, Curns A, Formenty P, Esposito JJ, Regnery RL, Damon IK. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(Pt 10):2661–72. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 8.Aragon TJ, Ulrich S, Fernyak S, Rutherford GW. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26. doi: 10.1186/1471-2458-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23(1718):2078–81. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5(2):84–90. [PubMed] [Google Scholar]
- 11.Sodeik B, Krijnse-Locker J. Assembly of vaccinia virus revisited: de novo membrane synthesis or acquisition from the host? Trends Microbiol. 2002;10(1):15–24. doi: 10.1016/s0966-842x(01)02256-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83(Pt 12):2915–31. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 13.Smith GL, Law M. The exit of vaccinia virus from infected cells. Virus Res. 2004;106(2):189–97. doi: 10.1016/j.virusres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Payne LG. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 15.Chung CS, Chen CH, Ho MY, Huang CY, Liao CL, Chang W. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J Virol. 2006;80(5):2127–40. doi: 10.1128/JVI.80.5.2127-2140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoder JD, Chen TS, Gagnier CR, Vemulapalli S, Maier CS, Hruby DE. Pox proteomics: mass spectrometry analysis and identification of Vaccinia virion proteins. Virol J. 2006;3(1):10. doi: 10.1186/1743-422X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B. Protein composition of the vaccinia virus mature virion. Virology. 2007;358(1):233–47. doi: 10.1016/j.virol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Bogdanov B, Smith RD. Proteomics by FTICR mass spectrometry: top down and bottom up. Mass Spectrom Rev. 2005;24(2):168–200. doi: 10.1002/mas.20015. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer JS, Monroe ME, Qian WJ, Smith RD. Advances in proteomics data analysis and display using an accurate mass and time tag approach. Mass Spectrom Rev. 2006;25(3):450–82. doi: 10.1002/mas.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T, Belov ME, Jaitly N, Qian WJ, Smith RD. Accurate mass measurements in proteomics. Chem Rev. 2007;107(8):3621–53. doi: 10.1021/cr068288j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston JB, McFadden G. Poxvirus immunomodulatory strategies: current perspectives. J Virol. 2003;77(11):6093–100. doi: 10.1128/JVI.77.11.6093-6100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas A, McFadden G. Secreted immunomodulatory viral proteins as novel biotherapeutics. J Immunol. 2004;173(8):4765–74. doi: 10.4049/jimmunol.173.8.4765. [DOI] [PubMed] [Google Scholar]
- 23.Doms RW, Blumenthal R, Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J Virol. 1990;64(10):4884–92. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adkins JN, Mottaz HM, Norbeck AD, Gustin JK, Rue J, Clauss TR, Purvine SO, Rodland K, Heffron F, Smith RD. Analysis of the salmonella typhimurium proteome through environmental response towards infectious conditions. Mol Cell Proteomics. 2006;5(8):1450–61. doi: 10.1074/mcp.M600139-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Craig R, Beavis RC. A method for reducing the time required to match protein sequences with tandem mass spectra. Rapid Commun Mass Spectrom. 2003;17(20):2310–6. doi: 10.1002/rcm.1198. [DOI] [PubMed] [Google Scholar]
- 26.Lefkowitz EJ, Upton C, Changayil SS, Buck C, Traktman P, Buller RM. Poxvirus Bioinformatics Resource Center: a comprehensive Poxviridae informational and analytical resource. Nucleic Acids Res. 2005;33:D311–6. doi: 10.1093/nar/gki110. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004;32:W20–5. doi: 10.1093/nar/gkh435. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R. The International Protein Index: an integrated database for proteomics experiments. Proteomics. 2004;4(7):1985–8. doi: 10.1002/pmic.200300721. [DOI] [PubMed] [Google Scholar]
- 29.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19(3):242–7. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 30.Petritis K, Kangas LJ, Yan B, Monroe ME, Strittmatter EF, Qian WJ, Adkins JN, Moore RJ, Xu Y, Lipton MS, Camp DG, 2nd, Smith RD. Improved peptide elution time prediction for reversed-phase liquid chromatography-MS by incorporating peptide sequence information. Anal Chem. 2006;78(14):5026–39. doi: 10.1021/ac060143p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monroe ME, Shaw JL, Daly DS, Adkins JN, Smith RD. MASIC: a software program for fast quantitation and flexible visualization of chromatographic profiles from detected LC-MS(/MS) features. Bioinformatics. 2007 doi: 10.1016/j.compbiolchem.2008.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strittmatter EF, Kangas LJ, Petritis K, Mottaz HM, Anderson GA, Shen Y, Jacobs JM, Camp DG, 2nd, Smith RD. Application of peptide LC retention time information in a discriminant function for peptide identification by tandem mass spectrometry. J Proteome Res. 2004;3(4):760–9. doi: 10.1021/pr049965y. [DOI] [PubMed] [Google Scholar]
- 33.Qian WJ, Liu T, Monroe ME, Strittmatter EF, Jacobs JM, Kangas LJ, Petritis K, Camp DG, 2nd, Smith RD. Probability-based evaluation of peptide and protein identifications from tandem mass spectrometry and SEQUEST analysis: the human proteome. J Proteome Res. 2005;4(1):53–62. doi: 10.1021/pr0498638. [DOI] [PubMed] [Google Scholar]
- 34.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 35.Qian WJ, Jacobs JM, Camp DG, 2nd, Monroe ME, Moore RJ, Gritsenko MA, Calvano SE, Lowry SF, Xiao W, Moldawer LL, Davis RW, Tompkins RG, Smith RD. Comparative proteome analyses of human plasma following in vivo lipopolysaccharide administration using multidimensional separations coupled with tandem mass spectrometry. Proteomics. 2005;5(2):572–84. doi: 10.1002/pmic.200400942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiebel GR, Auberry KJ, Jaitly N, Clark DA, Monroe ME, Peterson ES, Tolic N, Anderson GA, Smith RD. PRISM: a data management system for high-throughput proteomics. Proteomics. 2006;6(6):1783–90. doi: 10.1002/pmic.200500500. [DOI] [PubMed] [Google Scholar]
- 37.Monroe ME, Tolic N, Jaitly N, Shaw JL, Adkins JN, Smith RD. VIPER: an advanced software package to support high-throughput LC-MS peptide identification. Bioinformatics. 2007;23(15):2021–3. doi: 10.1093/bioinformatics/btm281. [DOI] [PubMed] [Google Scholar]
- 38.Tolmachev AV, Monroe ME, Jaitly N, Petyuk VA, Adkins JN, Smith RD. Mass measurement accuracy in analyses of highly complex mixtures based upon multidimensional recalibration. Anal Chem. 2006;78(24):8374–85. doi: 10.1021/ac0606251. [DOI] [PubMed] [Google Scholar]
- 39.Jaitly N, Monroe ME, Petyuk VA, Clauss TR, Adkins JN, Smith RD. Robust algorithm for alignment of liquid chromatography-mass spectrometry analyses in an accurate mass and time tag data analysis pipeline. Anal Chem. 2006;78(21):7397–409. doi: 10.1021/ac052197p. [DOI] [PubMed] [Google Scholar]
- 40.Anderson KK, Monroe ME, Daly DS. Estimating probabilities of peptide database identifications to LC-FTICR-MS observations. Proteome Sci. 2006;4:1. doi: 10.1186/1477-5956-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petyuk VA, Qian WJ, Chin MH, Wang H, Livesay EA, Monroe ME, Adkins JN, Jaitly N, Anderson DJ, Camp DG, 2nd, Smith DJ, Smith RD. Spatial mapping of protein abundances in the mouse brain by voxelation integrated with high-throughput liquid chromatography-mass spectrometry. Genome Res. 2007;17(3):328–36. doi: 10.1101/gr.5799207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18(1):207–8. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 43.Kapp EA, Schutz F, Connolly LM, Chakel JA, Meza JE, Miller CA, Fenyo D, Eng JK, Adkins JN, Omenn GS, Simpson RJ. An evaluation, comparison, and accurate benchmarking of several publicly available MS/MS search algorithms: sensitivity and specificity analysis. Proteomics. 2005;5(13):3475–90. doi: 10.1002/pmic.200500126. [DOI] [PubMed] [Google Scholar]
- 44.Calderara S, Xiang Y, Moss B. Orthopoxvirus IL-18 binding proteins: affinities and antagonist activities. Virology. 2001;279(1):22–6. doi: 10.1006/viro.2000.0689. [DOI] [PubMed] [Google Scholar]
- 45.Baxby D. Jenner’s smallpox vaccine: The riddle of vaccinia virus and its origin. Heinemann; Oxford: 1981. [Google Scholar]
- 46.Price N, Tscharke DC, Hollinshead M, Smith GL. Vaccinia virus gene B7R encodes an 18-kDa protein that is resident in the endoplasmic reticulum and affects virus virulence. Virology. 2000;267(1):65–79. doi: 10.1006/viro.1999.0116. [DOI] [PubMed] [Google Scholar]
- 47.Gubser C, Hue S, Kellam P, Smith GL. Poxvirus genomes: a phylogenetic analysis. J Gen Virol. 2004;85(Pt 1):105–17. doi: 10.1099/vir.0.19565-0. [DOI] [PubMed] [Google Scholar]
- 48.Hu FQ, Smith CA, Pickup DJ. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology. 1994;204(1):343–56. doi: 10.1006/viro.1994.1539. [DOI] [PubMed] [Google Scholar]
- 49.Shchelkunov SN, Blinov VM, Sandakhchiev LS. Ankyrin-like proteins of variola and vaccinia viruses. FEBS Lett. 1993;319(12):163–5. doi: 10.1016/0014-5793(93)80059-4. [DOI] [PubMed] [Google Scholar]
- 50.Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13(6):1435–48. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alcami A, Khanna A, Paul NL, Smith GL. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J Gen Virol. 1999;80(Pt 4):949–59. doi: 10.1099/0022-1317-80-4-949. [DOI] [PubMed] [Google Scholar]
- 52.Bernet J, Mullick J, Singh AK, Sahu A. Viral mimicry of the complement system. J Biosci. 2003;28(3):249–64. doi: 10.1007/BF02970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang HW, Watson JC, Jacobs BL. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992;89(11):4825–9. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolffe EJ, Weisberg AS, Moss B. The vaccinia virus A33R protein provides a chaperone function for viral membrane localization and tyrosine phosphorylation of the A36R protein. J Virol. 2001;75(1):303–10. doi: 10.1128/JVI.75.1.303-310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.