Abstract
An important means of determining how amyloid-beta peptide (Aβ) affects cells is to identify specific macromolecular targets and assess how Aβ interaction with such targets impacts on cellular functions. On the one hand, cell surface receptors interacting with extracellular Aβ have been identified, and their engagement by amyloid peptide can trigger intracellular signaling cascades. Recent evidence has indicated a potentially significant role for deposition of intracellular Aβ in cell stress associated with amyloidosis. Thus, specific intracellular targets of Aβ might also be of interest. Our review evaluates the potential significance of Aβ interaction with a mitochondrial enzyme termed Aβ-binding alcohol dehydrogenase (ABAD), a member of the short-chain dehydrogenase-reductase family concentrated in mitochondria of neurones. Binding of Aβ to ABAD distorts the enzyme's structure, rendering it inactive with respect to its metabolic properties, and promotes mitochondrial generation of free radicals. Double transgenic mice in which increased levels of ABAD are expressed in an Aβ-rich environment, the latter provided by a mutant amyloid precursor protein transgene, demonstrate accelerated decline in spatial learning/memory and pathologic changes. These data suggest that mitochondria ABAD, ordinarily a contributor to metabolic homeostasis, has the capacity to become a pathogenic factor in an Aβ-rich environment.
Keywords: amyloid-beta peptide, crystallization, reactive oxygen species, short-chain dehydrogenase reductase, transgenic mouse
There have been many links between mitochondrial function/dysfunction and Alzheimer's disease (AD) (Arispe et al. 1994) over the years. Such a connection is rational, because meeting neuronal requirements for metabolic (especially energetic) homeostasis is a prerequisite for cognitive function. Regional hypometabolism has been assessed by imaging studies using positron emission tomography with 18F-fluorodeoxyglucose. A pattern of bilateral temporo-parietal hypometabolism, especially associated with dementia, is considered characteristic of AD (Silverman et al. 1999; Hoffman et al. 2000; Silverman et al. 2002). Such defects in glucose utilization suggest possible abnormalities in mitochondrial function in AD. In support of this possibility, a range of reports have shown altered mitochondrial properties in AD. For example, decreased activity of complex IV (cytochrome c oxidase or COX) has been observed in the central nervous system, and even in peripheral tissues, such as platelets, of patients with AD (Kish et al. 1992; Mutisya et al. 1994; Parker et al. 1994; Maurer et al. 2000; Bosetti et al. 2002). The latter is the most consistently noted mitochondrial abnormality in AD tissue. Indeed, studies with cybrids, in which mitochondria from patient tissue/cell samples (platelets) are transferred to a cell line depleted of mitochondrial DNA (thus, the only mitochondrial DNA is from the patient sample), have also demonstrated decreased COX activity (Khan et al. 2000). In addition, others have shown a decrease in the area of intact mitochondria in AD brain, vs. control, and evidence of mitochondrial morphologic abnormalities associated with oxidative modification of guanosine (8-hydroxyguanosine) and increased levels of nitrotyrosine (Castellani et al. 2002). Although such reports support the possibility of specific mitochondrial dysfunction, it is important to point out that complex IV activity can be inhibited by a range of environmental perturbations, such as a milieu rich in nitric oxide and ischemia (Lehninger et al. 1960; Lesnefsky et al. 2001; Chen et al. 2003). Still, studies in cell culture demonstrating a role for proapoptotic Bax in Aβ-induced apoptosis, mediated via Bax-induced increased permeability of the outer mitochondrial membrane, caspase activation and cell death (Giovanni et al. 2000; Zhang et al. 2002), indicate that mitochondria remain important suspects as pathogenic contributors to Aβ-mediated cellular dysfunction.
The counterpart of these studies on patient samples/intact cells is experiments using isolated mitochondria exposed to Aβ. For example, rat brain mitochondria incubated with Aβ showed decreased state 3 and 4 mitochondrial respiration (Casley et al. 2002). COX, β-ketoglutatrate dehydrogenase and pyruvate dehydrogenase activities were also diminished in mitochondria incubated with Aβ (Casley et al. 2002)). In another study, liver mitochondria exposed to Aβ displayed a drop in membrane potential and increased susceptibility to matrix swelling, though brain mitochondria, by comparison, were less vulnerable to the effects of Aβ (Moreira et al. 2002). As might be expected from studies using intact cells, in which Aβ can trigger apoptotic cascades (Loo et al. 1993; LaFerla et al. 1995; Mattson et al. 1998), incubation of Aβ with brain mitochondria has been shown to cause cytochrome c release, as well as mitochondrial swelling (Kim et al. 2002). The relevance of these results, from experiments in which isolated mitochondria were exposed to micromolar levels of synthetic Aβ peptides, to the in vivo situation is yet to be determined.
Taken together, these studies indicate the likelihood that there is an association of mitochondrial dysfunction with AD. However, it is difficult to discern cause–effect relationships from such data, especially in view of the ability of Aβ to non-specifically perturb membranes (Arispe et al. 1994; Pillot et al. 1996; Muller et al. 2001). Nonetheless, cell culture studies in which mitochondria were rendered non-functional by depletion of mitochondrial DNA, resulting in lack of essential subunits for the respiratory chain, demonstrated cytoprotection in the presence of Aβ (Cardoso et al. 2001). These findings, along with the results of the studies mentioned above, led us to consider the possibility that mitochondria might have particular macromolecular components rendering them vulnerable to the effects of Aβ. This review describes our studies to delineate a mitochondrial target of Aβ in terms of the consequences for neuronal function.
Recognition of ABAD as a binding protein for Aβ
Some years ago, we hypothesized that early in the course of AD, when levels of Aβ were lower, the amyloid peptide would seek out specific cellular targets through which it might modify neuronal function. In this regard, our laboratory identified Receptor for Advanced Glycation Endproducts (RAGE) as a multiligand receptor whose repertoire of ligands included crossed β-sheet fibrils (Yan et al. 1996; Schmidt et al. 2001). We have subsequently shown that the interaction of nanomolar levels of Aβ with RAGE-bearing cells induces stress with a number of outcomes, including, at least in vitro, induction of programmed cell death. Studies in transgenic mice overexpressing mutant amyloid precursor protein (mAPP) and wild-type RAGE under control of the platelet-derived growth factor (PDGF) B-chain promoter have demonstrated accelerated neuronal dysfunction at the level of behavioural studies, markers of synaptic plasticity and enhanced neuropathologic changes, compared with single transgenics (expressing only the mAPP transgene) and other control groups (Arancio et al. 2004). Other cell-surface receptors/binding sites, such as the macrophage scavenger receptor (type A), α7-nicotinic acetylcholine receptor, proteoglycans, α-5-β-1 integrins, serpin enzyme complex receptor and neurotrophin receptor (p75), have also been shown to bind Aβ (Narindrasorasak et al. 1991; Buee et al. 1993; Snow et al. 1994, 1995; Boland et al. 1996; El Khoury et al. 1996; Paresce et al. 1996; Watson et al. 1997; Yaar et al. 1997; Matter et al. 1998; Wang et al. 2000). Low density lipoprotein (LDL) receptor-related protein interacts with Aβ in complex with other molecules, such as apolipoproteins and α2-macroglobulin (Hammad et al. 1997; Narita et al. 1997). The pathophysiologic significance of these cell surface-binding sites/receptors for Aβ-induced cellular dysfunction is something that remains to be clarified.
Several lines of investigation have indicated that intracellular Aβ may be an important contributor to Aβ-mediated cytotoxic events. First, intracellular accumulation of Aβ has been demonstrated in the endoplasmic reticulum of cultured cells by several laboratories (Cook et al. 1997; Hartmann et al. 1997; Tienari et al. 1997; Wild-Bode et al. 1997), and intracellular Aβ has been shown in brains from patients with AD and Down's syndrome, as well as in transgenic mice overexpressing mAPP (Hammad et al. 1997; Gouras et al. 2000; D'Andrea et al. 2001; Gyure et al. 2001). Importantly, intracellular Aβ appears to accumulate prior to the appearance of neurofibrillary tangles and senile plaques, and to be present in AD-affected areas of the brain. Furthermore, the toxic effects of intracellular Aβ on cells has been shown, in certain cases, to exceed those of extracellular Aβ (Kienlen-Campard et al. 2002; Zschocke et al. 2000). These data are consistent with the relevance of intracellular Aβ to disturbances in cellular function associated with AD.
For this reason, we have sought possible intracellular targets of Aβ. Using the yeast two-hybrid system, our laboratory found that a member of the short chain dehydrogenase-reductase family, which we have termed amyloid-β alcohol dehydrogenase (ABAD), binds Aβ (Yan et al. 1997). From screening brain and HeLa libraries using the yeast two-hybrid system, we obtained four positives: three from the brain library and one from the HeLa library. In each case, the positive clones encoded ABAD. ABAD is identical to L-3-hydroxyacyl Coenzyme A dehydrogenase type II, first identified by investigators analysing fatty acid β-oxidation (Kobayashi et al. 1996; Furuta et al. 1997) in their analysis of 3-hydroxyacyl CoA dehydrogenase activity in liver. The enzyme shares many properties with other members of the superfamily of short-chain dehydrogenase reductases or short-chain alcohol dehydrogenase family, including dinucleotide cofactor binding and catalytic sites (Eaton et al. 1996; Krozowski 1994). Unique features of ABAD include its presence in endoplasmic reticulum and mitochondria, its broad substrate specificity, and its capacity to bind Aβ and promote Aβ-induced cell stress (Yan et al. 1997; Yan et al. 1999, 2000; Lustbader et al. 2004). In neurons, ABAD is especially enriched in mitochondria (Furuta et al. 1997; Torroja et al. 1998; He et al. 2001) where it appears to serve important functions in metabolic homeostasis.
Role of ABAD in metabolic homeostasis
ABAD is an enzyme with broad substrate specificity. The enzyme catalyses the reversible NAD/NADH-dependent oxidation/reduction of a range of substrates, linear alcohols and steroid substrates (such as 17β-estradiol), S-acetoacetyl-CoA and β-hydroxybutyrate (Yan et al. 1999 2000). Although these data provide insights into the repertoire of substrates with which ABAD can interact, they do not provide a clear concept of physiologic properties/substrates of ABAD. For example, mitochondrial d-β-hydroxybutyrate dehydrogenase displays Km approximately 1 mm towards its substrate (Lehninger et al. 1960; Bock & Fleischer 1975). Futhemore, Vmax of the latter enzyme for d-β-hydroxybutyrate is over 1000 times greater than that of ABAD. Thus, it is possible to conclude that ABAD might have little or no role in β-hydroxybutyrate metabolism in vivo. However, such a conclusion would not take into account the local environment, and we have found that ABAD expression is sensitive to situations such as ischaemic/nutritional stress, in which β-hydroxybutyrate becomes a critical substrate for metabolic homeostasis.
To analyse the role of ABAD in β-hydroxybutyrate metabolism, we have overexpressed the enzyme in COS cells and placed cultures in medium devoid of glucose using β-hydroxybutyrate as the principal energy substrate (serum in the culture medium was dialysed to remove glucose) (Yan et al. 2000). Whereas control (vector-transfected) COS cells displayed loss of viability over 2–4 days in culture under these conditions, accompanied by a rapid fall in ATP, COS cells overexpressing ABAD better maintained their viability and ATP content. NMR analysis of cultures following addition of [13C]d-β-hydroxybutyrate demonstrated increased flux of acetyl-CoA through the tricarboxylic acid/Krebs cycle in COS cells overexpressing ABAD, compared with controls (Yan et al. 2000). To determine the relevance of these in vitro data to neuronal stress in vivo, experiments were performed using a murine model of stroke, transient middle cerebral ischemia (Yan et al. 2000). First, wild-type animals were subjected to stroke, and expression of ABAD was assessed. Increased ABAD antigen was observed in neurons proximal to the infracted area, especially those in the penumbra, compared with controls. Image analysis of immunohistochemical data demonstrated apptoximately 5-fold increased levels of ABAD antigen in cortical neurones in the ischaemic territory compared with controls. These experiments, indicating the relevance of ABAD to the setting of ischaemic stress, led us to produce transgenic (Tg) mice overexpressing ABAD under control of the PDGF B-chain promoter (Yan et al. 2000). The latter animals, termed Tg ABAD mice, displayed increased neuronal expression of ABAD in the cerebral cortex, which was present in the appropriate subcellular locations, including mitochondria. To our surprise, Tg ABAD animals had higher baseline levels of brain ATP and showed increased flux of acetyl-CoA through the tricarboxylic acid cycle following administration of [13C]d-β-hydroxybutyrate in the fasted state (Yan et al. 2000). Following induction of stroke, Tg ABAD mice displayed infarcts of lower volume and decreased neurological deficit scores, consistent with greater resistance to ischaemic stress, compared with non-Tg littermates (Yan et al. 2000). These data are consistent with a role for ABAD in the metabolic defence against ischaemic stress.
Studies of ABAD deficiency have provided considerable insight into the role of the enzyme in development and neuronal homeostasis. When the ABAD homolog in Drosophila, scully, was functionally inactivated, a developmentally lethal phenotype with multiple abnormalities was observed (Torroja et al. 1998). Certain salient features of the scully/ABAD-deficient flies included mutant testes which were small and undeveloped. The testes displayed deficiency of 3-hydroxyacyl-CoA dehydrogenase activity, indicating that ABAD is the principal enzyme with such activity in this tissue. Spermatocytes displayed cytoplasmic accumulation of fat-containing vesicles and scarce mitochondria, consistent with a defect in fatty acid oxidation and scarce mitochondria. Photoreceptor cell mitochondria were small, few and showed swollen cristae compared with controls. Although these data do not necessarily assign ABAD activity to a particular metabolic pathway, the enzyme's central role in metabolic homeostasis is clear. Furthermore, its contribution to fatty acid β-oxidation is emphasized. However, ABAD clearly has properties in addition to the latter in the in vivo setting, in view of its role in the central nervous system where fatty acids are not a key energy substrate (it is possible that β-hydroxybutyrate is an essential substrate of ABAD in the brain).
Another important piece of evidence was derived from observations in patients with methyl-3-hydroxybutyryl-CoA dehydrogenase (MHBD). The latter enzyme identified based on its participation in the catabolism of isoleucine and branched-chain fatty acids turns out to be identical to ABAD. Five cases of clinical MHBD/ABAD deficiency have been observed as of 2004 (Zschocke et al. 2000; Ofman et al. 2003; Sass et al. 2004), based on elevated levels of two urinary metabolites (2-methyl-3-hydroxybutyrate and tiglyl glycine) and absence of MHBD activity (NAD-dependent conversion of 2-methyl-3-hydroxybutyryl-CoA to 2-methyl-acetoacetyl-CoA). Patients with defects in MHBD/ABAD activity showed severe neurologic symptoms, including psychomotor retardation and progressive infantile neurodegeneration.
Taken together, these data indicate that ABAD might have several important roles in metabolic homeostasis related to energy metabolism and catabolism of isoleucine and branched-chain fatty acids. Of course, there may be other properties of the enzyme that are also important for the protective effect associated with ABAD under homeostatic conditions and in response to stress. In this context, we have observed that ABAD binds the mitochondrial chaperonin molecule cyclophilin D (unpublished observed, Yan & Stern 2004), a peptidylprolyl cis-trans isomerase whose activity and association with the inner mitochondrial membrane have been linked to opening of the mitochondrial membrane permeability transition pore (MPT) (Crompton et al. 1998; Woodfield et al. 1998; Crompton 1999). Furthermore, in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of murine Parkinsonism, overexpression of ABAD in neurons had a cytoprotective effect (Tieu et al. 2004). It appeared that increased levels of ABAD mitigated MPTP-induced inhibition of oxidative phosphorylation and ATP production. Thus, there is reason to believe that ABAD might have multiple protective effects on cellular functions.
Role of ABAD in Aβ-induced cell stress
In order to assess the possible relevance of ABAD to AD, studies in AD brain (n = 19) and non-demented controls (n = 15), matched for age, were performed using samples harvested according to the rapid autopsy method developed at Sun Health Research Institute, in order to minimize postmortem delay (Lue et al. 2001). Immunoblots of brain extracts were compared by laser densitometry, and each band was normalized according to the intensity of the β-actin band. The results showed that the intensity of the ABAD band was increased approximately 28% (inferior temporal lobe gyrus) and 40% (hippocampus) in samples from AD patients vs. non-demented controls (Lustbader et al. 2004). These data are consistent with our previous work (Yan et al. 2000), demonstrating enhanced expression of ABAD in AD brain by immunoblotting with anti-ABAD antibody. In contrast, protein extracts prepared from the cerebellar region, a portion of the brain not affected by AD, showed no significant differences in ABAD expression compared with non-demented controls. These data indicate that ABAD expression increases in affected regions of AD brain. However, it was equally possible that such elevated levels of ABAD could reflect either a protective or deleterious response to the Aβ-rich environment.
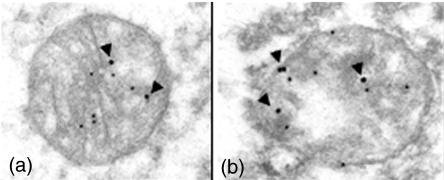
In view of the presence of ABAD in mitochondria, a compartment not previously shown to accumulate Aβ, it was important to determine if ABAD–Aβ interaction might occur in AD brain. Protein extracts of AD brain were subjected to immunoprecipitation with anti-Aβ imuunoglobulin G (IgG) followed by SDS-PAGE, transfer of proteins to nitrocellulose membranes and immunoblotting with anti-ABAD IgG (Lustbader et al. 2004). The results demonstrated a strong immunoreactive band in AD brain, though not in non-demented controls (the same result was obtained when the order of the antibodies was switched, though no specific band was observed when either antibody was replaced with with non-immune IgG). Because preparation of samples for immunoprecipitation disturbs tissue architecture, morphologic studies were performed. Confocal microscopy of affected regions of AD brain displayed colocalization of ABAD and Aβ antigens. In addition, ABAD antigen was colocalized with a mitochondrial marker, voltage-dependent anion channel. At the level of transmission electron microscopy, ABAD and Aβ were also colocalized to mitochondria using gold-labelled antibodies (Aβ, 12 nM gold particles; ABAD, 18 nM gold particles; Figure 1a,b) (Lustbader et al. 2004). These studies demonstrate that ABAD–Aβ interaction can occur in AD brain, and that Aβ is present in mitochondria, a previously unrecognized observation.
Figure 1.
Colocalization of amyloid-beta peptide alcohol dehydrogenase (ABAD) and Aβ in the brain of a patient with Alzheimer's disease. Double immunogold staining with anti-Aβ immunoglobulin G (IgG) and anti-ABAD IgG was employed. Twelve nanomolar gold particles denote sites of binding of the primary anti-Aβ(1–42) antibody, and 18 nM gold particles denote sites of binding of the primary anti-ABAD antibody. Arrowheads depict gold particles localizing ABAD antigen. Panels a and b show two representative fields. Modified from reference (Lustbader et al. 2004).
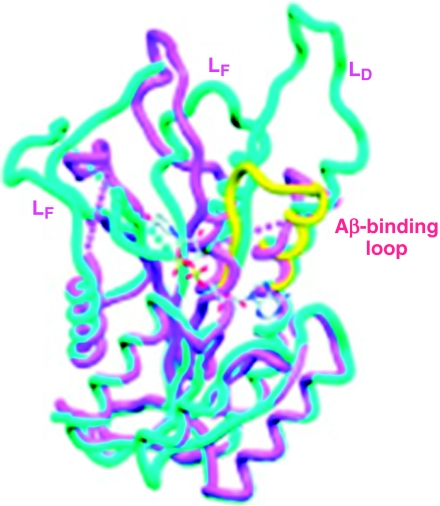
Results in the yeast two-hybrid system, indicating the possibility that Aβ bound to ABAD, lead us to perform further studies to determine parameters of this interaction. Using plasmon resonance, Aβ(1–40), Aβ(1–42) and Aβ(1–20) were found to bind to ABAD in a dose-dependent manner with half-maximal occupancy of binding sites at 40–80 nM (Lustbader et al. 2004). In contrast, Aβ(25–35), Aβ-derived peptides with a reversed sequence, amylin, and prion-derived peptide (109–141) displayed no specific binding. To further characterize this interaction, structural studies were performed on crystals of ABAD-Aβ mixtures formed in the presence of NAD (Lustbader et al. 2004). To our surprise, NAD was not bound to ABAD in the crystal structure suggesting that Aβ resulted in a considerable distortion of ABAD. This indeed proved to be the case; native ABAD displays an NAD-binding pocket and the expected catalytic triad associated with enzymatic activity (Figure 2; the native enzyme is shown in cyano). Note the prominent distortion of ABAD in the presence of Aβ, with deformation of the LD, LE and LF loops, as well as the NAD-binding pocket (Figure 2, Aβ-bound ABAD is shown in magenta). Another salient feature of ABAD structure is the LD loop. Comparison of the LD loop of native ABAD with that of another similar member of the short-chain dehyrogenase reductase family, 3 β-hydroxysteroid dehydrogenase (Figure 2, shown in yellow) demonstrates the possibility of an insertion in this portion of the ABAD sequence. In fact, extensive sequence comparisons of ABAD from five different species with four other members of this enzyme superfamily indicate the likelihood of an 11 amino acid insertion in the LD loop of ABAD. Taken together with the disordered structure of the LD in ABAD-Aβ crystals and the apparent absence (disorder) of Aβ in the crystals, we considered the possibility that this portion of ABAD (residence 91–119) might be the actual binding site for Aβ. Studies with this ABAD-derived peptide [termed ABAD (91–119) or ABAD decoy peptide or DP] indicated that it served as an inhibitor of ABAD–Aβ interaction, whereas a peptide with the same amino acids, but reversed sequence [termed ABAD(119–91) or ABAD reversed peptide or RP] was inactive.
Figure 2.
Structure of Aβ-bound human amyloid-beta peptide alcohol dehydrogenase (ABAD)/superposition of Aβ-bound human ABAD (magenta) and rat ABAD in complex with NAD (cyano). The LD loop of 3β-hydroxysteroid dehydrogenase (3β-HSD) is shown in yellow. The proposed Aβ-binding loop is indicated. The LD, LE and LF loops of ABAD are shown, along with a ball and stick model for the binding site of NAD in rat ABAD–NAD complex. Modified from reference (Lustbader et al. 2004).
An important prediction of these structural studies was that Aβ would inhibit ABAD enzymatic activity towards all of its substrates. This prediction was borne out in experiments with multiple substrates, including S-acetoacetyl-CoA, octanol and 17β-estradiol (Yan et al. 1999). However, the concentration of Aβ necessary for half-maximal inhibition of ABAD was in the low micromolar range (1–3 µm), rather than in the nanomolar range (40–80 nm), the latter corresponding to concentrations of Aβ which bind ABAD. Our concept is that the N-terminus of the peptide serves to localize Aβ at critical sites within the cell (in this case, associated with ABAD), and the C-terminal portion of the peptide is free to interact with additional molecules of Aβ forming oligomers, potentially fibrillar structures that further deform protein structure. Thus, binding of an initial molecule of Aβ to ABAD probably does not affect the enzyme's activity significantly. However, when oligomers of Aβ form, using the initial ABAD-bound Aβ as a nidus (at higher concentrations of Aβ, such as that used for our crystallization studies), the resulting macromolecular assembly distorts the enzyme and alters its function. In this context, it is important to note that from a structural standpoint, there is a large cavity (‘hole’) in the structure of the ABAD-Aβ complex which could accommodate a fibrillar assembly. Specifically, inspection of the crystal packing shows that ordered ends of the LD loop point into interconnected huge solvent channels with estimated dimensions of 70 Å. We estimate that the ordered part of the crystal only occupies about 30% of the total crystal volume. Sufficient space is therefore left available for the disordered loops and the bound Aβ (as well as macromolecular assemblies of Aβ), which could drift freely in the large solvent channels in the crystal to cause disorder and/or non-specifically bind and clog the active site region to inactivate the enzyme.
For the purpose of developing an experimental system to use in future studies, Tg mice overexpressing ABAD in an Aβ-rich environment were made (Lustbader et al. 2004; Takuma et al. 2005). Overexpression of mAPP was achieved using mice made in the laboratory of Dr Lennart Mucke (Hsia et al. 1999). These animals (termed Tg mutant or mAPP) express an alternatively spliced human APP minigene that encodes human APP695, APP751 and APP770 bearing mutations linked to familial AD (V717F, K670M, N671L) under control of the PDGF B-chain promoter and have been backcrossed into the C57BL6 background for these studies (Yan et al. 2000). Tg mice overexpressing ABAD, also under control of the PDGF B-chain promoter and in the C57BL6 background, were crossed with Tg mAPP to generate four genotypes; Tg mAPP/ABAD (double transgenics), Tg mAPP, Tg ABAD (single transgenics) and nonTg littermates.
First, we analysed cortical neurones from Tg mAPP/ABAD mice to determine if they displayed exaggerated evidence of Aβ-induced stress in cell culture. Specifically, we evaluated induction of oxidant stress, which has a counterpart in AD brain and transgenic models of AD-type pathology (Wild-bode et al. 1997; Pappolla et al. 1998; Perry et al. 1998; Eckert et al. 2001; Tabner et al. 2002; Marques et al. 2003; Tamagno et al. 2003). Neurons from Tg mAPP/ABAD mice demonstrated evidence of free radical-induced stress based on oxidation of 2′,7′-dichlorofuorescin (Yaar et al. 1997). Next, we wanted to determine whether ABAD–Aβ interaction was involved in such generation of oxidant stress. In order to utilize ABAD-DP to antagonize this interaction, we had to produce a membrane permeable form. The latter was accomplished using the membrane transduction domain of the human immunodeficiency virus type 1 (Aarts et al. 2002). A fusion peptide, tat-ABAD-DP, was added to culture medium of neurones from Tg mAPP/ABAD mice. In the presence of tat-ABAD-DP, induction of oxidant stress and, specifically, generation of superoxide anion and hydrogen peroxide were inhibited, though the reversed peptide was without effect (Lustbader et al. 2004; Takuma et al. 2005).
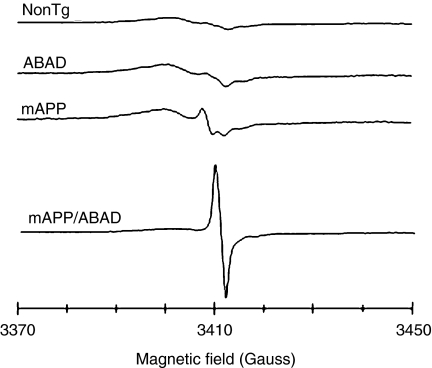
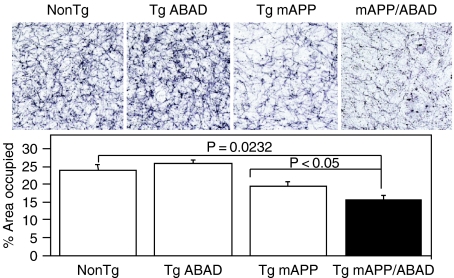
These data suggested that increased levels of ABAD in an Aβ-rich environment potentiate amyloid peptide-induced cell stress. To probe this issue further, brains from each of the genotypes of transgenic mice (as above) were studied using electron paramagnetic resonance (EPR) spectroscopy to identify free radicals (Figure 3). Higher levels of radicals were observed in the Tg mAPP/ABAD mouse brains, compared with brains from non-Tg, Tg ABAD or Tg mAPP mice. The species of free radicals, which could be from ascorbyl or a one-electron reduced ubiquinone radical, is likely to be generated because of the higher level of oxidant stress in the double transgenics. In addition to enhanced oxidant stress, Tg mAPP/ABAD mice displayed impaired spatial learning/memory in the radial arm water maze test to detect hippocampal-dependent learning/memory defects (Bliss & Collingridge 1993; Morgan et al. 2000). In contrast to non-Tg littermates, Tg mAPP and Tg ABAD mice, double transgenic animals at only 5 months of age already failed to learn efficiently. Furthermore, the area occupied by acetylcholinesterase-positive neurites in the subiculum, entorhinal cortex and CA-1 was significantly decreased in Tg mAPP/ABAD mice compared with the other genotypes (Figure 4). These data indicate that Tg mAPP/ABAD mice display exaggerated cytotoxicity of a type associated with AD-type pathology.
Figure 3.
Generation of free radicals in transgenic mutant amyloid precursor protein (Tg mAPP)/ amyloid-beta peptide alcohol dehydrogenase (ABAD) mouse brain is shown by the peak at 3410 Gauss in electron paramagnetic resonance spectra recorded on brain tissues froezen in liquid nitrogen using a quartz dewar at X-band. The amplitude of the spectra for Tg mAPP, Tg ABAD and non-Tg animals has been increased by 10-fold to display spectra, which showed only low-level changes. Modified from reference (Lustbader et al. 2004).
Figure 4.
AChE activity was determined histochemically in the subiculum of mice of the indicated genotype at 12 months old. The bar graph shows the results of the multiple images from numbers of mice. Upper panel indicates representative images from AChE histochemical staining from subiculum of mice.
Our findings in transgenic mice led us to analyse in further detail properties of cortical neurones cultured from each of the genotypes. Although these studies are ongoing, several findings have already been clearly delineated. First, neurones from Tg mAPP/ABAD mice spontaneously generate superoxide anion and hydrogen peroxide as a consequence of mitochondrial leakage of reactive oxygen species (Takuma et al. 2005). Production of oxygen radicals occurs at the level of complex III, and is also associated with opening of the MPT. These oxygen-free radicals trigger a programmed cell death pathway leading to activation of caspase-3, DNA fragmentation and, ultimately, loss of cell viability. Thus, neurones from mice overexpressing both mutant APP and ABAD display evidence of severe cellular stress. We propose that these mice are a model of accelerated and exaggerated cell stress relevant to the role of ABAD in AD. Further studies to link ABAD to the pathogenesis of cellular dysfunction in Alzheimer's-type pathology in murine models are underway through generation of animals deficient in ABAD and studies using agents to inhibit ABAD–Aβ interaction in vivo.
Hypothesis
ABAD is a member of the short-chain dehydrogenase reductase superfamily of intracellular enzymes present within neurones predominately in mitochondria. In the absence of Aβ, ABAD appears to have cytoprotective functions under homeostatic conditions and in response to stress. Result of studies in which the Drosophila counterpart of ABAD (scully) was inactivated displayed a phenotype resembling that observed in other defects in the fatty acid β-oxidation pathway (Eaton et al. 1996). The latter display cytoplasmic accumulation of lipid, because fatty acids are not effectively imported into mitochondria due to depletion of the mitochondrial CoA pool. Targeted overexpression of ABAD in neurones of transgenic mice caused an increase in baseline ATP and more effective utilization of β-hydroxybutyrate, a substrate mobilized and metabolized in response to nutritional and other stresses. Nonetheless, it is difficult to be certain which substrate of ABAD is physiologically relevant in the brain. For example, systemic fatty acids are not considered an important contributor to energy homeostasis in the brain. In the rodent brain, this may be due to the low levels of 3-ketoacyl-CoA thiolase activity (Yang et al. 1987). However, it is clear that higher levels of ABAD impacted positively on brain energetics. Consistent with cytoprotective properties of ABAD, recently described rare genetic deficiency states of MHBD/ABAD have been reported. The latter syndrome is associated with prominent clinical neurologic findings and pathologic neurodegeneration. Because MHBD/ABAD is in the catabolic pathway for isoleucine and branched-chain fatty acids, it is possible that accumulation of metabolites in this pathway exerts toxic effects on neurones. This hypothesis remains to be tested.
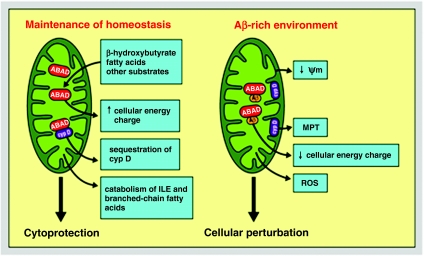
In terms of the cellular response to stress, ABAD also seems to have a beneficial role. Following induction of cerebral ischaemia, ABAD expression increased in neurones. In a murine stroke model, high levels of ABAD in transgenic mice were cytoprotective with respect to stroke volume and clinical neurologic deficit score. The latter findings paralleled higher levels of brain ATP and more effective utilization of β-hydroxybutyrate in Tg ABAD mice. Similarly, in the MPTP-induced murine model of Parkinsonism, overexpression of ABAD had a cytoprotective effect on neurones. Another contributor to the beneficial effects of ABAD on cellular functions might be related to its sequestration of cyclophilin D in the mitochondrial matrix. By maintaining cyclophilin D in the matrix compartment, this chaperone molecule is unable to translocate to the inner mitochondrial membrane, where it could interact with other components of the MPT and, potentially, destabilize mitochondrial function. Taken together, these data are consistent with cytoprotective properties of ABAD in health and disease (Figure 5).
Figure 5.
Schematic depiction of potential roles of amyloid-beta peptide alcohol dehydrogenase (ABAD) in physiologic and pathophysiologic settings. CypD, cyclophilin D; ILE, isoleucine; MPT, membrane permeability transition pore; Ψm, inner mitochondrial membrane potential; ROS, reactive oxygen species.
In an environment rich in Aβ, the protective properties of ABAD appear to be negated. Crystallographic studies demonstrated deformation of ABAD with loss of the NAD-binding site and a marked change in the enzyme's catalytic site. These structural changes were associated with inhibition of ABAD activity towards its diverse substrates. This leads to the hypothesis that decreased ABAD activity has negative effects on brain energetics and, possibly, other functions. For example, decreased MHBD/ABAD activity might result in accumulation of toxic metabolites that could have deleterious effects (as mentioned above). In addition, ABAD–Aβ interaction might displace cyclophilin D, resulting in its translocation to the inner mitochondrial membrane (as our preliminary findings suggest; Yan S-D. & Stern D., 2005). Others have hypothesized that loss of ABAD's activity as a hydroxysteroid dehydrogenase might alter metabolism of oestrogenic hormones involved in neuroprotection (He et al. 1999). Observations in double transgenic mice, overexpressing ABAD in an Aβ-rich environment, provided a means of testing the implications of Aβ for functional properties of the enzyme. Double transgenics demonstrated accelerated and more severe impairment of spatial learning/memory and loss of area occupied by acetylcholinesterase-positive neuronal fibers, compared with the other genotypes. In terms of the underlying mechanism, a prominent signal for free radicals was seen in brains from Tg mAPP/ABAD mice, and oxidant stress was evident in neurones cultured from these animals. These data lead us to hypothesize that ABAD–Aβ complex promotes leakage of oxygen-free radicals from mitochondria. Specifically, ABAD-Aβ-associated dysfunction of complex IV appears to result in leakage of oxygen-free radicals upstream at the level of complex III (Tieu et al. 2004). In vitro, oxidant stress initiates a cascade of events including reduced mitochondrial membrane potential and decreased cellular energy charge. The ultimate result is triggering of an apoptotic pathway that results in loss of cell viability. The latter pathway also appears to involve opening of the MPT (Tieu et al. 2004).
Thus, in the presence of Aβ, the properties of ABAD are considerably distorted from that of a cytoprotective enzyme involved in metabolic homeostasis on multiple levels (in the absence of Aβ) to a perturbant. We speculate that ABAD–Aβ complex causes cellular dysfunction by several potential mechanisms, including loss of enzymatic activity, accumulation of upstream toxic metabolites, stimulating leakage of mitochondrial oxygen-free radicals, loss of cellular energy charge and enhanced opening of the MPT (Figure 5).
In closing, it is also important to point out that these studies provide the first description of Aβ accumulation in mitochondria. In addition to ABAD, it is certainly reasonable to speculate that there may be other mitochondrial targets of Aβ. These results raise questions concerning the mechanism of Aβ import into mitochondria, and whether such mitochondrial Aβ has early and profound effects on mitochondrial function at the level of energy metabolism and control of apoptotic mechanisms. Although future studies will be required to address these issues, the current studies provide a first step in indicating a direct mechanism through which Aβ can impact on mitochondrial function.
Acknowledgments
This work was supported by grants from the USPHS (AG16736, AG17490, AG08702, NS42855). We gratefully acknowledge the secretarial assistance of Ms. Rita Lovering.
References
- Aarts M, Liu Y, Liu L, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD−95 protein interaction. Science. 2002;298:846–850. doi: 10.1126/science.1072873. 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Arancio O, Zhang HP, Chen X, et al. RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Pollard HB, Rojas E. The ability of Aβ (1–40) to form calcium channels provides a mechanism for neuronal death in Alzheimer's disease. Ann NY Acad Sci. 1994;15:256–266. doi: 10.1111/j.1749-6632.1994.tb44414.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bock J, Fleischer S. Preparation of a homogeneous soluble d-beta-hydroxybutyrate apodehydrogenase from mitochondria. J Biol Chem. 1975;250:5774–5781. [PubMed] [Google Scholar]
- Boland K, Behrens M, Choi D, Manias K, Perlmutter DH. The serpin-enzyme complex receptor recognizes soluble nontoxic Aβ but not aggregated, cytotoxic Aβ. J Biol Chem. 1996;271:18032–18044. doi: 10.1074/jbc.271.30.18032. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Buee L, Ding W, Delacourte A, Fillit H. Binding of secreted human neuroblastoma proteoglycans to Alzheimer's amyloid A4 peptide. Brain Res. 1993;601:154–163. doi: 10.1016/0006-8993(93)91706-x. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid β-mediated neurotoxicity. FASEB J. 2001;15:1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- Castellani R, Hirai K, Aliev G, et al. Role of mitochondrial dysfunction in Alzheimer's disease. J Neurosci Res. 2002;70:357–360. doi: 10.1002/jnr.10389. [DOI] [PubMed] [Google Scholar]
- Chen Q, VazqueZ EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Cook DG, Forman MS, Sung JC, et al. Alzheimer's Aβ (1–42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med. 1997;3:1021–1023. doi: 10.1038/nm0997-1021. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology. 2001;38:120–134. doi: 10.1046/j.1365-2559.2001.01082.x. 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- Eaton S, Bartlett K, Pourfarzam M. Mammalian mitochondrial β-oxidation. Biochem J. 1996;320:345–357. doi: 10.1042/bj3200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Steiner B, Marques C, et al. Elevated vulnerability to oxidative stress-induced cell death and activation of caspase-3 by the Swedish amyloid precursor protein mutation. J Neurosci Res. 2001;64:183–192. doi: 10.1002/jnr.1064. 10.1002/jnr.1064. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Furuta S, Kobayashi A, Miyazawa S, Hashimoto T. Cloning and expression of cDNA for a newly identified isozyme of bovine liver 3-hydroxyacyl-CoA dehydrogenase and its import into mitochondria. Biochim Biophys Acta. 1997;1350:317–324. doi: 10.1016/s0167-4781(96)00171-6. [DOI] [PubMed] [Google Scholar]
- Giovanni A, Keramaris E, Morris EJ, et al. E2F1 mediates death of B-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. J Biol Chem. 2000;275:11553–11560. doi: 10.1074/jbc.275.16.11553. 10.1074/jbc.275.16.11553. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, et al. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med. 2001;125:489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Hammad SM, Ranganathan S, Loukinova E, Twal WO, Argraves WS. Interaction of apoliprotein J-amyloid beta-peptide complex with LRP-2/megalin: a mechanism to prevent pathological accumulation of Aβ. J Biol Chem. 1997;272:18644–18649. doi: 10.1074/jbc.272.30.18644. 10.1074/jbc.272.30.18644. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Bieger SC, Bruhl B, et al. Distinct sites of intracellular production of A beta40/42 amyloid peptides. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- He XY, MerZ G, Mehta P, SchulZ H, Yang SY. Human brain short chain L-3-hydroxyacyl-Coenzyme A dehydrogenase is a single-domain multifunctional enzyme: Characterization of a novel 17 beta-hydroxysteroid dehydrogenase. J Biol Chem. 1999;274:15014–15019. doi: 10.1074/jbc.274.21.15014. 10.1074/jbc.274.21.15014. [DOI] [PubMed] [Google Scholar]
- He XY, MerZ G, Yang YZ, Mehta P, SchulZ H, Yang SY. Characterization and localization of human type10 17 beta-hydroxysteroid dehydrogenase. Eur J Biochem. 2001;268:4899–4907. doi: 10.1046/j.0014-2956.2001.02421.2421.x. 10.1046/j.0014-2956.2001.02421.2421.x. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Welsh-Bohmer KA, Hanson M, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucleic Med. 2000;41:1920–1928. [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Cassarino DS, Abramova NN, et al. Alzheimer's disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48:148–155. 10.1002/1531-8249(200008)48:2<148::AID-ANA3>3.0.CO;2-7. [PubMed] [Google Scholar]
- Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN. Intracellular amyloid-beta (1–42), but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem. 2002;277:15666–15670. doi: 10.1074/jbc.M200887200. 10.1074/jbc.M200887200. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee JH, Lee JP, et al. Aβ induces cytochrome c release from isolated mitochondria. Neuroreport. 2002;13:1989–1993. doi: 10.1097/00001756-200210280-00032. 10.1097/00001756-200210280-00032. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, et al. Brain cytochrome oxidase in Alzheimer's disease. J Neurochem. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Jaing L, Hashimoto T. Two mitochondrial 3-hydroxyacl-CoA dehydrogenases in bovine liver. J Biochem. 1996;119:775–782. doi: 10.1093/oxfordjournals.jbchem.a021307. (Tokyo). [DOI] [PubMed] [Google Scholar]
- Krozowski Z. The short-chain alcohol dehydrogenase superfamily: variations on a common theme. J Steroid Biochem Molec Biol. 1994;51:125–130. doi: 10.1016/0960-0760(94)90084-1. 10.1016/0960-0760(94)90084-1. [DOI] [PubMed] [Google Scholar]
- LaFerla F, Tinkle B, Bieberich C, Haudenschild C, Jay G. The Alzheimer's Abeta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet. 1995;9:21–30. doi: 10.1038/ng0195-21. 10.1038/ng0195-21. [DOI] [PubMed] [Google Scholar]
- Lehninger A, Sudduth H, Wise J. d-β-hydroxybutyric acid dehydrogenase of mitochondria. J Biol Chem. 1960;235:2450–2455. [PubMed] [Google Scholar]
- Lesnefsky E, Slabe T, Stoll M, Minkler P, Hoppel C. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol. 2001;280:H2770–H2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- Loo D, Copani A, Pike C, Whittemore E, Walencewicz A, Cotman C. Apoptosis is induced by Abeta in cultured central nervous system neurons. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Walker D, Brachova L, et al. Involvement of RAGE–microglia interactions in Alzheimer's disease: in vivo and in vitro studies. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- Lustbader J, Cirilli M, Lin C, et al. ABAD–Aβ interaction is a direct link to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Marques C, Keil U, Bonert A, et al. Neurotoxic mechanisms caused by the Alzheimer's disease-linked Swedish amyloid precursor protein mutation: oxidative stress, caspases, and the JNK pathway. J Biol Chem. 2003;278:28294–28302. doi: 10.1074/jbc.M212265200. 10.1074/jbc.M212265200. [DOI] [PubMed] [Google Scholar]
- Matter M, Zhang Z, Nordstadt C, Ruoslahti E. The alpha-5 beta-1 integrin receptor binds the Alzheimer disease amyloid-beta protein and mediates its internalization. Keystone Symp Proc. 1998;X5:101. (abstract). [Google Scholar]
- Mattson M, Partin J, Begley J. Abeta induces apoptosis-related events in synapses and dendrites. Brain Res. 1998;807:167–176. doi: 10.1016/s0006-8993(98)00763-x. 10.1016/S0006-8993(98)00763-X. [DOI] [PubMed] [Google Scholar]
- Maurer I, ZierZ S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. 10.1016/S0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Moreira P, Santos M, Moreno A, Rego A, Oliveira C. Effect of amyloid beta-peptide on permeability transition pore: a comparative study. J Neurosci Res. 2002;69:257–267. doi: 10.1002/jnr.10282. 10.1002/jnr.10282. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond D, Gottschall P, et al. Abeta vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Muller W, Kirsch C, Eckert G. Membrane-disordering effects of beta-amyloid peptides. Biochem Soc Transactions. 2001;29:617–623. doi: 10.1042/bst0290617. 10.1042/BST0290617. [DOI] [PubMed] [Google Scholar]
- Mutisya E, Bowling A, Beal M. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. J Neurochem. 1994;63:21769–22184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Narindrasorasak S, Lowery D, Gonzaelz-DeWitt P, Poorman R, Greenberg B, Kisilevsky R. High affinity interactions between the betaAPP and the basement membrane form of heparin sulfate proteoglycan. J Biol Chem. 1991;266:12878–12883. [PubMed] [Google Scholar]
- Narita M, Holtzman DM, SchwartZ AL, Bu G. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem. 1997;69:1904–1911. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- Ofman R, Ruiter JP, Feenstra M, et al. 2-methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency is caused by mutations in the HADH2 gene. Am J Hum Genet. 2003;72:1300–1307. doi: 10.1086/375116. 10.1086/375116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappolla MA, Chyan YJ, Omar RA, et al. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer's disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998;152:871–877. [PMC free article] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. 10.1016/S0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Parker WD, Parks J, Filley CM, Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994;44:1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- Pillot T, Goethals M, Vanloo B, et al. Fusogenic properties of the C-terminal domain of the Alzheimer beta-amyloid peptide. J Biol Chem. 1996;271:28757–28765. doi: 10.1074/jbc.271.46.28757. 10.1074/jbc.271.46.28757. [DOI] [PubMed] [Google Scholar]
- Sass JO, Forstner R, Sperl W. 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency: impaired catabolism of isoleucine presenting as neurodegenerative disease. Brain Dev. 2004;26:12–14. doi: 10.1016/s0387-7604(03)00071-8. 10.1016/S0387-7604(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern D. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:939–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DH, Cummings JL, Small GW, et al. Added clinical benefit of incorporating 2-deoxy-2-[18F]fluoro-D-glucose with positron emission tomography into the clinical evaluation of patients with cognitive impairment. Mol Imaging Biol. 2002;4:283–293. doi: 10.1016/s1536-1632(02)00016-1. 10.1016/S1536-1632(02)00016-1. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Phelps ME. Clinical Value of Neuroimaging in the Diagnosis of Dementia. Sensitivity Specificity Regional Cerebral Metabolic Other Parameters for Early Identification Alzheimer's Disease. Clin Positron Imaging. 1999;2:119–130. doi: 10.1016/s1095-0397(99)00020-5. 10.1016/S1095-0397(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Snow AD, Kinsella MG, Parks E, et al. Differential binding of vascular cell-derived proteoglycans (perlecan, biglycan, decorin, and versican) to the beta-amyloid protein of Alzheimer's disease. Arch Biochem Biophys. 1995;320:84–95. doi: 10.1006/abbi.1995.1345. 10.1006/abbi.1995.1345. [DOI] [PubMed] [Google Scholar]
- Snow AD, Sekiguchi R, Nochlin D, et al. An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A beta-amyloid in rat brain. Neuron. 1994;12:219–234. doi: 10.1016/0896-6273(94)90165-1. 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Tabner BJ, Turnbull S, El-Agnaf OM, Allsop D. Formation of hydrogen peroxide and hydroxyl radicals from A (beta) and alpha-synuclein as a possible mechanism of cell death in Alzheimer's disease and Parkinson's disease. Free Radic Biol Med. 2002;32:1076–10883. doi: 10.1016/s0891-5849(02)00801-8. 10.1016/S0891-5849(02)00801-8. [DOI] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, et al. ABAD enhances abeta-induced cell stress via mitochondria dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Guglielmotto M, et al. Multiple signaling events in amyloid beta-induced, oxidative stress-dependent neuronal apoptosis. Free Radic Biol Med. 2003;35:45–58. doi: 10.1016/s0891-5849(03)00244-2. 10.1016/S0891-5849(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Tienari PJ, Ida N, Ikonen E, et al. Intracellular and secreted Alzheimer beta-amyloid species are generated by distinct mechanisms in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1997;94:4125–4130. doi: 10.1073/pnas.94.8.4125. 10.1073/pnas.94.8.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu K, Perier C, Vila M, et al. L-3-hydroxyacyl-CoA dehydrogenase II protects in a model of Parkinson's disease. Ann Neurol. 2004;56:51–60. doi: 10.1002/ana.20133. 10.1002/ana.20133. [DOI] [PubMed] [Google Scholar]
- Torroja L, Ortuno-Sahagun D, Ferrus A, Hammerle B, Barbas JA. scully, an essential gene of Drosophila, is homologous to mammalian mitochondrial type II L-3-hydroxyacyl-CoA dehydrogenase/amyloid-beta peptide-binding protein. J Cell Biol. 1998;141:1009–1017. doi: 10.1083/jcb.141.4.1009. 10.1083/jcb.141.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, ReitZ AB. Beta-amyloid (1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000;278:5626–5632. doi: 10.1074/jbc.275.8.5626. 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Lander AD, Selkoe DJ. Heparin-binding properties of the amyloidogenic peptides Abeta and amylin. Dependence on aggregation state and inhibition by Congo red. J Biol Chem. 1997;272:31617–31624. doi: 10.1074/jbc.272.50.31617. 10.1074/jbc.272.50.31617. [DOI] [PubMed] [Google Scholar]
- Wild-Bode C, Yamazaki T, Capell A, et al. Intracellular generation and accumulation of amyloid beta-peptide terminating at amino acid 42. J Biol Chem. 1997;272:16085–16088. doi: 10.1074/jbc.272.26.16085. 10.1074/jbc.272.26.16085. [DOI] [PubMed] [Google Scholar]
- Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Pilch PF, et al. Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer's disease. J Clin Invest. 1997;100:2333–2340. doi: 10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yan SD, Fu J, Soto C, et al. An intracellular protein that binds amyloid-beta peptide and mediates neurotoxicity in Alzheimer's disease. Nature. 1997;389:689–695. doi: 10.1038/39522. 10.1038/39522. [DOI] [PubMed] [Google Scholar]
- Yan SD, Shi Y, Zhu A, et al. Role of ERAB/L-3-hydroxyacyl-coenzyme A., dehydrogenase type II, activity in Abeta-induced cytotoxicity. J Biol Chem. 1999;274:2145–2156. doi: 10.1074/jbc.274.4.2145. 10.1074/jbc.274.4.2145. [DOI] [PubMed] [Google Scholar]
- Yan SD, Zhu Y, Stern ED, et al. Amyloid beta-peptide-binding alcohol dehydrogenase is a component of the cellular response to nutritional stress. J Biol Chem. 2000;275:27100–27109. doi: 10.1074/jbc.M000055200. [DOI] [PubMed] [Google Scholar]
- Yang SY, He XY, SchulZ H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem. 1987;262:13027–13032. [PubMed] [Google Scholar]
- Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid beta peptide1–42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J, Ruiter JP, Brand J, et al. Progressive infantile neurodegeneration caused by 2-methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency: a novel inborn error of branched-chain fatty acid and isoleucine metabolism. Pediatr Res. 2000;48:852–855. doi: 10.1203/00006450-200012000-00025. [DOI] [PubMed] [Google Scholar]