Abstract
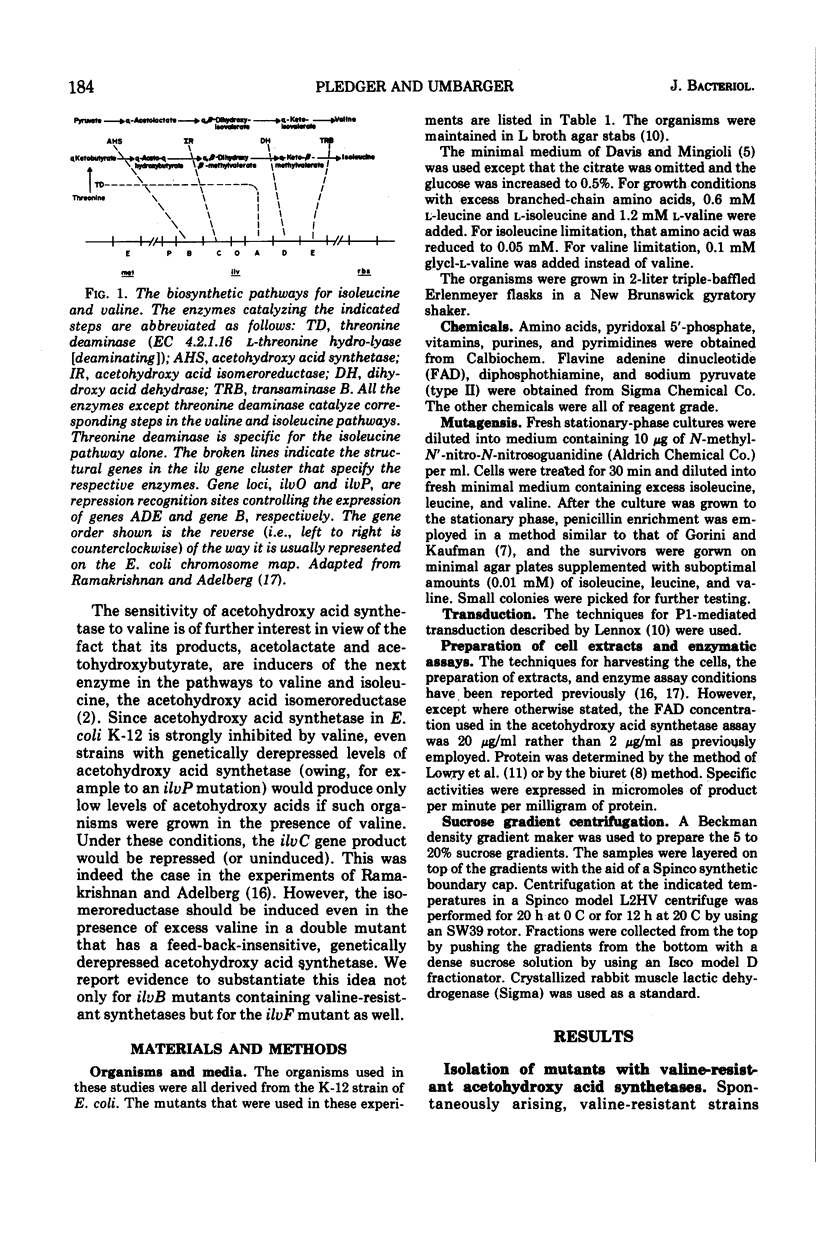
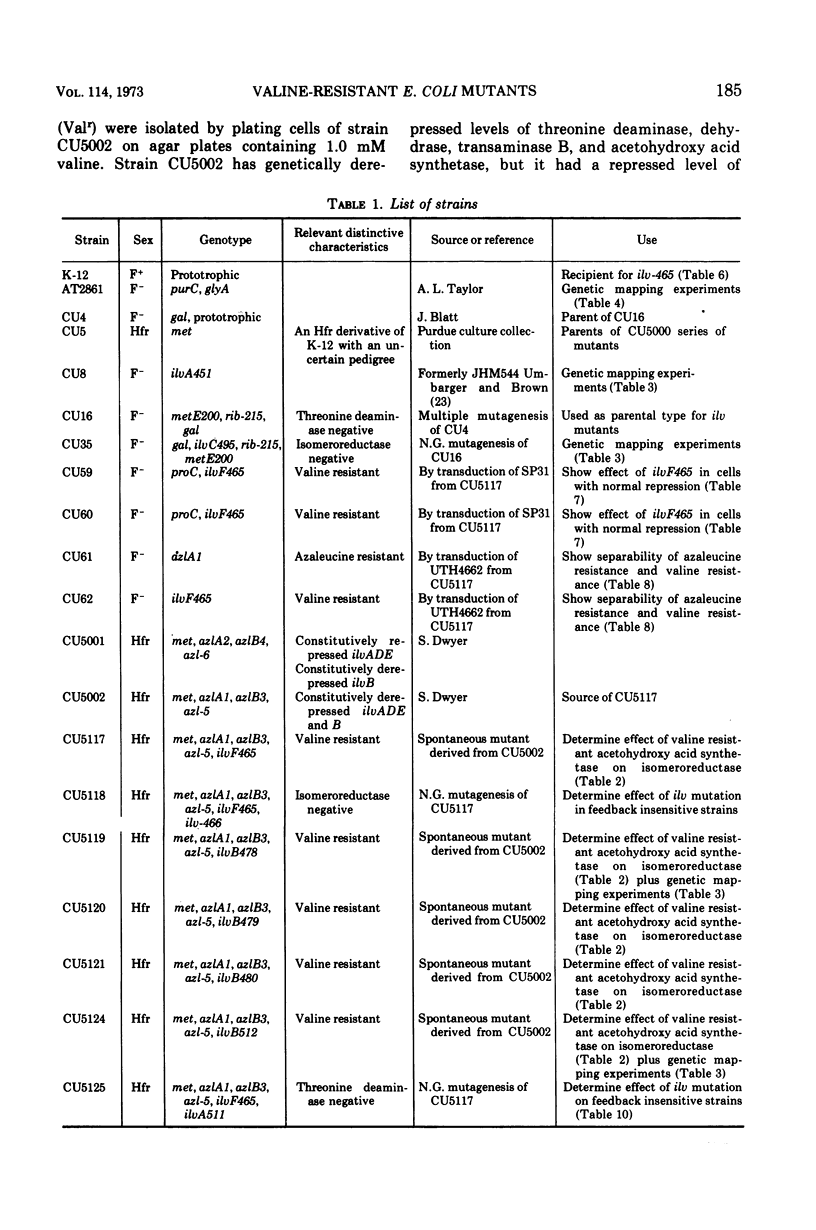
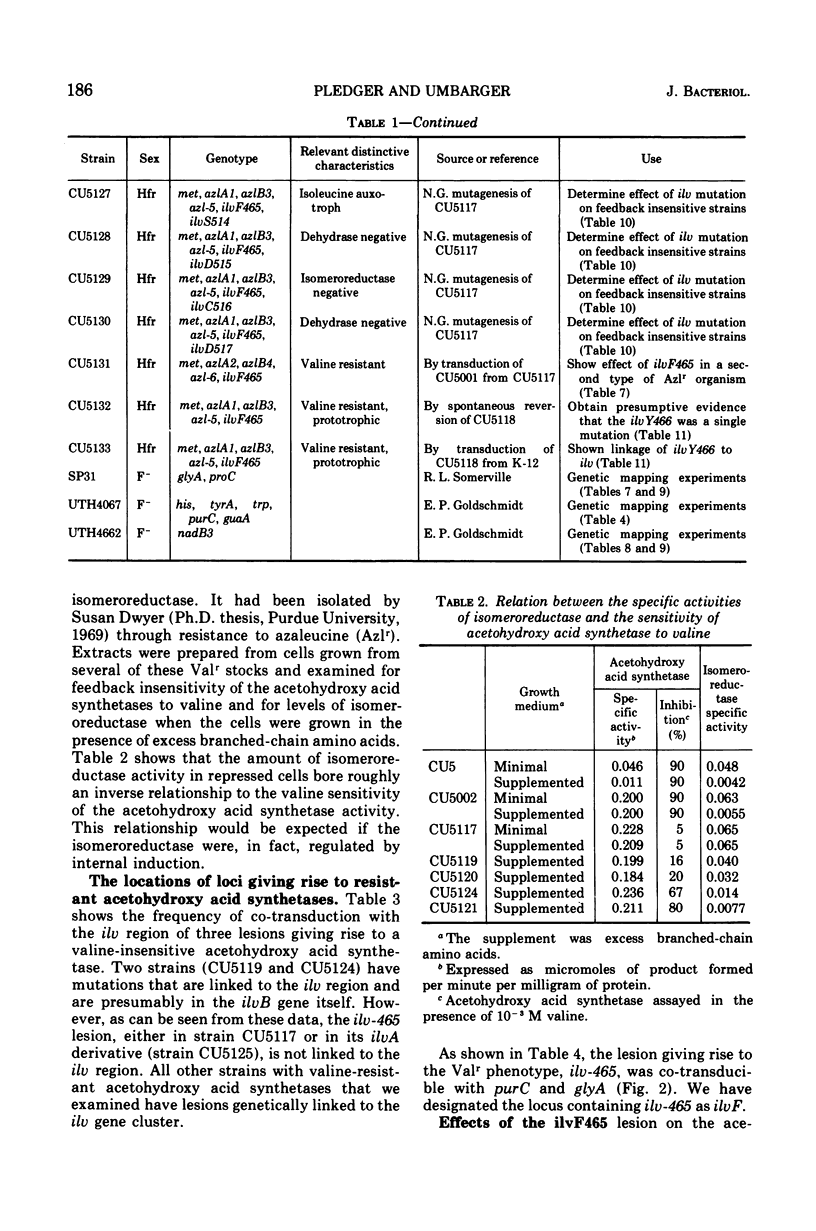
The activity of acetohydroxy acid isomeroreductase, an essential enzyme for isoleucine and valine biosynthesis in Escherichia coli, was examined in a series of mutants containing derepressed levels of acetohydroxy acid synthetase activity but which differed from each other in the sensitivity of the synthetases to valine inhibition. The finding that isomeroreductase was highest in the strain with the synthetase that was least sensitive to valine inhibition supported the model of internal induction of the isomeroreductase by its acetohydroxy acid substrates. The mutation leading to the acetohydroxy acid synthetase least sensitive to valine was found to be unlinked to the ilv gene cluster and appeared to result in a synthetase that differed from the normal enzyme in several properties. The locus of this mutation is designated ilvF. The loci leading to derepression were designated azl. A pleiotropic, apparently single-step, mutation was found that led to restoration of end-product sensitivity to the synthetase, loss of end-product sensitivity of threonine deaminase [EC 4.2.1.16, l-threonine hydro-lyase (deaminating) and loss of isomeroreductase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. R., Calvo J. M. A Salmonella typhimurium locus involved in the regulation of isoleucine, valine and leucine biosynthesis. Genetics. 1969 Mar;61(3):539–556. doi: 10.1093/genetics/61.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfin S. M., Ratzkin B., Umbarger H. E. The metabolism of valine and isoleucine in Escherichia coli. XVII. The role of induction in the derepression of acetohydroxy acid isomeroreductase. Biochem Biophys Res Commun. 1969 Dec 4;37(6):902–908. doi: 10.1016/0006-291x(69)90216-2. [DOI] [PubMed] [Google Scholar]
- Blatt J. M., Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XX. Multiple forms of acetohydroxy acid synthetase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):444–450. doi: 10.1016/s0006-291x(72)80071-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lansford E. M., Jr, Lee N. M., Shive W. Regulation by lysine of production of threonine-sensitive aspartokinase. Biochem Biophys Res Commun. 1966 Nov 22;25(4):468–472. doi: 10.1016/0006-291x(66)90229-4. [DOI] [PubMed] [Google Scholar]
- Nakae T. Multiple molecular forms of uridine diphosphate glucose pyrophosphorylase from Salmonella typhimurium. 3. Interconversion between various forms. J Biol Chem. 1971 Jul 25;246(14):4404–4411. [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Two forms of biosynthetic acetohydroxy acid synthetase in Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Jul 25;48(2):437–443. doi: 10.1016/s0006-291x(72)80070-6. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXII. A pleiotropic mutation affecting induction of isomeroreductase activity. J Bacteriol. 1973 Apr;114(1):195–207. doi: 10.1128/jb.114.1.195-207.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., SNELL E. E. Biosynthesis of valine and isoleucine. 2. Formation of alpha-acetolactate and alpha-aceto-alpha-hydroxybutyrate in Neurospora crassa and Escherichia coli. J Biol Chem. 1960 Aug;235:2316–2321. [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VIII. The formation of acetolactate. J Biol Chem. 1958 Nov;233(5):1156–1160. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


