Abstract
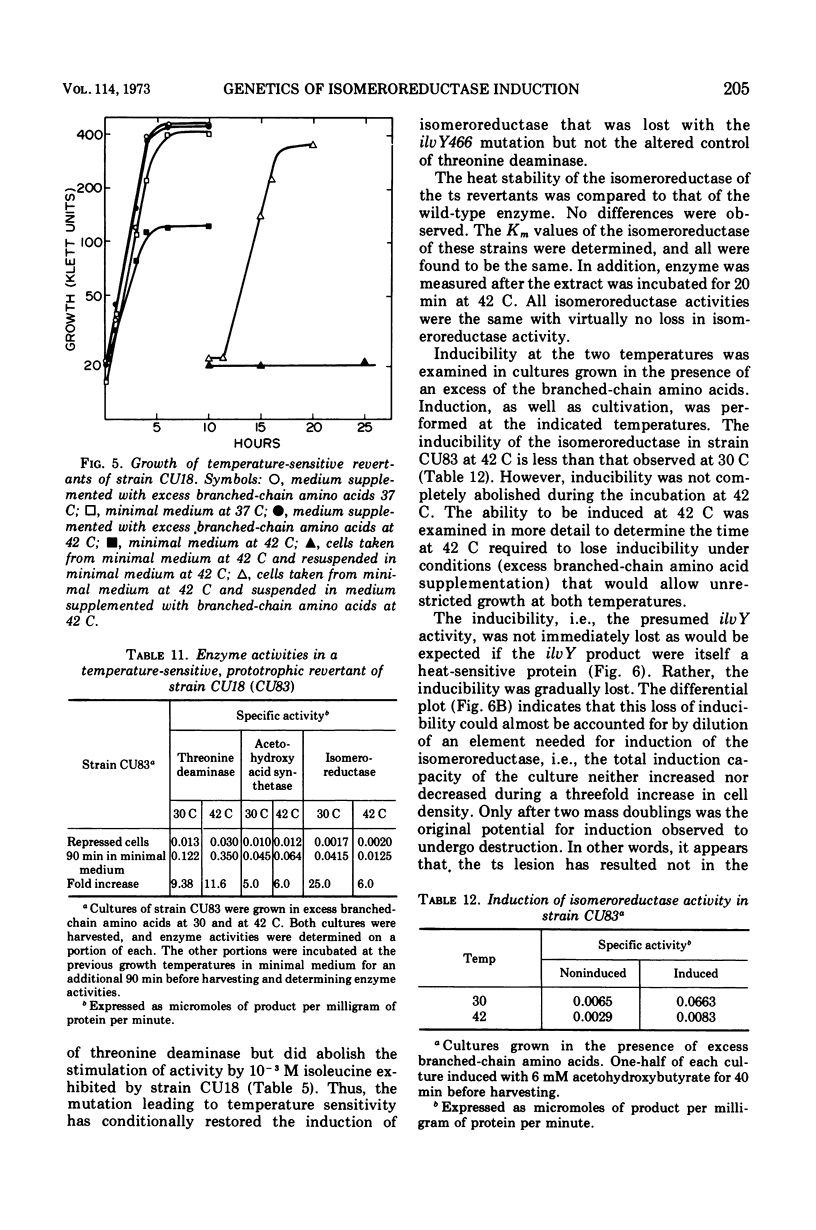
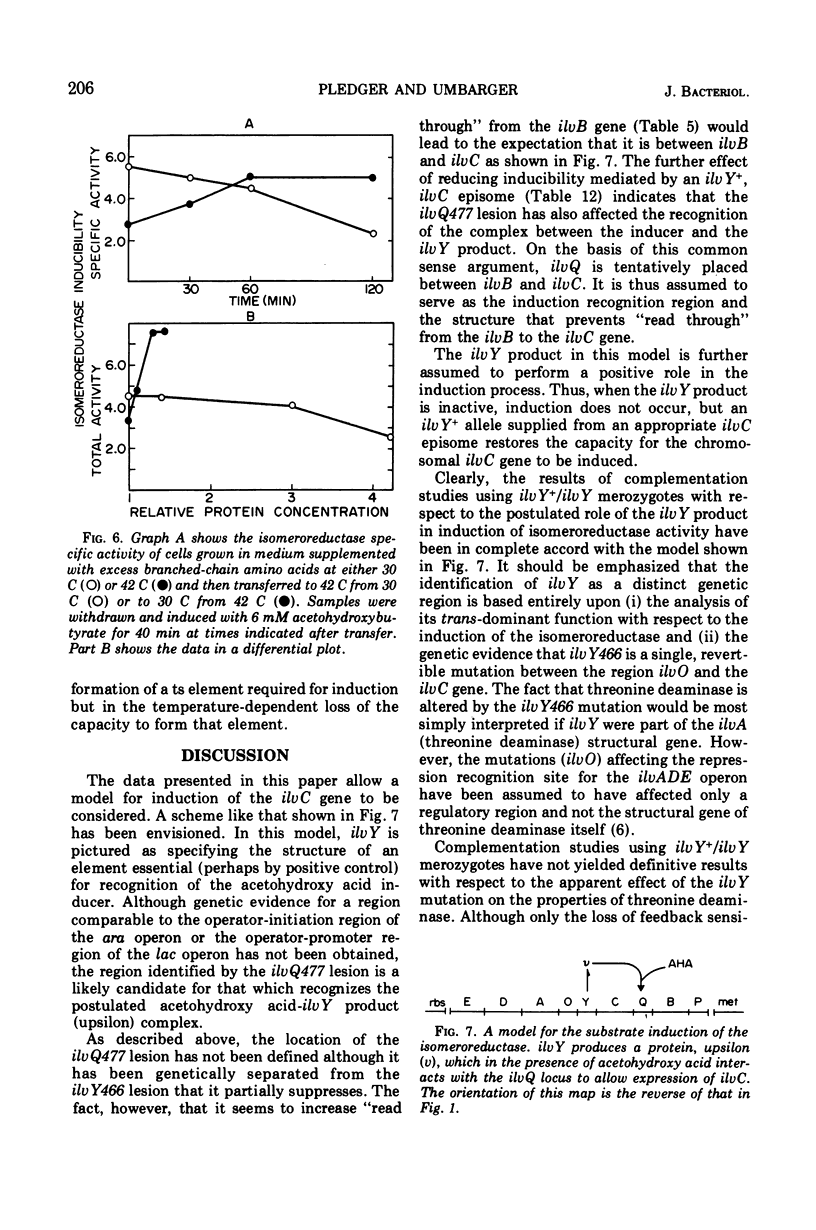
The ilvC gene product, acetohydroxy acid isomeroreductase, an enzyme essential for isoleucine and valine formation, is subject to substrate induction in Escherichia coli. We have isolated a mutant of E. coli K-12 with a mutation that renders the ilvC gene product noninducible by its substrates, the acetohydroxy acids. This mutation, ilvY466, has been shown to be in a previously undiscovered locus that lies between ilvC and ilvO. The ilvY product, upsilon, is thought to be a regulatory element involved in the induction of ilvC. We postulate the recognition site, ilvQ, or upsilon and suggest that it lies between ilvC and ilvB. A possible model, involving upsilon, in the positive control of isomeroreductase is presented. Pleiotropic effects of the ilvY466 mutation have been recognized from changes in the end-product inhibition of threonine deaminase and of acetohydroxy acid synthetase. In addition, pleiotropic effects of this lesion on the regulation of threonine deaminase and the physical properties of threonine deaminase and acetohydroxy acid synthetase have been observed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Umbarger H. E. Purification and properties of the acetohydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem. 1969 Mar 10;244(5):1118–1127. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXI. Mutations affecting derepression and valine resistance. J Bacteriol. 1973 Apr;114(1):183–194. doi: 10.1128/jb.114.1.183-194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Arfin S., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. 18. Induction of acetohydroxy acid isomeroreductase. J Bacteriol. 1972 Oct;112(1):131–141. doi: 10.1128/jb.112.1.131-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Kohno T., Roth J. R. Enzyme evolution: generation of a bifunctional enzyme by fusion of adjacent genes. Nature. 1970 Nov 28;228(5274):820–824. doi: 10.1038/228820a0. [DOI] [PubMed] [Google Scholar]


