Abstract
The technique of differential display (DD) has been used widely to identify potentially interesting overexpressed or repressed genes in a variety of compared samples. When used in studying tissue samples, it inevitably confronts problems of limited amount of input material and cell-type heterogeneity. We report here the application of in situ hybridization as a method of confirmatory test for DD as well as definition of cell type expressing differential cDNA. This procedure employed material derived from a single case of human mammary, grade III, infiltrating ductal carcinoma, using free-hand microdissection, where we have compared gene expression profiles in invasive tumour with those in adjacent normal tissue. A total of 21 cDNAs were found to be differentially expressed between the two tissue types; 11 upregulated in the tumour sample and 10 upregulated in the normal sample. Six cDNAs were utilized as probes for in situ hybridization analysis of a further five cases of comparably staged breast cancer. One of these clones, 11AT1, which was found to be homologous to Hsc70, was shown to be overexpressed in tumour cells relative to adjacent normal stroma and to benign glandular epithelium in all five cases; an increase in expression was further confirmed at protein level by immunohistochemistry. The study demonstrated the applicability of in situ hybridization as a screening test in DD strategy for studying tissue material and a reasonable technique combination of identifying changes in gene expression associated with tumour development.
Keywords: breast cancer, differential display, gene expression, heat-shock cognate 70, in situ hybridization, microdissection
Breast cancer continues to be a formidable life-threatening disease in women around the world. There were 33 100 new breast cancers in women in England & Wales in 1997; one in nine women will develop breast cancer at some time in their life (National Statistics, 2001). Internationally, the highest rates have been found in Western Europe, North America and Australia, with much lower rates in China, Japan and in most parts of the Third World (Parkin 1989).
The molecular basis of most targeted breast cancer treatment has been focused on oestrogen receptor (ER) blockade through the administration of Tamoxifen and, more recently, the use of Herceptin™ to target c-erbB-2-amplified tumours (Slamon et al. 2001). However, novel targets might be still identified through the characterization of genes, which are expressed differentially by tumour relative to normal tissue. To this end, a number of gene expression comparative techniques such as differential display (DD), SAGE, RDA and microarray have been developed in the last decade (Kao et al. 1999). Given these gene expression comparative tools, researchers must choose methods suitable for their own studies in terms of purposes and laboratory facilities. There has been a surge in the utilization of microarray analysis in the last few years (Perou et al. 1999; Nocito et al. 2001; van'tVeer et al. 2002). The technique has limiting factors such as the requirement for relatively large quantities of RNA for preparation of the probe (Kao et al. 1999), incapability to discover novel sequences in a close system and possible creation of ‘self-organizing data’ (Liang 2002). Differential display which is a polymerase chain reaction (PCR)-based RNA fingerprinting technique (Liang & Pardee 1992), is ideally suited to the identification of differentially expressed transcripts when only limited amounts of tissue are available. A potential disadvantage of DD is the possible isolation of a large number of candidate transcripts with an attendant high rate of false-positives (Liang 2002). A confirmatory test in transcript expression thus constitutes an important component of the DD technique. Northern blot analysis, which has been the conventional confirmatory test in the last 10 years, becomes problematic in studying tissue material regarding the requirement of relatively large amount of RNA input for each candidate differential cDNA. Determining the cellular provenance of the altered transcript level poses another difficulty. In order to overcome these hurdles, we integrated in situ hybridization into DD strategy. Using a single case of breast cancer as an illustrative example, we demonstrated that in situ hybridization could be acting as a confirmatory test as well as assignment of cell type expressing the differential cDNA; we identified that the overexpression of the constitutive heat-shock protein, heat-shock cognate 70 (Hsc70), was more predominant in malignant, but not in normal, epithelial cells.
Materials and methods
Tissue microdissection and RNA extraction
A single case of human mammary, grade III, infiltrating ductal carcinoma was used as the initial starting material. Frozen sections, cut 20 µm thick from the tumour part and the normal part, were taken and mounted on glass slides. The histology of the consecutive sections was examined by staining one in every 10 sections with haematoxylin, and the slides were checked under a light microscope to ensure that both tumour and normal tissue were present for microdissection. Microdissection was performed, free-hand, using a scalpel to scrape away the area of interest, which already had been determined by examination of the consecutive haematoxylin-stained sections. This tissue was collected into a 1.5 ml microcentrifuge tube containing 1 ml of RNA Stat-60 (Biogenesis, Poole, UK). The standard procedures of RNA extraction, as described in the manufacturer's instructions, were followed. The RNA was treated with RQ1 RNase-Free DNase (1 U/µl; Promega, Madison, WI, USA), as reported previously (Francia et al. 1996) and stored at −70 °C until required.
Differential display reverse transcriptase-polymerase chain reaction
The procedure was performed essentially as described before using the RNAimage Kit (GeneHunter Corporation, Brookline, MA, USA) (Francia et al. 1996). Briefly, three reverse transcription (RT) reactions using three one-base anchored primer (HT11A, HT11G and HT11C) for each RNA sample were undertaken in the following mixture: 2.0 µl of RNA sample (0.1 µg/µl), 2.0 µl of ×10 RT buffer (0.5 m Tris–HCl, pH 8.3, 0.75 m KCl, 0.03 m MgCl2; Stratagene, La Jolla, CA, USA), 1.6 µl of dNTP mix (dATP, dTTP, dCTP and dGTP, 250 µm each; Promega), 2.0 µl of anchored primer (2 µm), 1 µl of 0.1 m DTT and 9.4 µl of dH20. The tube was placed in a hot-lid thermocycler (Techne), and the programme was set as: 65 °C for 5 min, 37 °C for 1 h and 75 °C for 5 min. Two microlitres of Moloney Murine Leukaemia Virus reverse transcriptase (50 U/µl, Stratagene) was added after 5 min at 37 °C.
PCR reactions were carried out in the mixture of 2 µl of RT reaction mix, 2 µl arbitrary primer (HAP1-40, 2 µm), 2.0 µl of ×10 PCR buffer (0.1 m Tris–HCl, pH 8.3, 0.5 m KCl; Perkin-Elmer, Norwalk, CT, USA), 1.2 µl of MgCl2 (25 mm; Perkin-Elmer), 1.6 µl of dNTP mix (dATP, dTTP, dCTP and dGTP, 25 µm each), 2.0 µl of HT11M (2 µm) (the same one as used in the RT reaction), 0.04 µl of [α-33P]-dATP (2500 Ci/mmol; Amersham Life Science, Little Chalfont, UK), 0.2 µl of Pic Taq DNA polymerase (5 U/µl, ICRF) and 9.0 µl of dH2O. The HAP1-8 primers were from GeneHunter corporation and HAP9-40 were as designed (Francia et al. 1996). The programme was set as follows: 1 cycle of 95 °C for 5 min, 40 cycles of 94 °C for 30 s, 40 °C for 2 min, 72 °C for 30 s and 1 cycle of 72 °C for 5 min.
The PCR reactions were resolved by non-denaturing polyacrylamide gel electrophoresis, and autoradiography was carried out. By alignment of the film to the dried gel, the differential bands were cut out and the cDNAs were recovered. The reamplification was performed by making up the following reaction mix: 4.0 µl of recovered cDNA, 4.0 µl of ×10 PCR buffer, 3.2 µl of dNTPs (250 µm each), 2.4 µl of MgCl2 (25 mm), 4.0 µl of HT11M (2 µm), 4.0 µl of HAP (2 µm), 0.4 µl of Pic Taq DNA polymerase and 18.0 µl of dH2O. The primer pair and PCR conditions were the same as those used in the DD PCR. The reamplified cDNAs were cloned into pCR2.1 by TA cloning kit (Invitrogen, Leek, The Netherlands). The sequence of cDNA was determined using an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Warrington, UK) and analysed on an ABI 310 analyser according to the manufacturer's instructions.
In situ hybridization
The procedure followed was essentially the protocol of Poulsom (Poulsom et al. 1998). Briefly, paraffin-embedded tissue sections were prepared by permeabilizing with proteinase K (20 µg/ml in phosphate-buffered saline) at 37 °C and acetylated. The riboprobes were produced in 6.25 µl reaction volumes consisting of ×1 transcription buffer (Promega), 1.5 U/µl of RNase (20 U/µl; Promega), 11.2 mm DTT, 1.0 mm each of ATP, GTP, CTP, 0.5–1.0 µg of template (pCR2.1 containing the cDNA of interest), 14 µm of [35S]-UTPαS (800 Ci/mmol; Amersham), and 4 U of RNA polymerase, which was incubated at 37 °C for 1 h. Hybridization mix was made of the following components: 10% v/v of 0.2% Denhardt's solution in ×10 salt mix [3 m NaCl, 100 mm Na2HPO4, 100 mm Tris–HCl, 50 mm ethylenediaminetetraacetic acid (EDTA)], 50% v/v formamide, 3% v/v rRNA (10 mg/ml), 10% v/v dextran sulphate, 10 mm DTT and 16% v/v dH2O containing the required volume of riboprobe. The hybridization was carried out at 55 °C overnight. The following day, the slides were washed with buffer containing 0.5 m NaCl, 10 mm Tris–HCl, pH 7.6, 5 mm EDTA, pH 7.5 at 37 °C and ×2 standard saline citrate at 65 °C. Autoradiography was undertaken by dipping slides into Ilford K5 emulsion and exposing the slides in darkness for 7–21 days at 4 °C. After development, the slides were counterstained by Giemsa's stain and examined by alternating between light and dark field microscopy.
Immunohistochemistry
Tissue sections were microwaved in 0.1% sodium citrate for 10 min and then incubated with mouse antihuman Hsc70 antibody (B6, Santa Cruz Biotechnique, Santa Cruz, CA, USA) at 1 : 1000 of dilution. For detection, a standard streptavidin–biotin complex with diaminobenzidine and hydrogen peroxide was used, and results were interpreted by a specialist pathologist (A.H).
Results
DD of breast cancer tissue RNA
In total, 30 frozen sections from the tumour region and 60 frozen sections from the normal tissue region were used for RNA extraction and 33.8 µg and 16.8 µg of total RNA were obtained, respectively. Based on the dimension of the area utilized for the extraction, the approximate RNA yields from each source were calculated. For the tumour areas, the yield was 3.5 µg/cm2/20 µm thick section and for the normal area, 0.42 µg/cm2/20 µm thick section. These data are close to those values calculated previously by another group (Ansari-Lari et al. 1996).
In total, 120 different pairs of primers (three anchored primers: HT11A, HT11C and HT11G and 40 arbitrary primers: HAP1-40) were used, producing approximately 6000 bands in each of the tumour and the normal RNA samples. The DD processes were repeated and only reproducibly differential bands (i.e. those observed in replicate experiments) were considered for further characterization. Altogether, 21 differentially expressed bands were detected, of which 11 were upregulated in the tumour and 10 were upregulated in the normal sample. All the differential cDNA fragments were recovered and reamplified from the gel. Sixteen of them were successfully cloned into pCR2.1 vectors and sequenced. The sequences were searched for homology by the fasta programme in Genembl and Tags DNA databases. Four were known genes: mitochondrial DNA fragment M1 encoding tRNA and cytochrome oxidase I, human Hsc70, human NADH dehydrogenase subunit 2, human dehydrogenase; two were expression sequence tags (EST) and 10 were novel sequences.
In situ hybridization
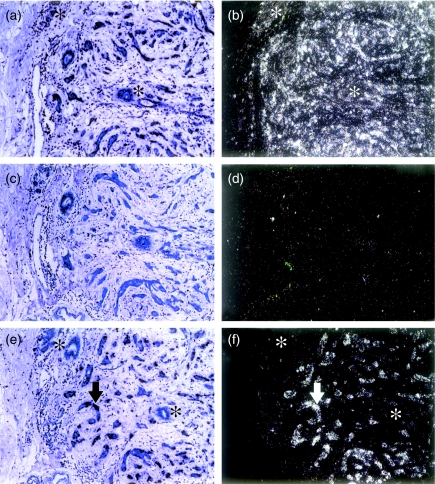
Six clones of differential cDNAs were selected first, according to the orientation of the inserts in the pCR2.1 vector, for RNA in situ hybridization. The poly(A) tails of the cDNA inserts were adjacent to the T7 promotor of pCR2.1 vector so that antisense riboprobes could be created by T7 RNA polymerase. Five were upregulated in the tumour part and one was upregulated in the normal part. One of them, designated as 11AT1, was found to be homologous to Hsc70; the rest were novel sequences. Paraffin-embedded tissue blocks of five human mammary, grade III, infiltrating ductal carcinomas, including part of the original one used for DD, were studied. The β-actin probe acted as a positive control and a totally negative result case acted as a negative control (Fig. 1). Among the five clones, which were selected because they were upregulated in the tumour part, one clone, 11AT1, showed differential cell expression pattern in all five tissue blocks in which tumour cells demonstrated silver grain staining (positive) while benign cells showed yellowish staining (negative) (Fig. 1); three clones showed the same differential cell expression pattern only in one block and were thus considered negative results; the remaining one, together with the cDNA upregulated in the normal part, showed negative staining in all blocks.
Figure 1.
In situ hybridization. Panels a and b, β-actin-positive control showing expression in both benign and malignant epithelium; panels c and d, negative control (of probe RHK3) negative for both tumour and benign compartments; panels e and f, 11AT1 riboprobe showing abundant expression in the invasive tumour epithelium (arrow) but not in the benign epithelium (asterisks). Panels a, c and e, under conventional light microscopy; b, d and f, under dark field reflected light microscopy.
Characteristics of 11AT1
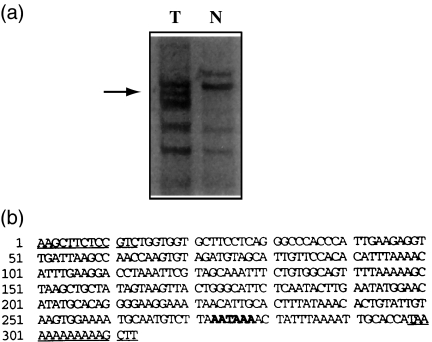
The 11AT1 differential band contained a 313 base pair (bp) cDNA, amplified by HT11A anchored primer and HAP11 arbitrary primer from tumour RNA. It contained a polyadenylation signal 36 bp 5′ to the 3′ end (Fig. 2). A search in the Genembl database by the fasta programme revealed 95.4% homology to the 3′ end of Hsc70 gene (access number: Y00371) with its 56 bp 5′ end overlapping the 3′ end of the ninth exon. This homology verifies the presumptive annealing site of the anchored primer to the poly(A) tail of the mRNA. An alignment of the sequence to Hsp70 (access number: M59828), which is 74% homologous to Hsc70 (Dwonrniczak & Mirault 1987), indicated 24% homology only, validating the specificity of 11AT1 to Hsc70.
Figure 2.
(a) Differential display gel. T, tumour part reaction; N, normal part reaction. The arrow indicates the 11AT1 band. (b) The sequence of 11AT1. The underlying sequences are HT11A anchored primer and HAP11 arbitrary primer areas, and the bold letters indicate the polyadenylation signal.
Immunohistochemistry
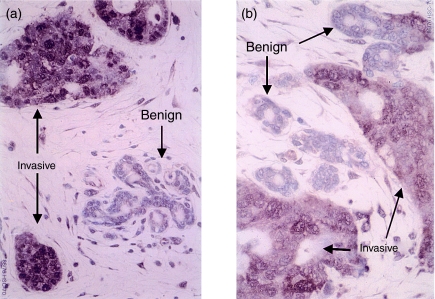
The preliminary immunostaining on five human mammary, grade III, infiltrating ductal carcinomas demonstrated that the tumour cells were stained strongly by the anti-Hsc70 antibody, B6, but no or only slight staining was observed in nearby stromal or benign glandular cells (Fig. 3). The result is concordant with the in situ hybridization findings.
Figure 3.
Immunohistochemistry of anti-HSC70 antibody in two samples of breast cancer tissue. Hsc70 was expressed predominantly in invasive cancer cells but not in benign glandular epithelial cells.
Discussion
The true relevance of detected differences in RNA level from different tissue samples may be difficult to determine for several reasons. First, the conventional confirmatory test for differentially expressed bands from DD is executed by Northern blot analysis, which requires at least 10 µg total RNA for each candidate, an amount usually not readily available from limited tissue material. When a DD shows a number of differential cDNAs, the required amount of RNA to screen those candidate cDNAs is overwhelming. Second, the presence of cellular heterogeneity in tissue poses another obstacle in the interpretation of DD results. In this study, we combined in situ hybridization with DD to overcome these problems. This strategy proved to be successful for one of the six bands assessed and serves as an illustration as to how this approach might be targeted to a range of clinical material. From a single case of breast cancer, a differential cDNA, 11AT1, was reproducibly identified from the tumour RNA reactions. The in situ hybridization data not only confirmed the differential overexpression of 11AT1 from the tumour part used in the DD procedure but also demonstrated its preferential expression in tumour cells rather than benign glandular or stromal cells at the tissue level in breast cancer. A limited immunohistochemical study further confirmed the differential expression pattern at the protein level.
The recovered gene, Hsc70, encoding a 73 kDa protein, belongs to heat shock protein 70 family whose members were considered as molecular chaperones in protein metabolism (Hartl 1996). However, emerging evidence indicates that Hsc70 may be involved in breast cancer pathogenesis in view of its association with p53 (Hinds et al. 1987), pRb110 (Nihei et al. 1993) and maturation process of steroid hormone receptors (Pratt & Welsh 1994). Interestingly, BAG-1, an antiapoptotic protein, has been found to bind to Hsc70 and modulate its chaperone activity (Höhfeld & Jentsch 1997) and Bakkenist et al. (1999) have described mutations in the N-terminal ATPase domain of Hsc70 in sporadic breast cancer (Bakkenist et al. 1999). These findings make Hsc70 an attractive candidate for further investigating its role in breast cancer biology.
The main aim of our study was not to pursue Hsc70 but to demonstrate that an integration of in situ hybridization into the strategy of DD, using limited clinical tissue samples, could be used to identify candidate genes for further investigation. However, the demonstration that five of the six bands identified by DD did not show consistent differential cell expression in the five breast cancer tissues reiterated the previous experience that the false-positive rate is inherently high in DD technique (Liang 2002). Effort has been made to reduce false-positive rate in DD (Liang 2002); however, there is still no reliable method to deal with this so far. The aim of the study was not to address this attendant problem in DD but to show that using in situ technique as screening method, DD is still a powerful tool to fish out potential target genes in cancer biology. It has been suggested that cDNA microarrays have significantly diminished the overall rationale for DD, but we suggest that the more low-key, tissue-sparing approach utilized here still may be employed by smaller, less resource intensive groups of investigators for elucidation of gene expression changes in tumour development.
Acknowledgments
The Tzu Chi Compassion Relief Foundation in Taiwan sponsored the work.
References
- Ansari-Lari MA, Jones SN, Timms KM, Gibbs RA. Microdissection RT-PCR analysis of gene expression in pathologically defined frozen tissue sections. BioTechniques. 1996;21:38–44. doi: 10.2144/96211bm07. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Koreth J, Williams CSM, Hunt NCA, McGee JO. Heat shock cognate 70 mutations in sporadic breast carcinoma. Cancer Res. 1999;59:4219–4221. [PubMed] [Google Scholar]
- Dwonrniczak B, Mirault ME. Structure and expression of a human gene coding for a 71 kd heat shock “cognate” protein. Nucleic. Acids Res. 1987;15:5158–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia G, Mitchell SD, Moss SE, Hanby AM, Marshall JF, Hart IR. Identification by differential display of Annexin-VI: a gene differentially expressed during melanoma progression. Cancer Res. 1996;56:55–58. [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hinds PW, Finlay CA, Frey AB, Levine AV. Immunological evidences for the association of p53 with a heat shock protein, Hsc70, in p53-plus-ras-transformed cell lines. Mol. Cell Biol. 1987;7:2863–2869. doi: 10.1128/mcb.7.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J, Jentsch S. GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO. J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RH, von Schlippe M, Francia G, Powell J, Hart IR. Identification of changes in gene expression associated with minimal residual disease. Cancer Metastasis Rev. 1999;18:3–13. doi: 10.1023/a:1006243917187. [DOI] [PubMed] [Google Scholar]
- Liang P. A decade of differential display. BioTechniques. 2002;33:338–346. doi: 10.2144/02332rv01. [DOI] [PubMed] [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic mRNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- National Statistics. SMPS No. 66 The Stationery Office: 2001. Cancer trends in England and Wales 1950–1999. [Google Scholar]
- Nihei T, Takahashi S, Sagae S, Sato N, Kikuchi K. Protein interaction of retinoblastoma gene product pRb110 with Mr 73,000 heat shock cognate protein. Cancer Res. 1993;53:1702–1705. [PubMed] [Google Scholar]
- Nocito A, Bubendorf L, Maria Tinner E, et al. Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J. Pathol. 2001;194:349–357. doi: 10.1002/1096-9896(200107)194:3<349::AID-PATH887>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Parkin DM. Cancers of the breast, endometrium and ovary: geographic correlations. Eur. J. Cancer Clin. Oncol. 1989;25:1917–1925. doi: 10.1016/0277-5379(89)90373-8. [DOI] [PubMed] [Google Scholar]
- Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl. Acad. Sci. USA. 1999;96:9212–9217. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R, Longcraft JM, Jeffery RM, Rogers L, Steel JH. A robust method for isotopic in situ hybridisation to localize mRNAs in routine pathology specimens. Eur. J. Histochem. 1998;42:143–150. [PubMed] [Google Scholar]
- Pratt WB, Welsh MJ. Chaperone functions of the heat shock proteins associated with steroid receptors. Cell Biol. 1994;5:83–93. doi: 10.1006/scel.1994.1012. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- van'tVeer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]