Abstract
Rejected pig-to-primate organ xenografts almost invariably exhibit significant microvascular thrombosis, believed to be due in part to several molecular incompatibilities affecting the regulation of coagulation. In this study, we tested one such proposed incompatibility: whether there is, at least in part, a functional incompatibility in pig tissue factor pathway inhibitor (TFPI) that impedes binding of human factor Xa and regulation of human tissue factor-initiated coagulation. TFPIα cDNA was cloned from pig aortic endothelial cells and found to encode a 279-residue mature protein with 79% overall identity to human TFPIα, increasing to 88–90% in the functional Kunitz-1 and Kunitz-2 domains. Transfected primate cells expressing equivalent levels of GPI-linked pig or human TFPIα were assayed for binding of human factor Xa and inhibition of the human factor VIIa/tissue factor complex. The activity of the expressed pig anticoagulant was equivalent to that of the human protein in both measures of TFPI function in these systems. These data indicate that there are no apparent incompatibilities between recombinant pig TFPI and the human tissue factor pathway. Other factors must account for the thromboregulatory failure of pig endothelium and aberrant tissue factor activity in xenograft rejection.
INTRODUCTION
Intragraft microvascular thrombosis and consumptive coagulopathy are recurring and important themes in pig-to-primate organ xenotransplantation. Although persistent activation of xenograft endothelium by elicited anti-pig antibodies has been suggested as the primary cause of these problems, putative molecular incompatibilities in the control of coagulation will compound xenograft rejection and may also play a role in systemic complications (1). At least two critical endothelial anticoagulant proteins, thrombomodulin and tissue factor pathway inhibitor, have been implicated. Thrombomodulin, an integral component of the protein C pathway, regulates the propagation phase of coagulation by binding thrombin and acting as a cofactor for its activation of protein C. We have recently cloned pig thrombomodulin and demonstrated that although it binds human thrombin, it is a poor cofactor for human protein C activation (2), confirming previous reports (3;4).
Tissue factor pathway inhibitor-1 (TFPI-1) is the key physiological regulator of the initiation phase of coagulation. The α isoform (TFPIα) has 3 Kunitz-type domains, the first of which (K1) binds to the active site of factor VIIa in the fVIIa/TF complex, and the second of which (K2) binds and inhibits factor Xa (5). Inhibition of fVIIa/TF is fXa-dependent; kinetic data favour a model in which TFPI interacts with fXa that has not yet been released from the activating fVIIa/TF complex, forming an inactive quaternary complex on the plasma membrane (6). The third Kunitz domain (K3) has no described inhibitory function but may mediate the ionic binding of TFPIα to cell surfaces (5). The β isoform of TFPI-1, generated by alternative splicing, contains only K1 and K2 and is directly attached to endothelial cells by a GPI linkage (7).
Prior studies using cultured pig and human endothelial cells have suggested that the xenogeneic combination of cells boosts TF activity (8) and that pig endothelium fails to efficiently bind human fXa, despite partial amino acid sequence data showing high identity (92%) between the pig and human TFPI K2 domains (9). In this study, we cloned and expressed pig TFPIα to directly examine its compatibility with components of the human tissue factor pathway. We found that pig and human TFPIα are highly conserved at the amino acid sequence level, and that the cell-associated recombinant proteins demonstrate similar efficiency in both binding of human fXa and inhibition of human fVIIa/TF activity in vitro. Thus while the failure of pig endothelial cells to regulate the initiation of human clotting remains incompletely understood, our results indicate that it is not due to molecular incompatibility in the control of the tissue factor pathway.
METHODS
Isolation of pig TFPI cDNA
The sequence of pig TFPI cDNA was determined by simultaneous 5′/3′ rapid amplification of cDNA ends (RACE) (10) as follows. Total RNA was isolated from cultured pig aortic endothelial cells (PAEC) with TRIzol (Invitrogen, Mount Waverley, Australia) and used to prepare cDNA by reverse transcription-PCR (RT-PCR) with the oligo(dT) anchor and T-S primers, using Superscript III reverse transcriptase (Invitrogen) as per manufacturer’s instructions. After further amplification using the T-S PCR and PCR anchor primers, the cDNA was purified, phosphorylated and ligated to generate a library containing circular full-length cDNA species. Degenerate PCR primers (pTFPI-Fdeg and pTFPI-Rdeg) were used to amplify an internal segment of pig TFPI cDNA from the library. Degenerate primer sequence was based on an alignment of the reported TFPI-1 cDNA sequences from six mammalian species (human, rhesus, bovine, rabbit, rat, and mouse) plus a partial pig TFPI amino acid sequence (9). The sequence of the resulting PCR product was used to design nested gene-specific primers (GSP) pointing away from the known sequence. Products from sequential PCR of the ligated cDNA library with GSP5′-1/GSP3′-1 and GSP5′-2/GSP3′-2 were cloned and their sequence was used to reassemble the pig TFPI cDNA sequence, which was subsequently amplified as a single product using the primers pTFPI-F and pTFPI-R and cloned into the ZEROBlunt vector (Invitrogen).
Construction of TFPI-GPI expression vectors
We have previously fused the human TFPIα open reading frame (ORF) to the glycosyl phosphatidylinositol (GPI) membrane attachment signal from human CD55 (unpublished results). A similar strategy was used to replace the human TFPIα ORF in this construct with the pig TFPIα ORF. TFPIα-GPI expression vectors were then constructed using the plasmid pEF-BOS-FLAG (11). Pig and human TFPIα-GPI fusion ORFs lacking their native signal peptide sequences were amplified using the primer sets pTFPI-MluI-F/TFPI-MluI-R and hTFPI-MluI-F/TFPI-MluI-R, respectively. After cloning of the products into ZEROBlunt, they were excised using MluI and cloned into pEF-BOS-FLAG. Both constructs were confirmed by sequencing.
Primers
Sequences of the oligo(dT) anchor, T-S, T-S PCR and PCR anchor primers are given elsewhere (10). Other primers were:
pTFPI-Fdeg = 5′-GCTTTTTGGAAGARGATCCTGG-3′
pTFPI-Rdeg = 5′-ATTTCCCCCACATCCASTGTA-3′
GSP5′-1 = 5′-AGATTGCCAAGGCAGCCACCATACT-3′
GSP3′-1 = 5′-GACTCTCCAGCCTACCAAAGCACCCAGC-3′
GSP5′-2 = 5′-GCGTTCACACTGCTTTGACTG-3′
GSP3′-2 = 5′-TTTTACGGCCCCTCCTGGTGTCTGACCC-3′
pTFPI-F = 5′-TCTTCAGAGATTTCACTCAGA-3′
pTFPI-R = 5′-CAGGAGAGAAAACTATAGGAGTCA-3′
pTFPI-MluI-F = 5′-ACGCGTATTCCTGAGGAAGGTGAAGA-3′
hTFPI-MluI-F = 5′-ACGCGTGATTCTGAGGAAGATGAAGA-3′
TFPI-MluI-R = 5′-ACGCGTCTAAGTCAGCAAGCCCAT-3′
Cell culture and transfection
COS-7 cells were cultured at 37ºC in Dulbecco’s Modified Eagle’s Medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (HyClone, Tauranga, New Zealand) and 15mM HEPES buffer (Invitrogen). 5×106 cells were transfected by electroporation with 10 μg of plasmid DNA as described (2). Transfectants were stained with fluoroscein isothocyanate-conjugated antibody KM5-1C5 (anti-FLAG, WEHI Monoclonal Laboratory, Bundoora, Australia) and analysed on a FACSCalibur flow cytometer (Becton Dickinson, Sydney, Australia).
Human factor Xa binding assay
24 h after transfection, cells were seeded at 3×104 cells per well in 96-well flat bottom plates and incubated at 37ºC for 24 h. Cultures seeded and grown in parallel were analysed by flow cytometry immediately prior to the binding assay to confirm equivalent expression of human and pig TFPI. The cells to be assayed were washed three times in wash buffer (0.15mM NaCl2, 4mM KCl, 11mM glucose, 10mM HEPES, 0.5% fatty acid-free BSA, 2μg/mL polybrene, pH 7.5), then incubated for 15 min at 25ºC in 50μL fXa assay buffer (wash buffer containing 5mM CaCl2). Human factor Xa (Merck, Kilsyth, Australia) was added at a range of amounts (0, 1, 2, 3 pmol/well) in a total final volume of 100μL, followed by incubation for a further 15 min at 25ºC. The reaction was terminated by the addition of 5μL stop buffer (wash buffer containing 100mM EDTA). 100μL of supernatant from each well was transferred to a 96-well flat-bottom plate and warmed to 37ºC in a FLUOStar Galaxy kinetic plate reader (BMG Labtech, Offenburg, Germany). Spectrozyme fXa chromogenic substrate (American Diagnostica, Stamford, CT) was added to a final concentration of 1mM, and the absorbance at 405nm was measured. The slope of the absorbance curve at each concentration of factor Xa was compared to that in the absence of cells with and without 12.5nM recombinant human TFPI (American Diagnostica). All assays were performed in triplicate.
Inhibition of human factor VIIa/TF activity
Transient transfectants were prepared and washed as described for the factor Xa binding assay. Recombinant human TF was prepared by dissolving the contents of one vial of Innovin (Dade Behring, Marburg, Germany) in 2mL fXa assay buffer. 50μL of 200pM human fVIIa (Merck) in fXa assay buffer was mixed on ice with a range of volumes (0–50μL) of Innovin in a final volume of 100μL, followed by incubation for 15 min at 37ºC. The resulting fVIIa/TF complexes were added at 100μL per well to the transfected cells, followed by incubation for 15 min at 37ºC. 100μL of 120nM human fX (Merck) in fXa assay buffer was then added, with incubation for a further 15 min at 37ºC. The reaction was terminated by the addition of 10μL stop buffer, and factor Xa activity was determined as described for the factor Xa binding assay. Inhibition of fVIIa/TF activity was measured as the decrease in fXa generated and compared to that observed in the presence of recombinant human TFPI. All assays were performed in triplicate.
Statistical analysis
Data were analysed for statistical significance using Student’s unpaired t-test. P values of <0.05 were considered significant.
Sequence analysis
Sequence analysis including ClustalW alignment was performed using Accelrys Gene 2.0 (Accelrys, Tokyo, Japan). Amino acid sequences were analysed for the presence of signal peptides and N- and O-linked glycosylation sites using the programs SignalP 3.0 (12), NetNGlyc 1.0 (www.cbs.dtu.dk/services/NetNGlyc), and NetOGlyc 3.1 (www.cbs.dtu.dk/services/NetOGlyc), respectively.
RESULTS
Cloning of pig TFPIα
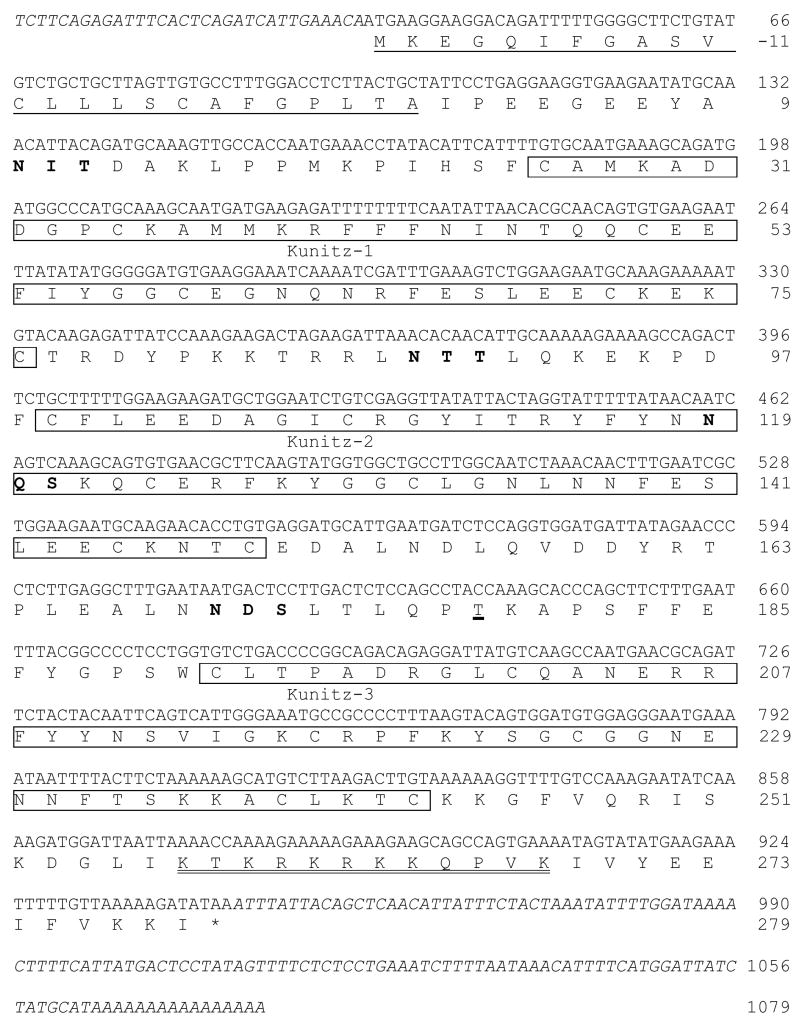
Simultaneous 5′/3′ RACE of PAEC total RNA was used to isolate a 1079-basepair (bp) cDNA containing an 870-bp open reading frame (Fig. 1). The deduced 303-residue protein showed significant amino acid sequence homology with TFPI-1 from various species and contained three Kunitz-type domains, indicating that it represented the α form of pig TFPI-1. The sequence of the mature pig TFPIα protein was 79% identical to that of human TFPIα (data not shown). Two N-linked glycosylation sites (one in K2 and one between K2 and K3) and one O-linked site in human TFPIα were present in pig TFPIα, which had two additional N-linked glycosylation motifs on either side of K1 (Fig. 1). Alignment of the nucleotide sequence with the incomplete sequence of the pig genome revealed identity with stretches within a chromosome 15 clone (EMBL Accession No. CU570823.1).
Figure 1. Nucleotide and deduced amino acid sequence of pig TFPIα cDNA.
Putative structural features of the protein are indicated as follows: signal peptide, underlined; Kunitz domains, boxed; N-linked glycosylation motifs, bold; O-linked glycosylation site, thick underlined; heparin binding site, double underlined. Amino acid numbers represent the position of residues in the deduced mature protein sequence. 5′- and 3′-untranslated regions of the cDNA are shown in italics. The cDNA sequence of pig TFPIα is available in GenBank with Accession No. EU090729.
The TFPIβ isoform results from the use of an alternate in-frame exon in the 3′ coding region, resulting in a protein with a C-terminus that is shorter than and distinct to the alpha isoform. We attempted to isolate TFPIβ cDNA by PCR of the ligated PAEC cDNA library using the PCR anchor primer with nested primers within the K2-encoding exon (common to both isoforms). However, only the longer TFPIα product was generated (data not shown), suggesting that PAEC express less TFPIβ than TFPIα at the transcriptional level.
The Kunitz domains of pig and human TFPIα are highly conserved
Alignment of K1, K2 and K3 of pig and human TFPIα revealed identity of 88%, 90% and 88%, respectively (Fig. 2). The critical P1 residues in K1 (Lys36 in human) and in K2 (Arg107 in human) were identical in seven mammalian species with the exception of the P1 of rabbit K1, which had a conservative (Lys → Arg) substitution. The P1 residue in K3, which has no inhibitory function, exhibited greater variation between species: Gln in pig, rabbit and bovine TFPI; Arg in human and rhesus TFPI; and Lys in mouse and rat TFPI.
Figure 2. The Kunitz domains of pig and human TFPI are highly conserved.
Amino acid alignments of the three Kunitz domains of pig TFPIα (P, upper) and human TFPIα (H, lower) are shown. Numbers represent the position of residues in the mature protein sequences. The residues in the P1 position of the active site cleft of each Kunitz domain of human TFPI (Girard 1989) are indicated in bold and underlined.
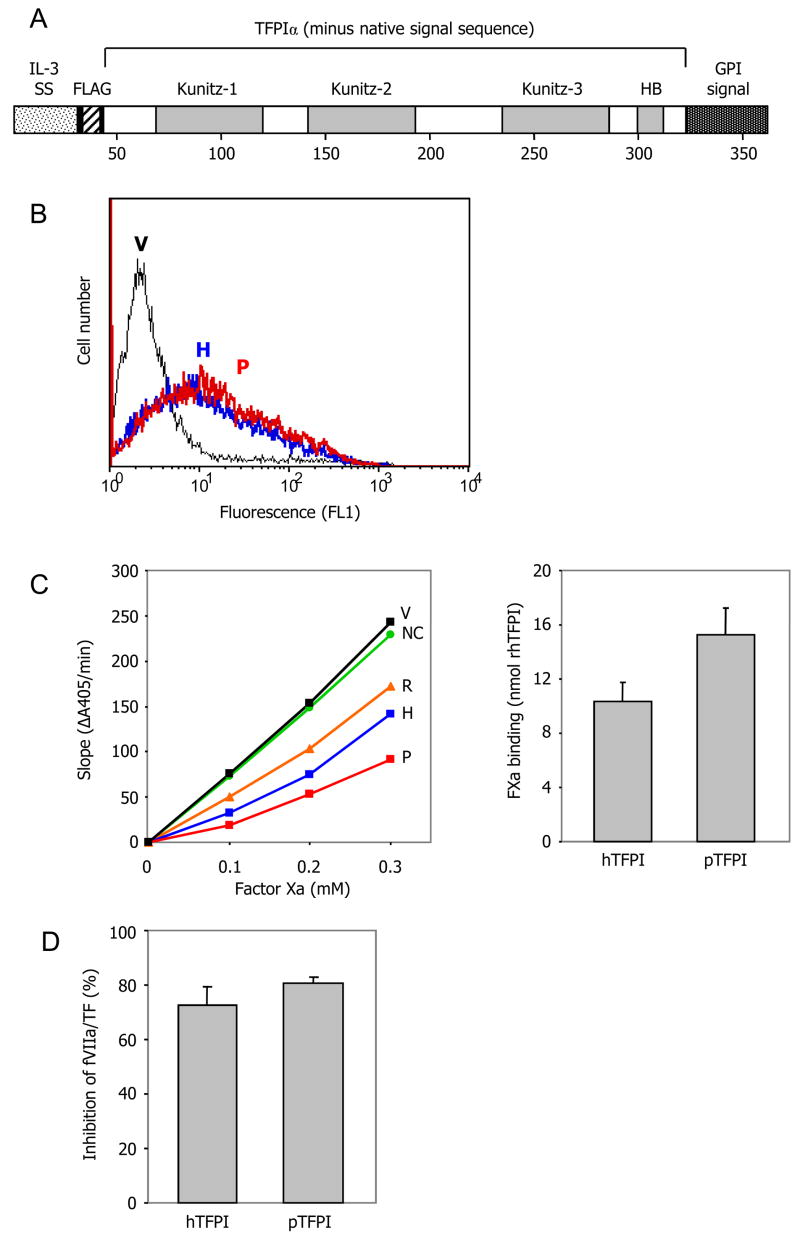
Pig TFPIα efficiently binds human factor Xa and inhibits human factor VIIa/TF
TFPI activity was measured in COS-7 cells transfected with expression vectors for pig or human TFPIα. Normally, attachment of TFPIα to the surface of endothelial cells is indirect, with a proportion binding to an unidentified GPI-linked co-receptor and the remainder associating with glycosaminoglycans (13). Since it is not known whether this co-receptor is expressed by COS-7 cells, we engineered directly membrane-bound forms of TFPIα by fusing a GPI attachment signal to the C-terminus of each open reading frame (Fig. 3A). We also added a FLAG epitope tag to the N-terminus to allow detection of pig TFPIα (which is not recognised by commercially available monoclonal antibodies to human TFPI) and to facilitate comparison of the expression levels of pig and human TFPIα. Strong and equivalent surface expression of pig and human TFPIα was detected by flow cytometry after transient transfection of COS-7 cells (Fig. 3B). Both pig and human TFPIα efficiently bound human factor Xa (Fig. 3C). Both also efficiently inhibited the activation of human factor X by the human factor VIIa/TF complex (Fig. 3D). These results indicate that there is no substantive molecular incompatibility between recombinant pig TFPIα and the human TF-mediated coagulation pathway.
Figure 3. Pig TFPIα binds human factor Xa and inhibits human factor VIIa/TF.
(A), Expression vectors were constructed for full length human and pig TFPIα, modified to incorporate a FLAG epitope tag at the N-terminus and the human CD55 GPI membrane attachment signal at the C terminus. IL-3 SS = IL-3 signal sequence; HB = heparin-binding domain of TFPIα. (B), The expression vectors were transiently transfected into COS-7 cells. Similar expression of human (H, blue line) and pig (P, red line) TFPIα was demonstrated by flow cytometric analysis using an anti-FLAG antibody (vector-transfected cells = V, black line). (C), Pig TFPIα efficiently bound human factor Xa. Left panel, representative experiment showing fXa binding by transfectants expressing human (H) or pig (P) TFPIα, relative to 12.5nM recombinant human TFPI (R) (vector-transfected cells = V, no cells control = NC). Right panel, comparison of fXa binding by human and pig TFPIα (mean ± SD of 2 independent experiments). (D), Pig TFPIα neutralised human factor VIIa/TF activity as efficiently as human TFPIα (mean ± SD of 3 independent experiments; p=0.12). Under the same conditions 3nM recombinant human TFPI inhibited fVIIa/TF activity by 66% (data not shown).
DISCUSSION
Molecular incompatibility affecting the function of porcine anticoagulants has long been suspected to contribute to thrombosis within pig-to-primate xenografts. The failure of pig cells to regulate human TF-initiated coagulation suggested an incompatibility of TFPI that was implied from in vitro experiments showing that human, but not pig, aortic endothelial cells inhibited human fXa activity (9). The TFPI protein undergoes ready glycosylation and is predominantly found in the vascular endothelium and plasma in both free forms and complexed with plasma lipoproteins. However in the Kopp studies, similar levels of TFPI protein expression on the surface of human aortic EC and PAEC could not be convincingly demonstrated.
We therefore focused on a major TFPI isoform and cloned pig TFPIα from PAEC, expressed it in epitope-tagged, GPI-linked form on transfected cells, and compared its capacity to regulate the human TF pathway with that of similarly tagged and expressed human TFPIα. We found that pig and human TFPIα are highly conserved, particularly in the critical K1 and K2 domains, and that there is in fact no significant inter-species functional incompatibility in the control of the human TF pathway by the recombinant engineered proteins. Due to the unavailability of suitable cross-reacting monoclonal antibodies, we could not directly confirm the structure and conformation of the expressed pig TFPIα; however, its presence on the cell surface and its activity in both TF binding and factor VIIa/TF inhibition imply that it must be processed and folded correctly.
How can our results be reconciled with the findings of Kopp et al (9)? It is possible that cultured PAEC express considerably lower levels of firmly attached TFPI on their surface than HAEC. Several alternatively spliced transcript variants of the TFPI gene have been described, but the full-length nature of some of these variants has not been confirmed. Furthermore, the major transcript identified in the prior study suggested that the TFPI-2 variant was expressed in PAEC. Therefore, unlike HAEC, PAEC in the quiescent state express very low levels of prototypic TFPI RNA (9). Furthermore, the ratio of the two forms of TFPI-1 – the directly membrane-anchored TFPIβ and the more loosely associated TFPIα – might differ in PAEC and HAEC. We showed here by RT-PCR that PAEC express TFPIα but we could not detect expression of TFPIβ; whether HAEC express more of one or the other forms has not been determined.
Another possibility is that different glycosylation of TFPIα by pig cells interferes with its interaction with human fXa, and the fact that the recombinant proteins were expressed in COS-7 primate cells and not α1,3-galactosyltransferase-expressing pig cells could impact this process. Still, we consider this to be an unlikely explanation for all of the observed effects because all of the glycosylation sites in human TFPIα, including one in K2, are conserved in pig TFPIα. The two novel N-linked glycosylation motifs in pig TFPIα flank K1 and would not be expected to disrupt binding of fXa to K2. Glycosylation of human TFPI has been postulated to influence its cell binding properties and plasma clearance, but not the direct inhibitory function (5).
Although our findings indicate that pig TFPI should function normally in the xenograft setting, they do not weaken the case for overexpression of some form of membrane-bound TFPI – human or pig – in porcine donor tissue. Our data at least suggest that the expression of either pig or human TFPIα engineered to be cell associated via a GPI anchor should have equivalent efficacy. TFPI has been shown to be lost in conjunction with heparan sulphates from the porcine vasculature during delayed rejection of pig-to-baboon cardiac xenografts, potentially promoting the development of a procoagulant environment (9). Furthermore, overexpression of human TFPIα in transgenic mice completely prevents acute humoral rejection of mouse-to-rat cardiac xenografts (14) and transgenic pigs are being developed to test efficacy in a primate model of xenotransplantation. Whether the remarkable protective effect of human TFPIα in mice can be reproduced in the pig-to-primate model is yet to be determined.
Acknowledgments
this study was funded in part by the Australian National Health and Medical Research Council (NHMRC) and NIH USA (to SCR and AJFd’A; U01 AI066331 and P01AI045897)
References
- 1.Chen D, Dorling A. Microcoagulation processes after xenotransplantation. Curr Opin Organ Transplant. 2005;10:240–245. [Google Scholar]
- 2.Roussel JC, Moran CJ, Salvaris EJ, Nandurkar HH, d’Apice AJF, Cowan PJ. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. doi: 10.1111/j.1600-6143.2008.02210.x. in press. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JB, Grey ST, Lesnikoski BA, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation. 1997;64:888–896. doi: 10.1097/00007890-199709270-00017. [DOI] [PubMed] [Google Scholar]
- 4.Kopp CW, Grey ST, Siegel JB, et al. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation. 1998;66:244–251. doi: 10.1097/00007890-199807270-00019. [DOI] [PubMed] [Google Scholar]
- 5.Crawley JTB, Lane DA. The haemostatic role of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2008;28:233–242. doi: 10.1161/ATVBAHA.107.141606. [DOI] [PubMed] [Google Scholar]
- 6.Lu G, Broze GJ, Jr, Krishnaswamy S. Formation of factors IXa and Xa by the extrinsic pathway: differential regulation by tissue factor pathway inhibitor and antithrombin III. J Biol Chem. 2004;279:17241–17249. doi: 10.1074/jbc.M312827200. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Piro O, Lu L, Broze GJ., Jr Glycosyl phosphatidylinositol anchorage of tissue factor pathway inhibitor. Circulation. 2003;108:623–627. doi: 10.1161/01.CIR.0000078642.45127.7B. [DOI] [PubMed] [Google Scholar]
- 8.Kopp CW, Robson SC, Siegel JB, et al. Regulation of monocyte tissue factor activity by allogeneic and xenogeneic endothelial cells. Thromb Haemost. 1998;79:529–538. [PubMed] [Google Scholar]
- 9.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 10.Huang JC, Chen F. Simultaneous amplification of 5′ and 3′ cDNA ends based on template-switching effect and inverse PCR. Biotechniques. 2006;40:187–189. doi: 10.2144/000112051. [DOI] [PubMed] [Google Scholar]
- 11.Kawakatsu T, Ogita H, Fukuhara T, et al. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem. 2005;280:4940–4947. doi: 10.1074/jbc.M408710200. [DOI] [PubMed] [Google Scholar]
- 12.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 30. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Maroney SA, Cunningham AC, Ferrel J, et al. A GPI-anchored co-receptor for tissue factor pathway inhibitor controls its intracellular trafficking and cell surface expression. J Thromb Haemost. 2006;4:1114–1124. doi: 10.1111/j.1538-7836.2006.01873.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Weber M, McVey JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4:1958–1963. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]