Abstract
One limitation of current biochemical or histologic analysis of advanced prostate cancer (PC; T3/T4 ± Nx Mx) is the ability to identify on first diagnostic biopsy patients who will make a durable response to hormone ablation therapy. The aim of this study was to assess the predictive value (sustained response to hormonal therapy and clinical outcome (relapse-free and overall survival)) of phosphatase and tensin homolog (PTEN) and the androgen receptor (AR) immunoexpression in the presenting biopsy. Analysis was performed on 47 samples (10 cases of benign prostatic hyperplasia and 37 hormone-naive PCs). Patients selected represented two stages in the natural history of PC: The “clinical metastatic androgen-responsive” (androgen-dependent PC, ADPC) and the “clinical metastatic androgen-resistant” (androgen-independent PC, AIPC). Reduced immunoreactivity (IR) of either or both PTEN/AR in the initial hormone-naive PC samples was observed with increased frequency in AIPCs. In the ADPC group, low PTEN and/or AR-IR was associated with a shorter median relapse-free survival, i.e., at 30 months after surgery, the probability of relapse-free survival for high expressors of PTEN and AR was 85.7% (SEM = 9.3) compared with only 16.6% (SEM = 15.2) in low expressors. At 36 months, only 28.5% (SEM = 9.3) of ADPC high expressors had experienced a biochemical relapse compared with 100% of low expressors (hazard ratio, 4.6; 95% confidence interval, 4.7–146.8). Further studies analyzing the coexpression of PTEN and AR should be undertaken to validate this pilot study and the utility of these biomarkers in routine histopathologic workup of patients with PC.
Introduction
Prostate cancer (PC) is the most common non dermatological epithelial cancer in men in developed countries accounting for approximately 15.3% of all male malignancies [1]. In up to 80% of patients with locally advanced (T3/T4 N0 M0) or metastatic (T3/T4 Nx M1), the disease responds to primary androgen ablation therapy [AAT; orchidectomy or administration of luteinizing hormone-releasing hormone (LHRH) agonists with or without antiandrogens]. A response to AAT averaging approximately 18 months is seen in most patients with androgen-dependent metastatic PC (ADPC) [2]. Disease relapse then ensues possibly because aberrant androgen receptor (AR) signaling and/or the activation of alternative signaling pathways and continues to progress as hormone refractory or androgen-independent PC (AIPC) [3,4].
Routine screening tests, in particular, measurement of the serum level of prostate-specific antigen (PSA), have significantly increased the rate of early detection of PC. Together with pathologic staging and Gleason grade, PSA level also governs decision making and prognosis in PC. However, due to the heterogeneity of tumors and variability in progression of PCs of different grades, commonly used diagnostic methods fail to discriminate between indolent tumors that do not require aggressive treatment from those that will progress after conventional treatments. There is, therefore, a need to identify markers that could aid in early differentiation between these two distinct clinical phenotypes, thus enabling better prognostication.
PTEN (phosphatase and tensin homolog deleted on chromosome 10), a negative regulator of phosphatidylinositol 3-kinase/Akt signaling, thus far, is the most frequently mutated tumor-suppressor gene in PC [5], where deletions, point mutations, and DNA methylation are reported to occur. However, because alterations of the PTEN gene are late events in PC progression, loss of protein expression correlates well with a high (>7) Gleason grade [5] but assessment of PTEN expression is of little value as a sole prognostic marker.
Increasing evidence points to a strong correlation between perturbations in the AR and PTEN/Akt signaling axis and PC progression [6–8]. Results from several in vitro studies using established PC cell lines have demonstrated that PTEN and Akt are, respectively, negative and positive modulators of AR transcriptional activity [7,9]. Under experimental conditions, PTEN and AR exert opposite effects on cell growth and apoptosis. Moreover, Wu et al. [10] in a recent study demonstrated that conditional expression of PTEN alters responsiveness of LNCaP, an androgen-responsive PC cell line, to antiandrogens.
The aim of this pilot study was to assess the potential benefit of analyzing immunohistochemical coexpression of PTEN and AR in the initial hormone-naive (not subjected to AAT) biopsies from patients with PC, as a possible predictor of sustained responsiveness to hormone therapy.
Materials and Methods
This is a retrospective pilot study of two distinct cohort groups, representing two clinical tumor phenotypes (ADPC and AIPC), as determined in a 2-year follow-up. To satisfy the criteria set up for the study, a relatively small number of cases could be selected from the hospital database and included in the analysis.
Samples and Patient Selection
The study included 47 transurethral prostatic resections samples [10 benign prostatic hyperplasia (BPH) and 37 advanced PCs] selected from the archives of the Hammersmith Hospital, London, from patients treated during the period from 1990 to 2001 (Local Research Ethics Committee approval no. 2001/6023). Age range was 52 to 65 years (median, 55.8 years) for patients with BPH and 55 to 90 years (median, 70.9 years) for patients with PC. All PC patients had stage III/IV disease (T3/T4 Nx M0 or Tx Nx M1) and the majority were of high Gleason grade (≥7). Prostate-specific antigen level at the time of surgery was ≤10 ng/ml in only 10 of 37 patients.
Groups. Prostate cancer patients were categorized by androgen sensitivity at the time of biopsy and ascribed to two groups:
Group 1: ADPC (20 patients) — patients who, after surgery, received and responded to AAT and, for at least 18 months, demonstrated no evidence of clinical or biochemical progression;
Group 2: AIPC (17 patients) — patients with documented clinical and biochemical progression while receiving AAT for at least 2 years after surgery.
Treatment
Treatment regimen included either a combination of an antiandrogen and LHRH analogues (18 patients), antiandrogens and orchidectomy (8 patients), LHRH analogues only (3 patients), and antiandrogen therapy (2 patients). Four patients underwent orchidectomy but did not receive any systemic hormone therapy, whereas two patients were treated by LHRH analogues, antiandrogens, orchidectomy, and diethylstilbestrol in the AIPC group. None of the patients received neoadjuvant hormonal therapy, radiotherapy, or cytotoxic chemotherapy.
Measurements
Evidence of clinical progression was defined as development of metastases and/or clinically assessed size increase of the prostate gland irrespective of PSA values. Follow-ups were available in all cases, and PC was defined as cause of death in patients dying after documentation of clinical progression. The main end point was survival duration, that is, the time between obtaining the specimen and death and median observation time was 36 months [95% confidence interval (CI), 33.65–55.05].
Clinicopathologic characteristics of AD and AI PC patients are summarized in Table 1.
Table 1.
Clinicopathologic Characteristics and Immunohistochemical LI of BPH and PC Cases.
| BPH | AD | AI | |
| No. of patients (% of total) | 10 (21%) | 20 (43%) | 17 (36%) |
| Age (years) | |||
| Median | 55.8 | 69.8 | 72 |
| Range | 52–65 | 55–90 | 60–85 |
| Gleason score | |||
| <7 | NR | 6 (30%) | 3 (18%) |
| 7 | 6 (30%) | 4 (24%) | |
| ≥7 | 8 (40%) | 10 (58%) | |
| TNM stage | |||
| T3/4 Nx M0 | NR | 16 (80%) | 7 (41%) |
| Tx Nx M1 | 4 (20%) | 10 (59%) | |
| Preoperative PSA (ng/ml) | |||
| <10 | 10 | 4 | 4 |
| ≥10 | 0 | 16 | 13 |
| Treatment | |||
| Monotherapy | NR | 4 | 5 |
| MAB | 16 | 12 | |
| Survival (months) | |||
| Median | NR* | 66 | 12 |
| 95% CI | 51.5–78.7 | 11.8–29.7 | |
| IHC LI (mean ± SEM) | |||
| AR | 220 ± 30.12 | 180.4 ± 15.78 | 130.6 ± 19.09 |
| PTEN | 284 ± 10.85 | 169.7 ± 21.88 | 110.6 ± 20.18 |
MAB indicates monoclonal antibody; NR, not relevant.
Only survival differed significantly between AD and AIPC patients (P < .001).
Immunohistochemistry
Paraffin sections (5 µm) cut from formalin-fixed tissue blocks were mounted on positively charged glass slides (VWR International, UK) and processed for conventional histologic diagnosis and immunohistochemistry (IHC) using the avidin-biotin-peroxidase complex (ABC) method [11]. Immunostaining for AR (mouse antihuman AR; BioGenex Laboratories, San Ramon, CA) and PTEN (mouse antihuman PTEN; Neomarkers, Fremont, CA) was carried out after antigen retrieval by boiling the sections in 10 mM citrate buffer (pH 6.0) in a conventional microwave oven for 15 minutes. The anti-PTEN monoclonal antibody recognizes an epitope at the C-terminus of PTEN, which has been shown to be lost in a range of human tumors [12].
To validate specificity of immunostaining, negative and positive PC cell line controls were included in the analysis: 1) LNCaP (AR+/PTEN-; PTEN deletion of one allele and a mutation of the other PTEN allele) and 2) DU145 (AR-/PTEN+; a heterozygous point mutation in exon 5 that does not affect PTEN protein expression) [13]. Before fixation of the pellets in formalin and processing for immunocytochemistry, cells were routinely grown in DMEM (Invitrogen, UK) medium supplemented with 10% fetal bovine serum (FBS; Invitrogen).
For each tissue sample, immunostaining was assessed by two independent observers. The entire section was assessed at low (100x) and high (400x) power, and 10 high-power fields (at least 1000 cells per patient) were analyzed. Selection of the most positive areas was avoided to prevent overestimation of reactivity. The fraction of immunoreactive cells was calculated as a percentage, and immunoreactivity in <10% of cells was considered as negative. Intensity of expression was evaluated and a score of 1, 2 or 3 was assigned to reflect focal/weak, moderate or high reactivity, respectively. An overall labeling index (LI) that ranged between 0 and 300 was calculated by multiplying the percentage (0–100%) of immunoreactive cells by the IHC intensity of expression (score range, 1–3).
Statistical Analysis
Scores were clustered into two groups using the median value as a cutoff point: high or low expressors. Fisher's exact test was used to compare clinicopathologic features of the two groups and to investigate whether combined expression of more than one variable was associated with development of AI state. Survival proportions in the groups were estimated by the Kaplan-Meier method and compared by log rank test. Statistical analyses were performed in either the Statistical Packaged for Social Science (SPSS v 9.0; SPSS, Inc., Chicago, IL) or in GraphPad Prism 3.2 (GraphPad Software, San Diego, CA).
Results
Androgen-Dependent PC and Androgen-Independent PC Groups
Androgen-dependent PC specimens were derived from hormone-naive tumors that were treated either immediately after biopsy (12/20 cases) or within 1 or 2 years (8 patients). These patients did not progress biochemically or clinically in the absence of androgen for 18 months or longer.
Androgen-Independent PC samples were derived from tumors, which, after an initial response to AAT, relapsed and continued to progress.
Fisher's exact test confirmed that clinicopathologic characteristics, including Gleason grade and stage, did not differ significantly between ADPC and AIPC groups, except for survival. This secured the elimination of the potential impact of these factors on the expression of the evaluated markers, thus, ascribing any differences detected to hormonal responsiveness rather than to disease progression.
Immunohistochemistry—Cellular Localization of AR and PTEN
Controls.
-
Cell lines
LNCaP: there was no detectable immunoreactivity for PTEN (Figure 1F), and AR was expressed in the nuclei of the cells (Figure 1G).
DU145: PTEN expression was strong and restricted to the cytoplasm (Figure 1D), and cells were found not to express the AR (Figure 1E).
-
Benign prostatic hyperplasia cases
Immunoreactivity for 1) AR was observed in the nuclei and 2) PTEN in the cytoplasm of luminal cells. Some reactivity of the latter was also found in stromal cells. Immunohistochemical labeling indices (LIs) were 220 ± 30.12 for AR and 284 ± 10.85 for PTEN.
-
Prostate cancer cases
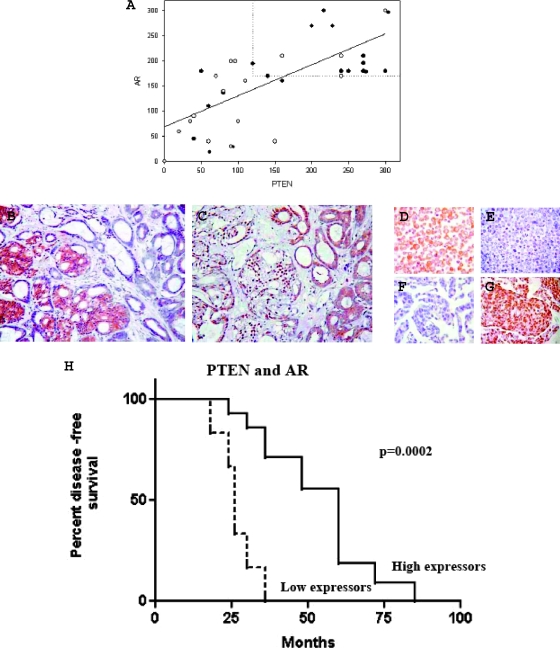
Androgen receptor was expressed in the nuclei of all except for two AIPC cases (cytoplasmic expression), whereas in all cases, PTEN expression was localized to the cytoplasm. Androgen receptor and PTEN expressions were higher in ADPC compared to AIPC, but these were not significant (P = .0503 and P = .0583, respectively). When stratified according to the median and analyzed together, 14 (70%) of 20 ADPC samples were found to be high expressors of both PTEN and AR compared to only 4 (24%) of 17 AIPC (Figure 1A). This difference was statistically significant (P = .0081; odds ratio, 7.583; 95% CI, 1.737–33.101).
Figure 1.
(A) Correlation between PTEN and AR expression in PC and increased frequency of high expressors of PTEN and AR in AD PC. Immunohistochemistry LI of PTEN and AR were directly (continuous line) correlated in ADPC (closed circle) and AIPC (open circle; rs = 0.6604, P = .0015 and rs = 0.6320, P = .0065). Dotted lines passing through the median value of each IHC LI identify the region where most ADPC and only a few of AIPC cases are observed (high expressors). Most of AIPC cases are scattered outside this area. (B and C) Expression of AR (right) and PTEN (left) in serial sections of PC. Photomicrographs show adjacent PC areas where AR and PTEN are differentially expressed. Cytoplasmic expression of PTEN is associated with strong AR nuclear reactivity. In areas where PTEN is negative, AR is confined to the cytoplasm (original magnification, x200). (D–G) DU145 and LNCaP as controls for PTEN and AR antibodies. DU145 cells expressed cytoplasmic PTEN (D) but were AR-negative (E). Conversely, nuclear expression of AR was observed in LNCaP cells (G), whereas PTEN was absent (F). (H) Kaplan-Meier curve showing relapse-free survival in ADPC patients stratified by PTEN and AR coexpression levels. High expressors and low expressors are represented by a continuous and an interrupted line, respectively. Log rank test was used to compare curves. P < .05 was considered significant. Time until disease recurrence (relapse-free survival) was measured from the date of surgery until the development of metastases and/or bladder outlet obstruction (clinical relapse) or three consecutive increases in the PSA level greater than the nadir (biochemical relapse). At 30 months after surgery, the probability of relapse-free survival for high expressors of PTEN and AR among AD patients was 85.71% (SEM = 9.35) compared to only 16.67% (SEM = 15.21) in low expressors. At 36 months, only 28.57% (SEM = 9.35) of high expressors had experienced a biochemical relapse compared to 100% of low expressors (hazard ratio, 4.65; 95% CI, 4.719–146.8).
In addition, a positive linear association was identified between PTEN and AR in both ADPC and AIPC cases (rs = 0.6604, P = .0015 and rs = 0.6320, P = .0065). Immunostaining of serial sections showed that strong AR expression was observed in areas where PTEN was strongly positive and vice versa (Figure 1, B and C).
Relationship between AR/PTEN and Survival
The median survival time was 66 months (95% CI, 51.5–78.7) for the ADPC patients and 12 months (95% CI, 11.86–29.3) for the AIPC patients (Table 1). In the ADPC group, two groups of patients with different prognosis could be identified according to the coexpression of AR and PTEN. To compare the prognostic impact of AR and PTEN on survival, high and low expressors were categorized according to the median of each group and a Kaplan-Meier survival curve was plotted for each of these variables. The median relapse-free survival for low and high expressors (of both AR and PTEN) was 27 and 60 months, respectively (ratio, 0.45; P = .0002; 95% CI, 0.07469–0.8253; Figure 1H). In the group with reduced expression of either AR and/or PTEN, only 49% (95% CI, 33–66%) were alive at 6 years (P = .008). There was a trend to reduced survival in AI patients with reduced expression of AR and/or PTEN but it was not statistically significant.
Discussion
The complementary role of PTEN and AR in prostatic epithelium is corroborated by evidence derived from in vivo and in vitro studies. Benign columnar prostatic epithelium, which requires androgen for its continuous maintenance, expressed both PTEN and AR; however, basal cells, which are considered to be AI [14], lacked both markers. Molecular studies have suggested an antagonistic interaction between PTEN and AR in controlling proliferation and apoptosis of prostatic cells, maintaining homeostasis of prostatic epithelium in adult males [7,15,16]. In PC, the transcriptional activity of the AR is inhibited by PTEN in ADPC, but the role of PTEN in AIPC is still unclear. In a recent study, Wu et al. [10] showed that PTEN induction may confer enhanced responsiveness to the antiproliferative effects of antiandrogens in the LNCaP PC cell line. These data together with our initial observations suggest a functional relationship between PTEN and AR and responsiveness to AAT.
We observed heterogeneous PTEN/AR immunoexpression within the same tumor; areas of cells strongly immunoreactive for both PTEN and nuclear AR were often juxtaposed to foci of PTEN-negative cells with AR expression restricted to the cytoplasm. The AR has been implicated in nontranscriptional activity outside the nucleus [17] such as direct protein-protein interactions with forkhead [18]. It is conceivable that in PTEN deficient cells, AR/FKHR complex is sequestered in the cytoplasm [19]. Although our study implies an association between PTEN/AR coexpression and responsiveness to AAT, our data are observational and do not allow any mechanistic interpretation of the PTEN and AR protein distribution.
Despite a considerable number of publications assessing the ability of biomarkers to predict PC relapse, clinicians still rely on conventional prognostic indicators in routine decision making. In breast cancer, immunohistochemical assessment of estrogen, progesterone and HER2 receptors is part of the routine histopathologic workup.
In this pilot report, we have demonstrated a correlation between coexpression of PTEN and AR and the response to hormonal treatment and survival in ADPC. Reduced expression of either marker was associated with the development AI and reduced survival in patients whose tumors remained AD. Our data suggest that assessment of PTEN/AR coexpression might prove useful in distinguishing PCs with a more favorable prognosis from those with a high likelihood of developing recurrence and AI disease. Our data also suggest that restoration of PTEN activity or function could be used jointly with AAT to maintain androgen responsiveness and possibly delay the emergence of AIPC.
Further investigation on a larger patient cohort should be undertaken to validate our initial results to assess reproducibility and usefulness of this potential new predictive indicator.
Abbreviations
- AAT
androgen ablation therapy
- AD
androgen dependence/dependent
- AI
androgen independence/independent
- AR
androgen receptor
- BPH
benign prostatic hyperplasia
- IHC
immunohistochemistry
- LI
labeling index
- PC
prostate cancer
References
- 1.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Auclerc G, Antoine EC, Cajfinger F, Brunet-Pommeyrol A, Agazia C, Khayat D. Management of advanced prostate cancer. Oncologist. 2000;5:36–44. doi: 10.1634/theoncologist.5-1-36. [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, DeWeese TL, Platz EA, Meeker AK, Nakayama M, Epstein JI, Isaacs WB, Nelson WG. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–477. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 4.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 5.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 6.El Sheikh SS, Domin J, Abel P, Stamp G, Lalani E. Androgen-independent prostate cancer: potential role of androgen and ErbB receptor signal transduction crosstalk. Neoplasia. 2003;5:99–109. doi: 10.1016/s1476-5586(03)80001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nan B, Snabboon T, Unni E, Yuan J, Whang YE, Marcelli M. The PTEN tumor suppressor is a negative modulator of androgen receptor transcriptional activity. J Mol Endocrinol. 2003;31:169–183. doi: 10.1677/jme.0.0310169. [DOI] [PubMed] [Google Scholar]
- 8.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 9.Lin HK, Hu YC, Yang L, Altuwaijri S, Chen YT, Kang HY, Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Conaway M, Gioeli D, Weber MJ, Theodorescu D. Conditional expression of PTEN alters the androgen responsiveness of prostate cancer cells. Prostate. 2006;66:1114–1123. doi: 10.1002/pros.20447. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981;75:816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- 12.Torres J, Navarro S, Rogla I, Ripoll F, Lluch A, Garcia-Conde J, Llombart-Bosch A, Cervera J, Pulido R. Heterogeneous lack of expression of the tumour suppressor PTEN protein in human neoplastic tissues. Eur J Cancer. 2001;37:114–121. doi: 10.1016/s0959-8049(00)00366-x. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz M, Grignard G, Margue C, Dippel W, Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R, Kieffer N. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer. 2007;120:1284–1292. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 14.Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24:114–118. doi: 10.1002/pros.2990240303. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Nicosia SV, Bai W. Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J Biol Chem. 2001;276:20444–20450. doi: 10.1074/jbc.M010226200. [DOI] [PubMed] [Google Scholar]
- 16.Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol. 2004;18:2409–2423. doi: 10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]
- 17.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23:104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]