Abstract
The role of tumour suppressor genes in the development of human cancers has been studied extensively. In viral carcinogenesis, the inactivation of suppressor proteins such as retinoblastoma (pRb) and p53, and cellular oncogenes overexpression, such as c-myc, has been the subject of a number of investigations. In uterine-cervix carcinomas, where high-risk human papillomavirus (HPV) plays an important role, pRb and p53 are inactivated by E7 and E6 viral oncoproteins, respectively. However, little is known about the in situ expression of some of these proteins in pre-malignant and malignant cervical tissues. On the other hand, it has also been demonstrated that c-myc is involved in cervical carcinogenesis, and that pRb participates in the control of c-myc gene expression. By using immunostaining techniques, we investigated pRb immunodetection pattern in normal tissues, squamous intraepithelial lesions (SILs) and invasive carcinomas from the uterine cervix. Our data show low pRb detection in both normal cervical tissue and invasive lesions, but a higher expression in SILs. C-Myc protein was observed in most of the cellular nuclei of the invasive lesions, while in SILs was low. These findings indicate a heterogeneous pRb immunostaining during the different stages of cervical carcinogenesis, and suggest that this staining pattern could be a common feature implicated in the pathogenesis of uterine-cervix carcinoma.
Keywords: retinoblastoma, uterine-cervix, neoplasia, invasive, HPV
Introduction
The retinoblastoma tumour suppressor gene (rb) encodes a nuclear phosphoprotein, termed p105Rb or pRb, which has been found, mutated or deleted in several types of human malignant tumours (Friend et al. 1987; Lee et al. 1987; Harbour et al. 1988; Varley et al. 1989; Horowitz et al. 1990; Furukawa et al. 1991). Introduction of pRb into tumour cells lacking the rb gene in vitro stops cell proliferation (Uzvolgyi et al. 1991; Huang et al. 1988), suggesting that pRb acts in normal cells to constrain growth and its loss in cancer cells allows for uncontrolled proliferation.
In epithelial cultured cells, hypophosphorylated forms of pRb predominate in G1 phase of the cell cycle (Hu et al. 1992; DiCaprio et al. 1992). As cells enter S phase, phosphorylated forms become more abundant and persist through S, G2 and M phases. When cells enter anaphase, pRb dephosphorylation begins, reaching its lowest phosphorylation level in G1, at which time a block in the progression of the cell cycle from G1 to S phase is evident (Ludlow et al. 1993). Dephosphorylated pRb appears to regulate cycle progression by inactivating several growth-promoting proteins, such as E2F-1 and c-Myc transcription factors (Shirodkar et al. 1992; Rustgi et al. 1992) as well as certain G1 phase cyclins (Resnitzky et al. 1992). When viral oncoproteins bind to dephosphorylated forms of pRb (Whyte et al. 1988; Dyson et al. 1989), they release cell proliferation factors (Chen et al. 1992).
Epidemiological and experimental studies have implicated high-risk human papillomavirus (HPV) genotypes 16 and 18 in the development of uterine-cervix carcinoma (Pfister 1987; zur Hausen 1991). The E6 and E7 oncoproteins encoded by HPV bind to p53 and pRb, respectively. E7 interacts with the dephosphorylated form of pRb (Dyson et al. 1989), thereby releasing growth-promoting factors such as E2F-1, cyclins, Cdks and probably Myc (Chen et al. 1992). It has also been proposed that HPV-16/E7 induces the degradation of pRb through the ubiquitin pathway (Boyer et al. 1996). This mechanism appears to be crucial for the development of cervical carcinoma in infected patients with oncogenic HPV strains. Even in the presence of HPV infection, other cellular oncogene alterations (Ocadiz et al. 1996; Gariglio et al. 1987) and overexpression (Gariglio et al. 1993) could also represent important steps in cervical carcinogenesis.
Loss of pRb function in different types of tumour cells have been determined by blotting techniques such as Southern, Northern or Western Blots (Friend et al. 1987; Lee et al. 1987; Harbour et al. 1988; Varley et al. 1989; Horowitz et al. 1990; Furukawa et al. 1991). These methods, however, require the use of tissue extracts from tumour biopsies that include not only malignant cells but various proportions of normal cells such as fibroblasts, inflammatory cells and endothelial cells (Xu et al. 1991). Immunohistochemical techniques allow the detection of pRb expression in individual cells and specific cell populations in tissue sections (Benedict et al. 1990; Geradts et al. 1994). A low frequency of rb gene alterations has been found in cervical carcinoma by Southern blot (Choo & Chong 1993; Wrede et al. 1991) and so far few reports have been focused on the cell per cell immunohistochemical expression and localization of pRb in normal cervical epithelium (Cordon-Cardo & Richon 1994; Amortegui et al. 1995;Sano et al. 1998).
The main goal of this study was to make a description of retinoblastoma protein distribution in normal, squamous intraepithelial lesions (SILs) and invasive cervical cancer tissues harbouring high-risk HPV. We also analysed a possible correlation between the presence and location of this protein to prognosis. Our results show different intensity levels of pRb in SILs and invasive carcinomas. A low concentration of pRb was observed in normal uterine-cervix and invasive tumours. We also analysed c-Myc protein presence in cervical tissue, because it has been demonstrated that pRb is involved in c-myc oncogene expression (Hamel et al. 1992; Salcedo et al. 1995). We found that pRb-positive and pRb-negative malignant cells showed low and high positive reaction for c-Myc protein, respectively, supporting the idea that functional pRb, associated with E2F-1, inhibits c-myc gene expression (Hamel et al. 1992; Salcedo et al. 1995). The heterogeneous pRb detection found could reflect the great clonal diversity of cells present during cervical carcinogenesis.
Materials and methods
Patients and tissues
We studied biopsies from 21 patients admitted to the Gynecology and Obstetrics Hospital #4-IMSS and Oncology Unit, Mexico General Hospital, with squamous cell carcinomas, clinical stages I, II, III and IV (Table 1) and biopsies from 20 squamous intraepithelial lesions (SIL) (13 low grade and 7 high grade) from patients who were treated at the Clinic of Dysplasias of the National Cancer Institute (Ministry of Health, Mexico). All samples were high-risk HPV positive. Fifteen normal cervical fresh tissues from autopsies or surgical specimens with uterine myomatosis of individuals with normal cervical cytology completed the samples. All tissues were fixed in methacarnoy fixative, dehydrated, paraffin-embedded, cut at 5-µm thick sections and mounted in poly d-lysine coated slides.
Table 1.
Immunoreactivity for Prb in different uterine-cervix tissues
| NORMAL* | HPV | SIL** | TISSUE HPV | INVASIVE*** | HPV | G |
|---|---|---|---|---|---|---|
| 1. (1) | 16 | 11.l (0) | 16 | 31.I (0) | 16/18 | P |
| 2. (0) | 16/18 | 12.l (2) | 16 | 32. (1) | 16 | M |
| 3. (0) | (−) | 13.l (2) | 16 | 33. (0) | 16 | M |
| 4. (1) | 16 | 14.l (1) | 16/18 | 34.II (2) | 16 | P |
| 5. (1) | (−) | 15.l (0) | 16/18 | 35. (0) | 18 | M |
| 6. (1) | (−) | 16.l (2) | 18 | 36. (2) | 16 | M |
| 7. (1) | (−) | 17.l (2) | 16/18 | 37. (2) | 18 | P |
| 8. (0) | 18 | 18.l (1) | 16 | 38. (0) | 31 | M |
| 9. (0) | (−) | 19.l (2) | 16 | 39. (0) | 16 | M |
| 10. (0) | 18 | 20.l (2) | 16 | 40. (0) | 16 | M |
| 11. (0) | (−) | 21.l (2) | 16 | 41. (0) | 33 | M |
| 12. (1) | (−) | 22.l (2) | 18 | 42. (0) | 16 | M |
| 13. (1) | (−) | 23.l (2) | 18 | 43. (2) | 16 | M |
| 14. (0) | (−) | 24.h (0) | 16 | 44. (0) | 16 | M |
| 15. (1) | (−) | 25.h (2) | 16 | 45. (2) | 16 | M |
| 26.h (2) | 16 | 46. (1) | 18 | M | ||
| 27.h (2) | 16 | 47.III (1) | 16 | M | ||
| 28.h (2) | 16 | 48. (0) | 16 | M | ||
| 29.h (2) | 16 | 49. (2) | 18 | M | ||
| 30.h (2) | 18 | 50.IV (0) | 16 | M | ||
| 51. (2) | 18 | P |
0 = less than 10%; 1 = 10-50% and 2=more than 50% positive cells/tissue sample. The indicated value represents the most frequent finding among the positive cell population. I-IV: Carcinoma clinical stage. SIL: squamous intraepithelial lesion, l: low grade and h: high grade. HPV: HPV (−): HPV negative.
G: differentiation grade; P (poorly), M (moderately) or W (well) differentiated squamous carcinoma. Mean age, year (range):
44(35–53)
51(24–78)
48(26–71).
Immunohistochemistry technique
After deparaffinization, tissue sections were preincubated in 3% hydrogen peroxide for 10 min, washed in running water and covered with 10% nonimmune-blocking swine serum for 30 min at room temperature. Tissues were then incubated with the primary antibody for 18 h at room temperature in a moist chamber. The primary antibodies used in this study were rabbit polyclonal antipRb C-15 (Santa Cruz Inc., CA, USA), which was raised against a 15 aminoacid peptide (aa. 914–928) of the carboxy terminal end domain of the human Rb protein (1 : 200) and recognizes both the unphosphorylated and phosphorylated forms of pRb, and the monoclonal antibody anti-Myc 1 (Oncogene Sciences Diagnostic, Uniondale, NY, USA) which recognizes a carboxy terminal peptide (aa. 408–439) of the Myc protein (1 : 100). Subsequently, the slides were washed with PBS buffer and treated according to the instructions of the DAKO PAP kit (DAKO Co., Carpinteria, CA, USA). Briefly, the slides were incubated with the secondary IgG antibody (swine antirabbit 1 : 100 for pRb or goat antimouse 1 : 100 for Myc) for 30 min, washed in PBS, incubated with specific peroxidase antiperoxidase complexes (rabbit or mouse, respectively) for 30 min, washed again in PBS and treated with 0.06% Diaminobenzidine (DAB) in 3% hydrogen peroxide colour developer solution. The reaction was stopped by washing with distilled water and tissue sections were counterstained with haematoxylin. Specific staining for pRb or c-Myc was indicated by a brown colour.
Controls
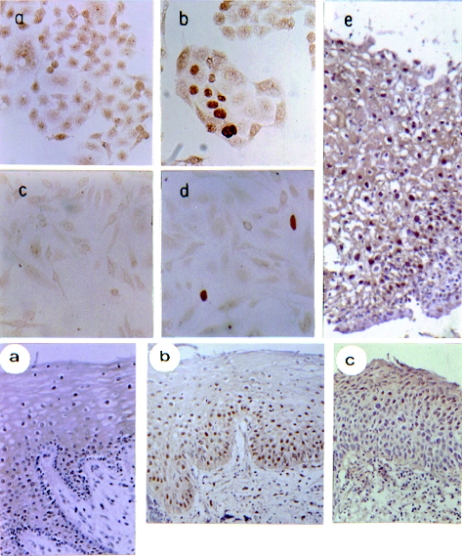
Immunohistochemical controls were produced by substitution of the primary antibody with an irrelevant rabbit IgG polyclonal antibody. Other controls used for specificity of the pRb reaction were as follows: (1) the rb−/−C33A cervical carcinoma cell line expressing a mutated protein showed low staining (Scheffner et al. 1991; Fig. 1a, Panel A). After transfection with rb cDNA this cell line gave a strong signal in some nuclei (Fig. 1b, Panel A). (2) Saos-2 cell line lacking pRb due to a homozygous rb deletion (Shew et al. 1990; Fig. 1c, Panel A) and then transfected with rb cDNA presented a strong signal in some nuclei (Fig. 1d, Panel A).
Figure 1.
Immunohistochemical detection of pRb in normal human cervical tissues and squamous intraepithelial lesions. Immunohistochemical analysis of the rb gene product was performed employing Polyclonal antipRb C-15 (see Materials and methods). Paraffin-embedded tissue sections were used to detect the pRb protein by PAP technique; DAB was used as chromogen and samples were haematoxylin counterstained. Panel A. As control, pRb immunostaining of C33-A (a, b) or Saos-2 (c, d) cell lines were employed; (a) and (c) non transfected C33-A and Saos-2 cell lines; (b) and (d) rb transfected cells, respectively, X400; (e) low grade squamous intraephitelial lesions (SIL) Ki-67 immunostained, X200. The cells and tissues were haematoxylin counterstained. Panel B, normal tissue: (a) section showing a high percentage of cells with nuclear pRb staining, SIL: (b) nuclear staining predominantly in the lower (basal layer) and medium (suprabasal) part of the epithelium from low grade SIL (c) majority of cells presenting pRb staining in the upper third part of the epithelium from a high grade SIL. All haematoxylin counterstained specimens (X200 amplification) were orientated in such a way that the top represents the differentiated cell layer and the bottom the nondifferentiated basal cell layers.
The monoclonal antibody Ki-67 (1 : 50; DAKO Co.) was used as a marker of cellular proliferation (Fig. 1e, Panel A). This antibody reacts with cells at all stages of the cell cycle, but not with cells in G0 phase. The reaction with this antibody serves as an indicator of the growth fraction; that is the number of cells undergoing active division.
Interpretation and quantification
Cells were noted as positive for pRb when they showed nuclear immunoreactivity (brown precipitate). Cytoplasmic staining was disregarded. Only the neoplastic region of each tissue section was evaluated. Two slides were analysed for each sample. To assess the stained nuclei, the slides were reviewed at ×40 magnification. The percentage of positive cells in each tissue section was estimated on a semi-quantitative scale where O: less than 10%, 1: 10–50% and 2: more than 50% of the total tumoural area. After the examination of the slides by three independent observers, a global agreement was reach regarding the results.
Invasive lesions were classified as poorly (P), moderately (M) or well (W) differentiated squamous cell carcinomas using standard histopathological criteria. Mitotic figures were counted in 10 medium power fields (MPF; ×200 magnification) in each section.
HPV typing
Paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated through graded ethanols (100%, 90%, 70% and 30%) to distilled water, and were scraped into Eppendorff tubes. Specimens were treated with 100 µL Proteinase K (100 µg/mL), 10 mm Tris-HCl pH 7.2 for 2 h at 50 °C, then boiled for 5 min, cooled and centrifuged. Supernatants (20 µL) were directly used for PCR reactions employing the L1C1/L1C2 oligonucleotides according to Yoshikawa et al. (1991a). One fourth of the PCR product was analysed for restriction fragment length polymorphism among HPVs (Yoshikawa et al. 1991a). In order to avoid cross-contamination the detection of HPV, DNA sequences were performed in two other laboratories in a blind study achieving similar results (Dr Berumen, Military Medical School, Mexico City and Dr Cossette Wheeler, NIH, USA). As negative controls we always used no DNA or DNA from C33A cells (cervical cells that do not contain HPV DNA), as well as DNA from lymphocytes obtained from healthy individuals.
Results
HPV detection
HPV-16 was the most common type found even in normal cervical tissue, 13.3%, SILs, 65%, and invasive lesions, 62%. HPV-18 was found in 13.3% of normal tissue, 20% SILs, and 23% invasive samples. Co-infection of HPV-16/18 was detected in 6.66% of normal tissue, 15% in SILs, and 4% in invasive specimens. One carcinoma had HPV-31 and another the 33 type (see Table 1).
pRb in normal uterine-cervix
Normal cervical squamous epithelium showed pRb nuclear immunoreactivity in parabasal cell layers. Cells in the upper (mature) and lower (basal) layers of the epithelium displayed low to undetectable levels of pRb, whereas cells in the middle layers showed strong nuclear staining for this suppressor protein (Fig. 1a, Panel B). Nuclear staining was detected either in HPV-16 positive or HPV negative normal cervices. C-Myc immunostaining was seen mainly in the nuclei of the highly proliferative basal cell layer of the normal epithelium (Hanson et al. 1994) (not shown). Proliferative activity, measured by Ki-67 antigen staining, was observed approximately in 50% of the epithelial cells from basal and parabasal layers (data not shown).
pRb expression in squamous intraepithelial lesions
All the pre-cancerous cervical lesions (SIL) showed numerous pRb positive cells (Fig. 1c,d, Panel B; see Table 1). In most SILs, pRb staining was seen in the basal and parabasal layer of the epithelium. The percentage of pRb positive cells was higher in SIL than in normal cervical epithelium (compare Fig. 1a,b, Panel B). Three SIL samples showed an extremely low pRb reaction (less than 10% of positive cells); two samples were given a value of 1 (10–50% of positive cells) and 15 samples showed more than 50% of positive cells. As can be observed in Fig. 1(b), Panel B, basal cells present variable pRb concentration, but frequently higher than the low level observed in the normal epithelium basal layer (Fig. 1a,b, Panel B). For c-Myc protein, nuclear immunoreaction was observed in most of the dysplastic epithelial cells present in SIL. However, in some serial sections differences between c-Myc and pRb expression and location were observed (data not shown).
pRb expression in invasive cervical carcinoma
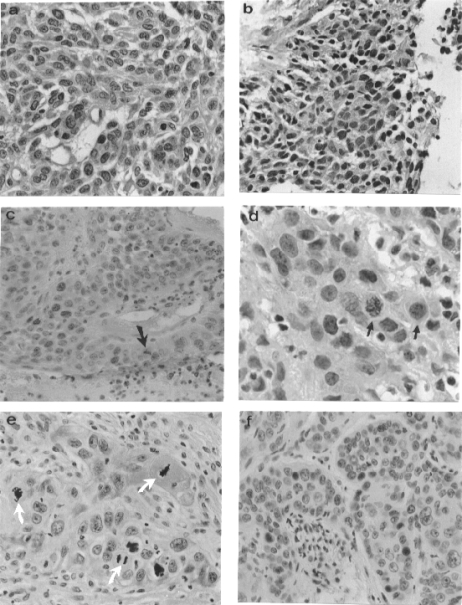
In general, the intensity of reaction and the number of pRb positive cells in invasive uterine-cervix lesions were significantly lower than in SIL specimens but similar to normal samples. However, most of the positive invasive samples were classified as 2 (more than 50% of positive cells). Eleven invasive samples showed the lowest pRb immunoreactivity (see Table 1). A relation between pRb expression and clinical stage was not observed. The positive pRb immunostaining was quite heterogeneous; some samples showed negative pRb reaction in the majority of cells but slightly positive reaction in the cytoplasm (Fig. 2a), other samples contained intense nuclear staining (Fig. 2b,c) and few specimens showed nuclear and cytoplasmic immunostaining (Fig. 2d). In some specimens a weak nuclear immunoreaction was observed (Fig. 2e; indicated as 0 in Table 1). In a few samples, some positive cells formed clusters of variable size or were scattered throughout the section (Fig. 2f). Mitotic figures were found in 7/21 samples (Fig. 2c–e); among these, some presented frequent mitoses (20–30 mitotic figures per 10 LPF), and colleagues showed sporadic mitoses (2–5 mitotic figures detected also per 10 LPF). Approximately 10–20% of mitoses showed pRb immunoreactivity while the remaining were negative (Fig. 2e, see arrows). Most squamous carcinomas (Chen et al. 1995; Boyer et al. 1996) were classified as moderately differentiated (M), the remaining carcinomas were poorly differentiated (P). We observed in most of the poorly differentiated carcinomas a strong nuclear pRb immunoreaction, furthermore no relation between moderately differentiated carcinomas and pRb expression was observed.
Figure 2.
Immunodetection of pRb in invasive cervical carcinomas. Clinical stage I: (a) cytoplasmic pRb staining with a few positive nuclei, X400; (b) strong nuclear pRb immunoreaction, X400. Clinical stage II: (c) mostly nuclear pRb staining, X400; the arrow indicates a pRb positive staining mitosis; (d) nuclear staining, X800; the arrows show positive mitoses; (e) mitoses showing negative pRb staining (white arrows) in a cervical carcinoma presenting low pRb concentration, X400; (f) high intensity of pRb immunostaining in scattered nuclei, X400. All samples were haematoxylin counterstained.
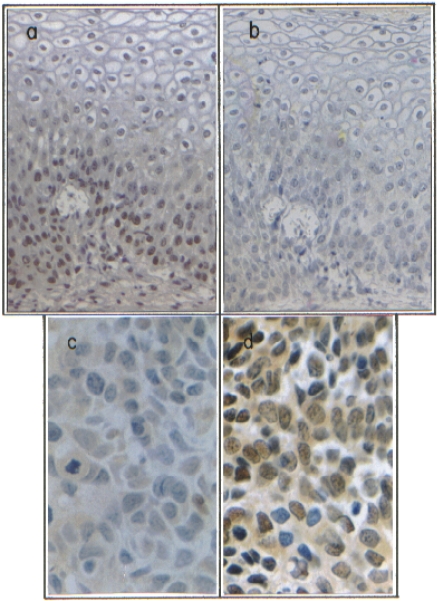
In order to compare the presence of a protein that promotes cellular growth (Myc) with pRb, the pRb and Myc proteins were evaluated employing serial tissue sections from SILs (Fig. 3a,b) and invasive tumours (Fig. 3c,d).
Figure 3.
Differential immunodetection of pRb and c -Myc in human cervical specimens.Immunohistochemical analysis of pRb in a SIL, X160 (a) or in an invasive cervical carcinoma clinical stage II, X800 (c), showing different immunoreactivity. Immunodetection of Myc in SIL, X160 (b) and invasive cervical carcinoma, X800 (d), showing strong variations of Myc protein concentration. Brownish colour shows a positive immunoreaction. Tissue sections were haematoxylin counterstained.
As shown in Fig. 3, strong variations of immunoreaction intensity to c-Myc could be observed both in SIL (Fig. 3b) and in cervical carcinoma (Fig. 3d). Different immunostaining patterns were observed in the SIL and invasive samples; pRb negative/Myc slightly positive cells; pRb positive/Myc extremely low positive cells and pRb positive/Myc positive cells. This differential staining pattern of these proteins was confirmed employing double immunostaining technique in the samples (not shown). In general, proliferative activity was markedly increased in most tumour samples showing strong reactivity (70–90% of the cells), in contrast to SIL and normal tissues 50–70% and 40–50% of the cells, respectively (data not shown).
Discussion
Several studies in different normal or cancerous tissues, employing both protein and mRNA analysis, have indicated that rb gene is ubiquitously expressed (Friend et al. 1987; Harbour et al. 1988; Varley et al. 1989; Horowitz et al. 1990; Furukawa et al. 1991; Uzvolgyi et al. 1991; Cordon-Cardo & Richon 1994). However, these studies were unable to distinguish between different cell types within the tissues analysed. A few immunohistochemical studies on pRb in human cancer (Cance et al. 1990; Cordon-Cardo et al. 1992; Benedict et al. 1990), and in normal tissues, including the uterine-cervix, have been published, showing that pRb is expressed in mature and differentiated cells, in the basal third epithelium in 90% of normal/reactive atypia or in the scattered nuclei of normal cells in all cases investigated, respectively (Cordon-Cardo & Richon 1994; Amortehio et al. 1995; Sano et al. 1998). In the present work the percentage of pRb positive cells and cellular location in normal, SIL and invasive human uterine-cervix specimens was determined by immunohistochemical assays. As was previously found (Cordon-Cardo & Richon 1994; Sano et al. 1998), we observed that the majority of cells in the proliferating basal layer of the normal epithelium displayed low levels of pRb, whereas cells in the maturing suprabasal layers presented nuclear pRb staining, suggesting that pRb may inhibit proliferation of mature differentiating epithelial cells, rather than controlling cell-cycle progression of their dividing progenitors. In addition to previous reports (normal pRb expression in cervical carcinomas; Amortehio et al. 1995; Sano et al. 1998; Parker et al. 1997), we observed that pRb staining in invasive cervical lesions is frequently lower than in SIL, suggesting that rb gene downregulation could be involved in cervical carcinogenesis (Ludlow et al. 1993; Boyer et al. 1996; invasive carcinomas contained less than 10% of positive cells). Low expression of rb may also be related to pRb inactivation resulting from complex formation with high risk HPV E7 oncoprotein and its degradation (Whyte et al. 1988; Dyson et al. 1989; Boyer et al. 1996), downregulation mechanisms or rb gene mutations (deletions or point mutations). It is also possible that variable levels of E7 oncoprotein expression from cell to cell might contribute to the heterogeneity. Different types of human cancers have been associated with altered pRb expression patterns (Furukawa et al. 1991; Cance et al. 1990; Cordon-Cardo et al. 1992). Invasive cancers from early clinical stages frequently express high pRb levels (Varley et al. 1989; Xu et al. 1991a; Cordon-Cardo et al. 1992; Xu et al. 1991b; Yoshikawa et al. 1991b). However, late clinical stages of invasive cancers express low to undetectable levels of pRb (Cance et al. 1990; Xu et al. 1991a; Cordon-Cardo et al. 1992). Those authors have suggested that pRb negative tumours might be clinically more aggressive and with a poorer prognosis than those tumours containing a variable pRb expression (Harbour et al. 1988; Hanson et al. 1994; Benedict et al. 1990). Supporting this possibility, it has also been reported that rb gene expression inhibits tumour cell invasion in vitro (Lij et al. 1996). In this scenario, we will expect similar results, that is that patients with invasive cervical tumours showing low levels of pRb will present a lower life expectancy than those with higher levels of this suppressor protein; however, we found that some patients with a very low number of pRb positive cells with low pRb levels are still alive. On the other hand, patients with high (cases 49 and 51) or low (cases 48 and 50) percentage of pRb positive cells are dead. Thus, there must be no correlation between clinical stage or prognosis of cervical tumours and pRb detection. The number of specimens evaluated in this study is too small to allow statistically significant conclusions to be drawn.
Most cells in SILs and invasive tumours showed nuclear pRb detection but in five specimens pRb location was clearly cytoplasmic. Most published reports indicate nuclear pRb staining in a variety of tumours and normal tissues (Cordon-Cardo & Richon 1994; Cance et al. 1990; Cordon-Cardo et al. 1992; Benedict et al. 1990; Xu et al. 1991b; Zacksenhaus et al. 1993), considering cytoplasmic localization as an artifact (Benedict et al. 1990) or related to rb gene mutation (Zacksenhaus et al. 1993). Additional studies should be carried out to support these observations. In addition, some of the actively dividing cells (mitotic cells) showed cytoplasmic immunoreactivity with or without staining of the chromosomes, indicating that in the absence of nuclear envelope pRb diffuses to other cellular locations. Recently, it has been suggested that during cellular division, pRb may have a mitotic spindle function and/or represents part of a nuclear protein transport system (Thomas et al. 1996) which could partly explain positive pRb mitoses.
It has been reported that the c-Myc gene is positively regulated by the transcription factor E2F-1 (Gu et al. 1994; Amortegui et al. 1995; Dang et al. 1995; Hoang et al. 1995), suggesting that the interaction of pRb with E2F-1 downregulates c-myc expression (Hamel et al. 1992). In addition, Adnane et al. (1995) have shown that pRb negatively regulates Myc-mediated transcriptional control in vivo, through protein–protein interactions. Thus, pRb blocks both c-Myc transcription and Myc function as transcriptional factor and alterations of pRb expression or function could lead to c-Myc upregulation. We have recently determined in SILs a pRb suppressor effect on c-Myc expression in vivo (Salcedo et al. 1995). It has been extensively demonstrated the important role of c-Myc (overexpression and amplification) in the development of the uterine-cervix carcinomas (Ocadiz et al. 1987; Gariglio et al. 1987; Gariglio et al. 1993; Hidalgo et al. 2000; Solinas-Toldo et al. 1997). In the present work the results indicate that some regions in both SILs and cervical carcinoma specimens showed pRb negative/Myc positive cells or pRb positive/Myc slightly positive cells, with a few cells expressing both pRb and Myc, indicating that pRb-dependent c-Myc downregulation determined in vitro could also occur in vivo, suggesting that pRb must be functional in the SILs samples analysed. Genome instability due to low p53 levels by the continuous presence of HPV E6 oncoprotein (Rangel et al. 1994) could favour a variety of cellular gene mutations important for tumour progression and partially responsible for the great heterogeneity of pRb detection observed within and among cervical tumours. For example, Benedict et al. (1990) suggest that during tumour progression the expression pattern of a group of genes implicated in this event, specifically pRb and p53, determined by immunohistochemistry staining, could be an important key to understand the spatio-temporal participation of these genes in tumour evolution.
Due to the multistep nature of cancer, this could suggest that in pRb negative carcinomas rb gene alterations might be related to neoplastic initiation. If reactivity were heterogeneous (mixture of positive and negative cells) a progressional role would be more likely (Benedict et al. 1990). In this context, our findings in the present work could suggest that in the majority of the cervical carcinomas, low levels or absence of pRb probably due to different molecular mechanism are involved in cancer progression. In conclusion, even though changes in pRb concentration in individual cells should be observed as they undergo G0/middle G1 phases of the cycle (Xu et al. 1991b), in those cells presenting rb gene alterations or HPV infection (different E7 oncoprotein concentrations), the pRb detection could be very low to undetectable as we found in cervical carcinoma samples.
Acknowledgments
This work was supported by grants from Consejo Nacional de Ciencia y Tecnologia F383-M9304, 3385P-M9608, 4895-N (México), UNIDO (Trieste, Italy), PNUD and Aaron Saenz Association. We thank Dr D. Jenkins (University of Nottingham, UK) and Nikki Baird-Salcedo for critical review of this manuscript. We also acknowledge Ms. G. Mora, A. Marroquín, E. García, R. Ocádiz, O. López (Hospital General de Naucalpan, México) and Dr M. Bonilla (UNAM) for technical assistance. We thank Dr F. Cruz (Centro de Referencia Nacional de Displasias, Hospital General de México, S.S.) and Dr C. Aranda (Clínica de Displasia, Instituto Nacional de Perinatología, S.S.) for providing tissue samples. We would also like to thank Dr E. Garrido (CINVESTAV, México) for kindly providing the pCMV-Rb plasmid and Saos-2 cells.
REFERENCES
- Adnane J, Robbins PD. The retinoblastoma susceptibility gene product regulates myc-mediated transcription. Oncogene. 1995;10:381–387. [PubMed] [Google Scholar]
- Amortegui AJ, Meyer MP, Elborne VL, Amin RM. P53, retinoblastoma gene product, and cyclin protein expression in human papillomavirus DNA-positive cervical intraepithelial neoplasia and invasive cancer. Mod Pathol. 1995;8:907–912. [PubMed] [Google Scholar]
- Beijersbergen RL, Hijmans EM, Zhu L, Bernards R. Interaction of C-Myc with the pRb-related protein p107 results in inhibition of C-Myc-mediated transactivation. EMBO J. 1994;13:4080–4086. doi: 10.1002/j.1460-2075.1994.tb06725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict WF, Xu HJ, Hu SX, Takahashi R. The role of the retinoblastoma gene in initiation and progression of human cancer. J. Clin. Invest. 1990;85:988–993. doi: 10.1172/JCI114575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer SN, Wazer DE, Band V. E7 protein of Human Papilloma Virus-16 induces degradation of Retinoblastoma protein through the Ubiquitin-Proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- Cance WG, Brennan MF, Dudas ME, Huang CM, Cordon-Cardo C. Altered expression of the retinoblastoma gene product in human sarcomas. N. Engl. J. Med. 1990;323:1457–1462. doi: 10.1056/NEJM199011223232105. [DOI] [PubMed] [Google Scholar]
- Chen P-L, Riley DJ, Lee W-H. The retinoblastoma protein as a fundamental mediator of growth and differentiation signals. Crit. Rev. Eukaryot. Gene. Expr. 1995;5:79–95. [PubMed] [Google Scholar]
- Choo KB, Chong KY. Abscence of mutation in the p53 and the retinoblastoma susceptibility genes in primary cervical carcinoma. Virol. 1993;193:1042–1046. doi: 10.1006/viro.1993.1224. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, Wartinger D, Petrylak D, et al. Altered expression of the retinoblastoma gene product is prognostic indicator in bladder cancer. J. Natl. Cancer Inst. 1992;84:1251–1256. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, Richon VM. Expression of the retinoblastoma protein is regulated in normal human tissues. Am. J. Pathol. 1994;144:500–510. [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Lee LA. C-Myc function in neoplasia. Medical Intelligence Unit. Georgetown, TX: R.G. Landes Company; 1995. Structure of the c-myc gene and its transcription; pp. 73–84. [Google Scholar]
- DeCaprio JA, Furukawa Y, Ajechenbaum F, Griffin JD, Livingston D. The retinoblastoma-susceptibility gene product becomes phosphorylated in multiple stages during cell cycle entry and progression. Proc. Natl. Acad. Sci. USA. 1992;89:1795–1798. doi: 10.1073/pnas.89.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N, Howley PM, Münger K, Harlow E. The human papillomavirus 16, E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Friend SH, Horowitz JM, Gerber MR, et al. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc. Natl. Acad. Sci. USA. 1987;84:9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, DeCaprio JA, Belvin M, Griffin JD. Heterogeneous expression of the product of the RB gene in primary human leukemia cells. Oncogene. 1991;6:1343–1346. [PubMed] [Google Scholar]
- Gariglio P, Ocadiz R, Sauceda R. Human papillomavirus DNA sequences and C-myc oncogene alterations in uterine cervix carcinoma. Cancer Cells, Papillomavirus, CSH. 1987;5:343–348. [PubMed] [Google Scholar]
- Gariglio P, Salcedo M. Molecular genetics of uterine cervix carcinoma. Involvement of C-myc oncogene. In: Santoscoy G, editor. XVII World Congress of Anatomic and Clin. Pathol. Bologna, Italy: Monduzzi Editoire; 1993. pp. 295–300. [Google Scholar]
- Geradts J, Hu SX, Lincoln CE, Benedict WF, Xu HJ. Aberrant RB gene expression in routinely processed, archival tumor tissues determined by three different anti-RB antibodies. Int. J. Cancer. 1994;58:161–167. doi: 10.1002/ijc.2910580203. [DOI] [PubMed] [Google Scholar]
- Gu W, Bhatia K, Magrath IT, Dang CV, Dalla-Favera R. Binding and suppression of the Myc transcriptional activation domain by p107. Science. 1994;264:251–254. doi: 10.1126/science.8146655. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Lai SL, Whang PJ, Gazdar AF, Minna JD, Kaye FJ. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988;241:353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel P, Gill R, Phillips R, Gallie B. Transcriptional repression of the E2F-containing promoters E1aE, C-myc, and RB1 by the product of the RB1 gene. Mol Cell Biol. 1992;12:3431–3438. doi: 10.1128/mcb.12.8.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KD, Shichiri M, Follansbee MR, Sedivy JM. Effect of C-myc expression on cell cycle progression. Mol Cell Biol. 1994;14:5748–5755. doi: 10.1128/mcb.14.9.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A, Schewe C, Petersen S, et al. Human papillomavirus status and chromosomal imbalances in primary cervical carcinomas and tumour cell lines. Eur J. Cancer. 2000;36:542–548. doi: 10.1016/s0959-8049(99)00323-8. [DOI] [PubMed] [Google Scholar]
- Hoang AT, Lutterback B, Lewis BC, et al. A link between increased transforming activity of lymphoma-derived MYC mutant alleles, their defective regulation by p107, and altered phosphorylation of the C-Myc transactivation domain. Mol Cell Biol. 1995;15:4031–4042. doi: 10.1128/mcb.15.8.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JM, Park SH, Bogenmann E, et al. Frequent inactivation of the retinoblastoma antioncogene is restricted to a subset of human tumor cells. Proc. Natl. Acad. Sci. USA. 1990;87:2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Yee JK, Shew JY, et al. Suppression of the neoplastic phenotype by replacement of the Rb gene in human cancer cells. Science. 1988;242:1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Hu Q, Lees JA, Buchkovich KJ, Harlow E. The retinoblastoma protein physically associates with the human cdc2 kinase. Mol Cell Biol. 1992;12:971–980. doi: 10.1128/mcb.12.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins LA. The biology of human papillomaviruses: from warts to cancer. Infect. Agents Dis. 1993;2:74–86. [PubMed] [Google Scholar]
- Lee WH, Shew JY, Hong FD, et al. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987;329:642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lij Hu SX, Perng GS, Zhou Y, et al. Expression of the retinoblastoma (RB) tumor suppressor gene inhibits tumor cell invasion in vitro. Oncogene. 1996;13:2397–2405. [PubMed] [Google Scholar]
- Ludlow JW, Glendening CL, Livingston DM, DeCaprio JA. Specific enzymatic dephosphorylation of the retinoblastoma protein. Mol Cell Biol. 1993;13:367–372. doi: 10.1128/mcb.13.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocadiz R, Sauceda R, Cruz M, Graef A, Gariglio P. High corrrelation between molecular alterations of the C-myc oncogene and uterine cervix carcinoma. Cancer Res. 1987;47:4173–4177. [PubMed] [Google Scholar]
- Parker MF, Arroyo GF, Geradts JB, et al. Molecular characterization of adenocarcinoma of the cervix. Gynecol. Oncol. 1997;64:242–251. doi: 10.1006/gyno.1996.4580. [DOI] [PubMed] [Google Scholar]
- Pfister H. Human papillomavirus and genital cancer. Adv. Cancer Res. 1987;48:113–147. doi: 10.1016/s0065-230x(08)60691-0. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Ramirez M, Torroella M, Pedroza A, Ibarra V, Gariglio P. Multistep carcinogenesis and genital papillomavirus infection. Implications for diagnosis and vaccines. Arch. Med. Res. 1994;25:265–272. [PubMed] [Google Scholar]
- Resnitzky D, Reed S. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol. Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustgi AK, Dyson NJ, Bernards R. Amino-terminal domains of C-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature. 1992;353:541–544. doi: 10.1038/352541a0. [DOI] [PubMed] [Google Scholar]
- Salcedo M, Garrido E, Taja L, Gariglio P. The retinoblastoma gene product negatively regulates cellular or viral oncogene promoters in vivo. Arch. Med. Res. 1995;26:S157–S162. [PubMed] [Google Scholar]
- Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Immunohistochemical overexpression of p16 protein associated with intact retinoblastoma protein expression in cervical cancer and cervical intraepithelial neoplasia. Pathol. Int. 1998;48:580–585. doi: 10.1111/j.1440-1827.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Munger K, Byrne JC, Howley P. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew JY, Lin BT, Chen PL, Tseng BY, Yang-Feng TL, Lee WH. C-terminal truncation of the retinoblastoma gene product leads to functional inactivation. Proc. Natl. Acad. Sci., USA. 1990;87:6–10. doi: 10.1073/pnas.87.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar S, Ewen M, DeCaprio JA, Morgan J, Livingston DM, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Solinas-Toldo S, Dürst M, Lichter P. Specific chromosomal imbalances in human papillomavirus-transfected cells during progression toward immortality. Proc. Natl. Acad. Sci. USA. 1997;94:3854–3859. doi: 10.1073/pnas.94.8.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Edwards M, Marks R. Translocation of the retinoblastoma gene product during mitosis. Exp Cell Res. 1996;223:227–232. doi: 10.1006/excr.1996.0076. [DOI] [PubMed] [Google Scholar]
- Uzvolgyi E, Classon M, Henriksson M, et al. Reintroduction of a normal retinoblastoma gene in osteosarcoma cells inhibits the replication associated function of SV40 large T antigen. Cell Growth Differ. 1991;2:297–303. [PubMed] [Google Scholar]
- Varley JM, Armour J, Swallow JE, et al. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumors. Oncogene. 1989;4:725–729. [PubMed] [Google Scholar]
- Whyte P, Buchkovich KJ, Horowitz JM, et al. Association between an oncogene and an anti-oncogene: the adenovirus E1A protein binds to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Wrede D, Tidy JA, Crook T, Lane D, Vousden KH. Expression of RB and p53 proteins in HPV-positive and HPV-negative cervical carcinoma cell lines. Mol. Carcinog. 1991;4:171–175. doi: 10.1002/mc.2940040302. [DOI] [PubMed] [Google Scholar]
- Xu HJ, Hu SX, Cagle PT, Moore GE, Benedict WF. Abscence of retinoblastoma-protein expression in primary non-small cell lung carcinomas. Cancer Res. 1991a;51:2735–2739. [PubMed] [Google Scholar]
- Xu HJ, Hu SX, Benedict WF. Lack of nuclear RB protein staining in G0/middle G1 cells: correlation to changes in total RB protein level. Oncogene. 1991b;6:1139–1146. [PubMed] [Google Scholar]
- Yoshikawa H, Kawana T, Kitagawa K, Mizuno M, Yoshikura H, Iwamoto A. Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J. Cancer Res. 1991a;82:524–531. doi: 10.1111/j.1349-7006.1991.tb01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa J, Xu HJ, Hu SX, et al. Inactivation of the retinoblastoma gene in human bladder and renal cell carcinomas. Cancer Res. 1991b;51:5733–5743. [PubMed] [Google Scholar]
- Zacksenhaus E, Bremner R, Phillips RA, Gallie BL. A bipartite nuclear localization signal in the retinoblastoma gene product and its importance for biological activity. Mol Cell Biol. 1993;13:4588–4599. doi: 10.1128/mcb.13.8.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]