Abstract
Homozygous mice transgenic for αA-crystallin, one of the structural eye lens proteins, developed hindlimb paralysis after 8 weeks of age. To unravel the pathogenesis of this unexpected finding and the possible role of αA-crystallin in this pathological process, mice were subjected to a histopathological and immunohistochemical investigation. Immunohistochemistry showed large deposits of αA-crystallin in the astrocytes of the spinal cord, and in the Schwann cells of dorsal roots and sciatic nerves. Additionally, microscopy showed dystrophic axons in the spinal cord and digestion chambers as a sign of ongoing demyelination in dorsal roots and sciatic nerves. Apart from a few areas with slight αA-crystallin-immunopositive structures, the brain was normal. Because the αA-crystallin protein expression appeared in specific cells of the nervous system (astrocytes and Schwann cells), the most plausible explanation for the paralysis is a disturbance of cell function caused by the excessive intracytoplasmic accumulation of the αA-crystallin protein. This is followed by a sequence of secondary changes (demyelination, axonal dystrophy) and finally arthrosis. In conclusion, αA-crystallin transgenic mice develop a peripheral and central neuropathy primarily affecting spinal cord areas at the dorsal side, dorsal root and sciatic nerve.
Keywords: αA-crystallin, axonal dystrophy, demyelination, astrocyte, Schwann cell, transgenic mice
α-Crystallin, a structural eye lens protein ( Bloemendal 1982), is composed of two subunits of 20 kDa (αA and αB) which can form large aggregates of approximately 800 kDa. These aggregates form 40% of the total fibre protein in the lens. In healthy individuals the αB-subunit is not only expressed in the lens but also in heart, skeletal muscle, spleen, kidney and in nervous tissue. In the nervous system it is present in astrocytes and oligodendrocytes under normal conditions (Iwaki et al. 1990). Very little information is available about the function of αA-crystallin, which seems to be more specific for the eye lens, except for its essential role in maintaining lens transparency (Brady et al. 1997). However, extralenticular expression has been reported in the rat in significant amounts in spleen and thymus whereas low levels were found in retina, intestines, liver, kidney, adrenal, cerebellum and brainstem (Kato et al. 1991; Maeda et al. 1999).
The strong structural homology of α-crystallins with the small heat shock proteins of the hsp20–30 class (Ingolia & Craig 1982), points to a similar ‘heat-shock’ protein function for these proteins. Under pathological conditions, αB-crystallin is also abundantly present in neurones, suggesting that it may be involved in aggregation and remodelling of neurofilaments in disease (Lowe et al. 1992). This suggested function of αB-crystallin is mainly based on studies of neurodegenerative diseases like Alexander's disease, amyotrophic lateral sclerosis (ALS), Pick's disease, prion diseases and Alzheimer disease (Iwaki et al. 1989; Iwaki et al. 1992; Lowe et al. 1992; Renkawek et al. 1992; Kato et al. 1997). αB-crystallin appears to be abundantly present in the degenerating cells of many neurodegenerative diseases and seems to be a common factor in intermediate filament-containing inclusion bodies and in ballooned neurones which are also a feature of many neurodegenerative diseases (Lowe et al. 1992). However it should be noted that at least in some of the neurodegenerative diseases (Parkinson and Alzheimer) other proteins such as α-synuclein and tau, may play a more dominant role in neurodegeneration (reviewed by Goedert 1999).
Considering the strong structural resemblance of the two subunits it can be hypothesized that αA-crystallin behaves in a similar way to αB-crystallin when expressed in nonlenticular tissues. The issue of whether the αA-subunit has functions comparable with those of the αB-subunit of crystallin was addressed, and for this purpose a line of transgenic mice, generated by introducing the hamster αA-crystallin transgene driven by the vimentin promoter, was studied. Vimentin is normally involved in the cytoskeletal structuring of most eukaryotic cells and is expressed in mesenchymal tissue. In transgenic mouse models the vimentin promoter is used to obtain high expression levels in various tissues of the gene fused behind. Although initially several lines were constructed, offspring could be obtained from only one line. These homozygous αA-crystallin transgenic animals develop paralysis of the hindlimbs after two months of age.
To find the cause of this clinical alteration and to study the possible role of αA-crystallin in this pathological process, mice were killed and tissues were processed for histopathological analysis and immunohistochemistry.
Materials and methods
Animals
Mice from the FVB/N strain were obtained from The Netherlands Cancer Institute (Amsterdam, the Netherlands) and used for the generating of transgenic animals as described below. Transgenic and wildtype animals were housed in macrolon cages (3–5 animals per cage) with sterilized wood chip bedding and kept under standardized environmental conditions (room temperature of 20–22 °C, relative humidity 55% and an artificial light cycle on a 12 h light/dark base).
Generation of transgenic mice
Plasmid construction of the vector with the hamster αA-crystallin gene was performed as described earlier for a desmin transgenic mouse (Pieper et al. 1989). The αA-crystallin gene was subcloned in a pUC18 plasmid, and ligated with a 3.2 kbp vimentin promoter region. This pVimαA construct has been used for pronuclear microinjection as described previously (Krimpenfort et al. 1988). Plasmid sequences were removed by digestion (using the restriction enzymes BAM HI and Eco RI), isolated and purified. The DNA concentration was adjusted to 4 μg/ml and approximately 20 pVimαA copies were injected. Several weeks after the birth of the mice that had developed from microinjected eggs, tail DNA was isolated and analysed by Southern blotting.
SDS/PAGE and Immunoblotting
Samples for electrophoresis were prepared by homogenizing different tissues (heart, kidney, lung, liver, spleen, skeletal muscle, brain, uterus and spinal cord) in sodium-dodecylsulphate (SDS) sample buffer. Of each homogenate, 50 μg was diluted in 50 μl SDS-buffer with bromo-phenol blue and mercapto-ethanol and brought on the gel. Electrophoresis was carried out according to Laemmli (Laemmli 1970). After electrophoresis, Western blotting was performed according to Towbin and coworkers (Towbin et al. 1979). An affinity-purified anti-αA-crystallin antibody (diluted 1 : 2000) was used as first antibody, the goat-anti-rabbit alkaline phosphatase conjugate (Promega Benelux B.V., Leiden, The Netherlands) was used as detection system.
Tissue sampling
Four wildtype mice of the FVB/N strain (male and female, age 5–6 months) and six homozygous transgenic mice (male and female, age approximately 7 months) were killed by exsanguination under anaesthesia. The following tissues were sampled and processed for light-microscopy: skeletal muscle of fore-and hindlimbs, diaphragm, sternum, femur, knee joint, heart, aorta, spleen, lymph node, bone marrow, thymus, salivary gland, pancreas, liver, oesophagus, stomach, intestine, kidney, urinary bladder, testis, epididymis, prostate gland, seminal vesicle, ovary, uterus, vagina, mammary gland (+ skin), thyroid, adrenal gland, pituitary, brain (cross-sections at three different levels: 1) cerebellum (2) cerebrum at the level of the hippocampus (3) cerebrum at the level of caudate/putamen), spinal cord (cervical, thoracic and lumbar), eye with optic nerve and sciatic nerve. Tissues were fixed in 4% buffered formaldehyde (Klinipath, Duiven, The Netherlands), routinely processed through alcohol/xylene series and embedded in paraffin.
Histochemical staining
Sections of 5 μm thickness were cut, mounted on glass slides and stained with haematoxylin-eosin (HE). Some sections of spinal cord were additionally stained with periodic acid Schiff reagent (PAS; glycoprotein staining), with Congo red (amyloid staining) or with Holmes′-luxol fast blue (staining, respectively, neurites and myelin). Sections were examined with a light microscope.
Five μm thick paraffin sections from eye and optic nerve, spinal cord, brain, femoral nerve, skeletal muscle (forelimbs and hindlimbs), heart and knee (joint) of both wildtype and transgenic mice were immunostained using an antibody against αA-crystallin (a rabbit polyclonal antibody raised against bovine lens αA crystallin and affinity purified against bovine lens αA crystallin). In brief, sections were preincubated with 3.6% H2O2 in methanol (15 min) and normal swine serum in 1% bovine albumin in PBS/Tween (1 : 20, 1 h, 37 °C). Incubation with anti-αA-crystallin in 1% bovine albumin in PBS/Tween (1 : 100; 1 h, 37 °C) was followed by a HRP/streptavidin detection system (according to instructions of DAKO-kit LSAB2 for mouse and rabbit antibodies (Dako Diagnostics B.V., Glostrup, Denmark); cat. No. K0609) and 0.05% DAB-solution in PBS for 15 min at room temperature in the dark. Sections were slightly counterstained with Mayer's haematoxylin.
Sections from spinal cord, femoral nerve and brain of wildtype and transgenic mice were immunostained with an antibody to αB-crystallin (a rabbit polyclonal antibody raised against the polypeptide identical to the C-terminal 13 residues of mammalian αB-crystallins [LAP-70] and purified against LAP-70; a kind gift of Dr Joseph Horwitz, UCLA, USA) (rabbit-anticow) glial fibrillary acid protein (= GFAP; Dako code Z0334; dilution 1 : 250) (rabbit-anticow) ubiquitin (Dako code Z0458; dilution 1 : 100) and (mouse-anticalf lens) vimentin (Eurodiagnostics B.V., Arnhem, The Netherlands; code 2208 PVI; dilution 1 : 100). Skeletal muscle and spinal cord were additionally stained with antivimentin. All immunohistochemical procedures were performed identical to that described for αA-crystallin. For the antibodies against alpha A-crystallin, alpha B-crystallin, vimentin and ubiquitin the eye lens of the wildtype mouse was used as internal positive control tissue, whereas anti-GFAP was tested in the optic nerve. To exclude nonspecific staining of the primary antibodies used, spinal cord and brain sections were incubated with normal rabbit serum and the second antibody conjugate.
Electron Microscopy
Spinal cord was immediately fixed for 24 h in 2.5% glutaraldehyde with 0.1 m Na-Cacodylate. The material was postfixed in 1% osmium Na-cacodylate for one hour at room temperature, dehydrated and embedded in epon 812. Ultrathin sections were cut (80 nm) on an ultramicrotome (Reichert Jung, Vienna, Austria), collected on grids and contrasted with 3% uranyl-acetate (15 min) and lead-citrate (5 min). Sections were examined using an electron microscope (Jeol Ltd., Tokyo, Japan).
Results
SDS/PAGE and Immunoblotting
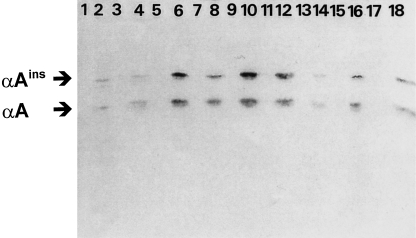
From the Western blot (Figure 1) it is clear that in all nonlenticular tissues sampled of the wildtype mice, the amount of αA-crystallin was below the detection limit (see odd lanes). However, although in different amounts, in many organs of transgenic mice (heart, kidney, lung, liver, spleen, muscle, brain, uterus and spinal cord) immunoblotting with antiαA-crystallin resulted in two bands of approximately 20.0 and 22.5 kDa (see even lanes). These bands have been described earlier as representing two different forms of αA-crystallin, the normal αA-crystallin and the elongated αA-crystallin αAins-crystallin (van Rijk et al. 1999).
Figure 1.
Immunoblot after SDS/PAGE of several tissues of wildtype (odd numbered lanes) and transgenic mice (even numbered lanes). Tissues in lanes are: 1,2: heart; 3,4: kidney; 5,6: lung; 7,8: liver; 9,10: spleen; 11,12: skeletal muscle; 13,14: brain; 15,16: uterus, and 17,18: spinal cord, with different expression levels of αA-crystallin and an elongated form αAins-crystallin of approximately 19.8 and 22.5 kDa, respectively. Detection was achieved with an affinity-purified anti-αA-crystallin antibody.
Histopathology and Electron Microscopy
Relevant findings in routine HE-stained sections of transgenic and wildtype mice are summarized per organ.
Brain
No abnormalities were observed in the brain.
Spinal cord
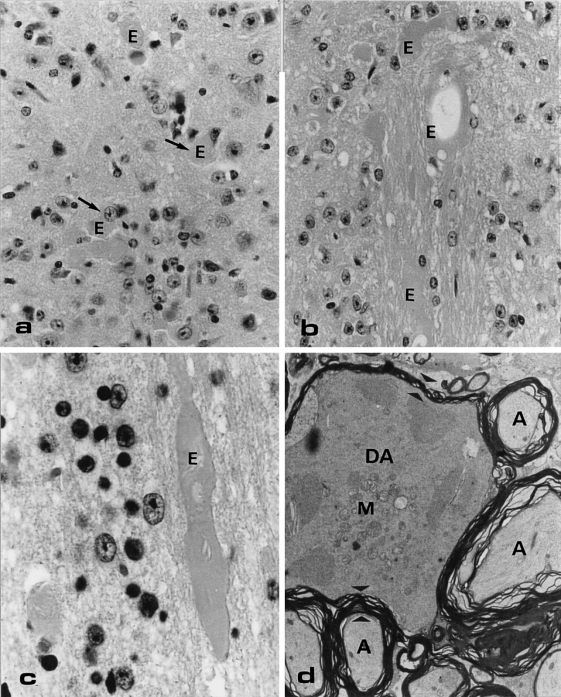
In the white and grey matter of only the transgenic mice, changes were noted (Figure 2). Many eosinophilic bodies of varying sizes were present in the grey matter of the thoracic and lumbar spinal cord as well as in the corticospinal tract and gracile fascicle (Figure 2a–c). Sometimes these bodies were in close contact with neuronal perikarya (Figure 2a), but the majority of them were found scattered in the grey and white matter especially at the dorsal side of the spinal cord. The largest eosinophilic bodies were present in the white matter of thoracic and lumbar spinal cord (Figure 2b). Some of these bodies had a torpedo-like shape (Figure 2c). The eosinophilic bodies were generally negative for PAS and Congo red, although some of the bodies showed dispersed PAS-positive granularity. The Holmes′-luxol fast blue method stained these structures black, which sometimes showed granular staining pattern. Electron microscopic examination of the spinal cord of the transgenic animals showed that the eosinophilic bodies represented swollen axons with accumulation of neurofilaments, mitochondria, dense bodies, vesicular elements, tubular structures (forming an irregular network) and thinning of the myelin sheets. In Figure 2d an example of such a large degenerating axon is given, which is surrounded by a few normal axons.
Figure 2.
Light microscopical (LM) haematoxylin-eosine (HE)-stained section (a, b, c) and electron microscopical section (d) of abnormalities at the dorsal side of a transgenic mouse lumbar spinal cord. (a) Eosinophilic bodies/axonal swelling (E) scattered throughout the grey matter. Original Magnification (OM) = × 100. (b, c) Large eosinophilic bodies in the white matter, sometimes showing a torpedo-like shape (c) OM = 100x and × 250, respectively. (d) The degenerating axon (DA) in this electron micrograph is surrounded by normal axons (A). A clear difference in size and density of the cytoplasm and in number of mitochondria (M) in the degenerating and normal axon is shown. Note also the difference in thickness of the myelin surrounding the normal and degenerating axon (electron dense layered material in between the small arrowheads). OM = × 4.500.
Spinal roots and sciatic nerve
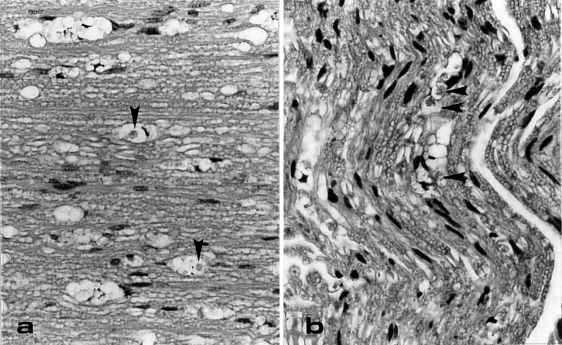
In the dorsal spinal roots and the sciatic nerves, vacuolated structures containing a small amount of debris and/or macrophages were found (Figure 3). In the Holmes′-luxol fast blue method, this debris was stained blue indicating that it was originated from myelin. In the ventral roots no abnormalities were present.
Figure 3.
Vacuolated structures in dorsal root (a) and sciatic nerve (b) of a transgenic mouse. In some of the vacuoles clear macrophages are present (arrowhead). LM-sections are HE-stained. OM = × 100.
Knee joint
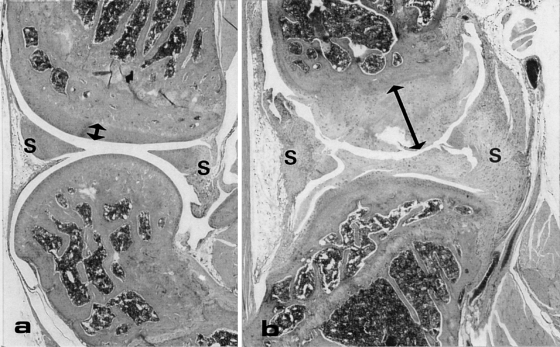
Compared to the wildtype mice (Figure 4a) the amount of joint-cartilage was significantly increased in the transgenic animals (Figure 4b). The mesenchymal cells in the synovia showed a clear hyperplasia and chondroid metaplasia.
Figure 4.
A longitudinal HE-stained sections of the knee joint of a wildtype (a) and transgenic (b) mouse. In the transgenic mouse the amount of cartilage covering the joint (arrow) is increased significantly and more synovial tissue (S) is present which consists largely of cartilage. OM = × 6.6.
Eye
As is known from the FVB/N mice, a strain-specific absence of the photoreceptor layer and outer nuclear layer in the retina was found. The other structures in the eye, with special interest in the eye lens, seemed to be normal.
Other organs/tissues
All other tissues examined, including skeletal muscle, did not reveal any abnormalities.
Immunohistochemistry
Controls
The eye lens of wildtype animals, which was used as internal control tissue, was strongly positive with the antibodies against αA-crystallin, αB-crystallin, vimentin and ubiquitin. The optic nerve showed strong immunopositive stellate cells after staining with anti-GFAP. On the negative control sections no staining was observed.
αA-crystallin
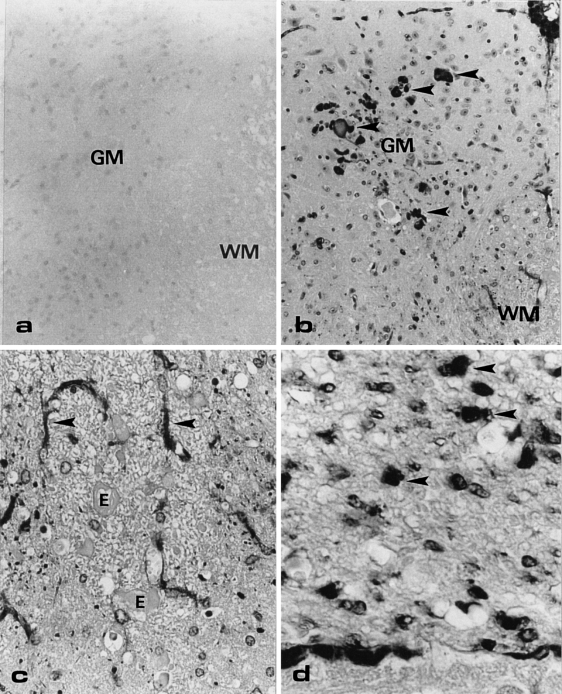
In both wildtype and transgenic mice, erythrocytes and endothelial cells were stained with the anti-αA-crystallin antibody in all tissues examined. Although wildtype mice showed immunopositive erythrocytes and endothelial cells, it should be noted that in the latter cells the staining was stronger in the transgenic mice. Incomplete blocking of the endogenous peroxidases probably causes this nonspecific staining. In the other tissues of the wildtype mice (e.g. the dorsal spinal cord in Figure 5a and sciatic nerve in Figure 6a), no staining was observed with anti-αA-crystallin.
Figure 5.
Cross-section of the lumbar dorsal spinal cord of a wildtype (a) and transgenic mouse (b, c, and d), immunostained with anti-αA-crystallin. (a) No immunostaining is observed in the wildtype mouse. OM = × 50. (b) Various sized anti-αA-positive structures are present in the grey matter of the transgenic mouse. OM = × 50. (c) In the white matter of the transgenic mouse, small dots and stellate cells (arrows) are positive with anti-αA-crystallin. The eosinophilic bodies/axonal swellings (E) are not immunopositive. OM = × 100. GM, grey matter; WM, white matter. (d) The dorsal root shows many large dot-like immunopositive structures (arrowheads). OM = × 250.
Figure 6.
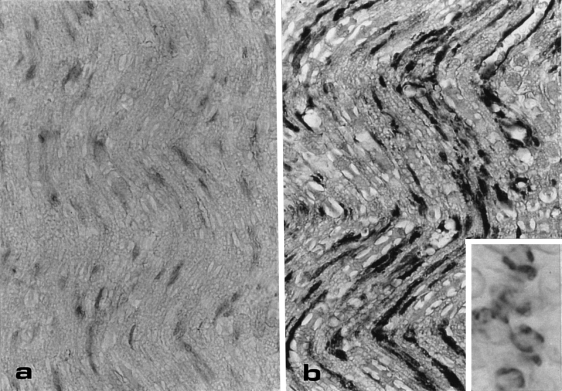
Longitudinal section of the sciatic nerve of a wildtype (a) and transgenic (b) mouse, immunostained with anti-αA-crystallin. (a) No immunostaining of nerve structures can be seen in the wildtype mouse. (b) The transgenic mouse shows a strong stripe-like staining which is running parallel to the nerve axons. OM = × 100. Inset: cross-section shows positive anti-αA-crystallin staining around the axon. OM = × 250.
Strong immunopositive staining with anti-αA-crystallin was observed in the dorsal spinal cord of the transgenic mice. The ventral side of the spinal cord was negative in these animals. At the dorsal side various sized dot-like structures were evident in the grey matter (Figure 5b) whereas stellate cells and fibre-like staining were present in the white matter (Figure 5c). The eosinophilic bodies that are prominently present in the routinely stained sections of the transgenic mice were not immunopositive for αA-crystallin. In the dorsal root also many positively stained dot-like structures were present (Figure 5d).
Longitudinal sections of the sciatic nerve of the transgenic mice showed a strong stripe-like staining (Figure 6b). Cross-sections showed that the immunostaining was located around the axon (inset, Figure 6b).
In the brain of transgenic mice, immunostaining with anti-αA-crystallin was demonstrated but the number of cells and the density of the staining were significantly less than in the dorsal spinal cord. Similar to the dorsal spinal cord, stellate cells and fibre-like staining was observed in the white matter of several brain areas (trigeminal and pyramidal tract, internal connection and pons). This is shown in Figure 7a. The positive staining in cartilage with anti-αA-crystallin was observed specifically in active newly formed chondroblasts and not in more mature chondrocytes. In the skeletal muscle of transgenic mice, the moderate anti-αA-crystallin-stained muscle fibres showed a mosaic distribution pattern.
Figure 7.
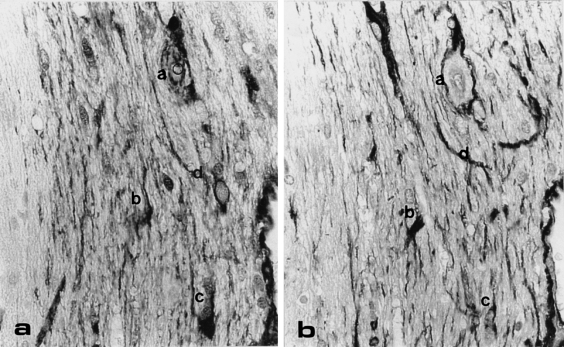
Consecutive cross-section of the white matter in a transgenic mouse cerebellum, immunostained with anti-αA-crystallin (a) and anti-GFAP (b). The same stellate cells are anti-αA-crystallin and anti-GFAP positive (compare a, b, c, d in Figure 7a, b). OM = × 100.
αB-crystallin, anti-GFAP and anti-Ubiquitin
For all tissues examined, no differences in immunostaining (pattern and density) using anti-αB-crystallin, anti-GFAP and antiubiquitin, were found between wildtype and transgenic mice. Serial sections of transgenic mice showed that astrocytes in the optic nerve, in the brain (Figure 7) and in the dorsal spinal cord stained with both anti-αA-crystallin (Figure 7a) and anti-GFAP (Figure 7b). In contrast to anti-GFAP, no similarity in spinal cord staining pattern was found between anti-αA-crystallin and anti-αB-crystallin. Serial sections of skeletal muscle showed immunostaining with both anti-αA-crystallin and anti-vimentin in the same muscle fibres. Anti-ubiquitin immunostaining in the brain and spinal cord showed weak immunopositivity in different cells, in the sciatic nerve the axons (and vacuolated structures in the transgenic mice) were weakly immunopositive.
Discussion
A transgenic mouse line for αA-crystallin clinically shows hind limb paralysis after 8 weeks of age. This unexpected finding raises the question of what possible role αA-crystallin plays in this pathological process. To unravel this role, αA-crystallin localization in transgenic mice was studied using immunohistochemistry with an antibody against the protein. The Western blot and the strong immunostaining with the anti-αA-crystallin in the eye lens of the wildtype mice suggest a high specificity of this antibody. Cross reactivity with αB-crystallin, which could be expected based on the high similarity of αA-and αB-crystallin, was excluded since the specific staining pattern using anti-αB-crystallin was completely different from that of anti-αA-crystallin.
In transgenic mice, αA-crystallin is expressed at low levels in the brain, moderately in the skeletal muscle and abundantly in the spinal cord, the sciatic nerve, the knee joint and the eye (for some tissues also shown in a Western blot). Many of the areas in brain and spinal cord in which αA-crystallin is detected run parallel to the mouse pyramidal tract (cerebral internal connection, the basal pons and the ventral part of the dorsal funiculus in the spinal cord) (Armand 1982; Kuypers 1982), which is known to be of major importance for motor functioning. This suggests that in these transgenic mice αA-crystallin expression in the pyramidal tract may contribute to the dysfunction of the hind limb.
In contrast to the brain and skeletal muscle, which did not show any pathological changes, the localization of the αA-crystallin immunostaining was correlated with the other histopathological changes in the spinal cord, dorsal root, the sciatic nerve and the knee joint.
The eosinophilic bodies that were found in the white matter (e.g. in the gracile fascicle) and in some of the spinal cord layers in the grey matter on the dorsal side of the thoracic and lumbar spinal cord reflect axonal degeneration without αA-crystallin accumulation. Electron microscopy revealed that eosinophilic bodies are morphologically similar to structures described in the nervous system of mammalian species as axonal dystrophy (Kretzschmar et al. 1987; Mukoyama et al. 1989; Lowe et al. 1997). Axonal dystrophy is a feature of neuronal degeneration and can occur, for example, during the process of ageing (Sung 1964; Dayan 1971) or as a pathological process (Lowe et al. 1997).
In longitudinal sections of the dorsal root and sciatic nerve of the transgenic mice, many vacuolated structures were observed. These structures contain remnants of axonal and myelin material and in some of them macrophages are also present, known as ‘digestion chambers’. Such structures are described in the process of Wallerian degeneration (Stenwig 1972) and are often formed in conjunction with severe neuronal degeneration. It can be concluded that such circumstances are present in the transgenic mice and an extensive degeneration of myelin and axons causes the hind limb paralysis. The localization of the severe changes and αA-crystallin accumulation in the dorsal roots strongly suggest the involvement of sensory neurones. A similar involvement of primary sensory neurones of the dorsal root ganglia in the pathogenesis of gracile axonal dystrophy has been described in a mutant mouse (Mukoyama et al. 1989).
Most of the transgenic mice showed a significant increase in cartilage in one or both knee joints, as well as chondroid metaplasia of the synovial tissue. The variability in occurrence and severity of the knee joint lesions among the mice was large, indicating that these changes were secondary to the paralysis caused by the lesions in the spinal cord and sciatic nerve. Similar joint lesions have been described in dogs with spinal cord injuries (Turbes 1998). However, since αA-crystallin is found in many active chondroblasts the possibility that the cartilage changes in the transgenic mice aggravates the symptoms cannot be excluded.
Immunostaining with anti-GFAP and anti-αA-crystallin shows that excessive amounts of αA-crystallin are primarily deposited in the astrocytes of the spinal cord and in the Schwann cells of the dorsal root and sciatic nerve. Ubiquitin, a stress protein involved in many neurodegenerative diseases (Goldman & Corbin 1991; Lowe et al. 1993), does not seem to play an important role in the pathogenesis of αA-crystallin transgenic mice since no difference in immunostaining was found between transgenic and wildtype mice. Vimentin was found in mesenchymal cells (transgenic and wildtype mice) and in all tissues in which αA-crystallin was expressed. By constructing an αA-crystallin gene using a hamster vimentin promoter region, it was to be expected that only cells normally expressing vimentin also express αA-crystallin. Skeletal muscle cells and astrocytes in the spinal cord also showed colocalization of vimentin and αA-crystallin. Although this was not expected it has been described earlier that, under pathological conditions, a high expression of vimentin can be found in skeletal muscle cells (e.g. in human distal myopathy; Uesaka et al. 1997) and astrocytes (e.g. in mice with cerebral injuries; Janeczko 1993). Moreover, both glial cells and muscle cells contain vimentin as intermediate filament in the embryonal phase, being replaced later by the mature cell-specific intermediate filaments desmin and glial fibrillary acid protein (GFAP), respectively (Dahl et al. 1981; Schnitzer et al. 1981; Lazarides et al. 1982; Eng 1985). Possibly, this switch which normally occurs postnatally in mice (Lewis & Cowan 1985; Mukono et al. 1989; Landry et al. 1990; Sarthy et al. 1991), has been disturbed by using this construct in the transgenic mice of this study.
Taking the results together, the following pathogenesis of the hindlimb paralysis in the αA-crystallin transgenic mice can be postulated. In the transgenic mice, αA-crystallin is synthesized and deposited in a variety of cells but accumulates especially in the astrocytes of the spinal cord and in Schwann cells of the sciatic nerve. Most likely, such extremely high intracytoplasmic deposition impairs the cell and its function, finally resulting in axonal dystrophy and demyelination.
In many neurodegenerative diseases αB-crystallin is abundantly present (Arrigo & Previll 1999) and it has been suggested that high expression of the protein is associated with the presence of abnormal neuronal cells (Lowe et al. 1992). In contrast to these αB-crystallin-associated diseases, in which αB-crystallin is accumulated especially within the abnormal cells and structures (swollen neuronal cells and inclusion bodies), the αA-crystallin in our transgenic mice is not found within the abnormal cells or structures (dystrophic axons). This suggests a different pathogenesis. The main question concerning the pathogenesis in our αA-crystallin transgenic mice is whether the pathology is caused specifically by high αA-crystallin expression, or whether any excessive protein deposit that disturbs the function of the same cells gives similar changes. A strong argument for the latter hypothesis is the publication of Smit and coworkers (SMit et al. 1996), describing a similar demyelination in the peripheral nerve and hindlimb paralysis in mice transgenic for a human MDR3 P-glycoprotein, having a function completely different from αA-crystallin. The construct was also made by ligating the hamster vimentin promoter to the gene, indicating that the cell type, in which the protein is expressed, may be important in the pathogenesis of hindlimb paralysis in both αA-crystallin-and the MDR3 P-glycoprotein transgenic mice.
The hypothesis of an excessive intracellular protein accumulation gives a plausible explanation for the pathological changes. However, the method of generating transgenic mice does not allow a precise localization of the inserted gene and thus it cannot be excluded that the pathological changes in our αA-crystallin transgenic mice are caused by disturbance of an endogenous gene by insertion of the αA-crystallin transgene. It would, however, be a coincidence that disruption of such a gene causes the observed severe pathology and increases transgene expression in the same tissues. This coincidence would be even more curious when in two independent transgenic mouse models (αA-crystallin and MDR3 P-glycoprotein), that have completely different transgenes inserted but are driven by the same promoter, the same endogenous gene is disrupted and an increase in expression of different proteins occurs (αA-crystallin and MDR3 P-glycoprotein).
The pathological characteristics of the αA-crystallin transgenic mouse do not cover any specific human neurological disease. However, the most prominent feature, presence of eosinophilic bodies in the spinal cord, is similar to the axonal swellings in axonal dystrophy in humans. The pathogenesis of axonal dystrophy in humans is largely unknown. Therefore, the αA-crystallin transgenic mouse may give a contribution to this pathogenesis by demonstrating that the excessive accumulation of proteins in cells such as astrocytes and Schwann cells may result in axonal dystrophy and, in our model, finally in hindlimb paralysis.
Acknowledgments
We would like to thank Dr P. Krimpenfort and Dr A. Berns for providing the transgenic animals. We thank H. Arnts for excellent animal care, J. Fundter, A. de Haze and W. van Ravesloot for the autopsies, histological preparations and immunohistochemistry. We are also grateful for the technical assistance of F. Rietveld in performing the electron microscopy. This work was supported by ECBIOMED (contract no. B14H4-CT96±1593).
References
- 1.Armand J. The origin, course and determination of corticospinal fibers in various mammals. In: KUypers HGJM, MArtin GF, editors. Progress in Brain Research: Anatomy of the Descending Pathways to the Spinal Cord. Amsterdam: Elsevier; 1982. pp. 329–360. [Google Scholar]
- 2.Arrigo A-P, Previll X. Role of hsp27 and related proteins. In: LAtchman DS, editor. Stress Proteins. Vol. 136. Berlin: Springer Verlag; 1999. pp. 101–132. [Google Scholar]
- 3.Bloemendal H. Lens proteins. Crit. Rev. Biochem. 1982;12:1–39. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- 4.Brady JP, Garland D, Douglas-Tabor Y, Jr Robinson WG, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc. Natl. Acad. Sci. USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahl D, Rueger DC, Bignami A, Weber A, Osborn M. Vimentin the 57000 daltons protein of fibroblast filaments is the major cytoskeletal component in immature glia. Eur. J. Cell Biol. 1981;24:191–196. [PubMed] [Google Scholar]
- 6.Dayan AD. Comparative neuropathology of aging: studies on the brain of 47 species and vertebrates. Brain. 1971;94:31–42. doi: 10.1093/brain/94.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Eng LF. Glial fibrillary acidic protein (GFAP): the major protein in of glial intermediate filaments of differentiated astrocytes. J. Neuroimmunol. 1985;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- 8.Goedert M. Filamentous nerve cell inclusions in neurodegenerative diseases: tauopathies and alpha-synucleinopathies. Philosoph Transactions Royal Soc. London. Series B: Biol. Sci. 1999;354(1386):1101–1118. doi: 10.1098/rstb.1999.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman JE, Corbin E. Rosenthal fibers contain ubiquitinated αB-crystallin. Am. J. Pathol. 1991;139:933–938. [PMC free article] [PubMed] [Google Scholar]
- 10.Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian α crystallin. Proc. Natl. Acad. Sci. USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwaki T, Kume-Iwaki A, Goldman JE. Cellular distribution of αB-crystallin in non-lenticular tissues. J. Histochem. Cytochem. 1990;38:31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- 12.Iwaki T, Kume-Iwaki A, Liem RK, Goldman JE. αB crystallin is expressed in nonlenticular tissues and accumulates in Alexander's disease brain. Cell 1989. 1989;57:71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- 13.Iwaki T, Wisniewski T, Iwaki A, et al. Accumulation of αB crystallin in central nervous system glia and neurons in pathological conditions. Am. J. Pathol. 1992;140:345–356. [PMC free article] [PubMed] [Google Scholar]
- 14.Janeczko K. Co-expression of GFAP and vimentin in astrocytes proliferating in response to injury in the mouse cerebral hemisphere. A combined autoradiographic and double immunocytochemical study. Int. J. Dev. Neurosci. 1993;11:139–147. doi: 10.1016/0736-5748(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 15.Kato S, Hayashi H, Nakashima K, et al. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am. J. Pathol. 1997;151:611–620. [PMC free article] [PubMed] [Google Scholar]
- 16.Kato K, Shinohara H, Kurobe N, Goto S, Inaguma Y, Ohshima K. Immunoreactive αA crystallin in rat-non-lenticular tissues detected with a sensitive immunoassay method. Bioch. Biophys. Acta. 1991;1080:173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- 17.Kretzschmar HA, Berg BO, Davis RL. Giant axonal neuropathy. Acta Neuropathol. (Berl). 1987;73:138–144. doi: 10.1007/BF00693779. [DOI] [PubMed] [Google Scholar]
- 18.Krimpenfort PJ, Schaart G, Pieper FR, et al. Tissue-specific expression of a vimentin-desmin hybrid gene in transgenic mice. EMBO J. 1988;7:941–947. doi: 10.1002/j.1460-2075.1988.tb02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers HGJM. A new look at the organization of the motor system. In: Kuypers HGJM, Martin GF, editors. Progress in Brain Research: Anatomy of the Descending Pathways to the Spinal Cord. Amsterdam: Elsevier; 1982. pp. 381–403. [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lazarides E, Granger BL, O'Connor CM, Breckler J, Price M, Danto SI. Desmin- and vimentin-containing filaments and their role in the assembly of the Z-disks in muscle cells. Cold Spring Harb. Symp Quant. Biol. 1982;46:351–378. doi: 10.1101/sqb.1982.046.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SA, Cowan NJ. Temporal expression of mouse glial fibrillary acidic protein mRNA studied by a rapid in situ hybridization procedure. J. Neurochem. 1985;45:913–919. doi: 10.1111/j.1471-4159.1985.tb04080.x. [DOI] [PubMed] [Google Scholar]
- 23.Lowe J, Lennox G, Leigh PN. Disorders of movement and system degenerations. In: Graham DI, Lantos PL, editors. Greenfield's Neuropathy. London: Arnold London; 1997. pp. 281–343. [Google Scholar]
- 24.Lowe J, Errington DR, Lennox G, et al. Ballooned neurons in several neurodegenerative diseases and stroke contain αB crystallin. Neuropathol. Appl. Neurobiol. 1992;18:341–350. doi: 10.1111/j.1365-2990.1992.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 25.Lowe J, Mayer RJ, Landon M. Ubiquitin in neurodegenerative diseases. Brain Pathol. 1993;3:55–65. doi: 10.1111/j.1750-3639.1993.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 26.Maeda A, Ohguro H, Maeda T, Nakagawa T, Kuroki Y. Low expression of alpha A-crystallins and rhodopsin kinase of photoreceptors in retinal dystrophy rat. Invest. Ophtalmol. Visual Sci. 1999;40(12):2788–2794. [PubMed] [Google Scholar]
- 27.Mukono K, Kamholz J, Behrman T, et al. Neuromodulation of Schwann cell glial fibrillary acidic protein (GFAP) J. Neurosci. Res. 1989;23:396–405. doi: 10.1002/jnr.490230405. [DOI] [PubMed] [Google Scholar]
- 28.Mukoyama M, Yamazaki K, Kikuchi T, Tomita T. Neuropathology of gracile axonal dystrophy (GAD) mouse. Acta Neuropathol. 1989;79:294–299. doi: 10.1007/BF00294664. [DOI] [PubMed] [Google Scholar]
- 29.Landry CF, Joy GG, Brown IR. Developmental expression of glial fibrillary acidic protein mRNA in the rat brain analyzed by in situ hybridization. J. Neurosci. Res. 1990;25:194–203. doi: 10.1002/jnr.490250207. [DOI] [PubMed] [Google Scholar]
- 30.Pieper FR, Scaart G, Krimpenfort PJ, et al. Transgenic expression of the muscle-specific intermediate filament protein desmin in non-muscle cells. J. Cell Biol. 1989;108:1009–1024. doi: 10.1083/jcb.108.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renkawek K, de Jong WW, Merck KB, van Workum FP, Bosman GJ. Alpha B-crystallin is present in reactive glia in Creutzfeldt-Jacob disease. Acta Neuropathol. 1992;83(3):324–327. doi: 10.1007/BF00296796. [DOI] [PubMed] [Google Scholar]
- 32.van Rijk AF, De Jong W, Bloemendal H. Exon shuffling mimicked in cell culture. Proc. Natl. Acad. Sci. USA. 1999;96:8074–8079. doi: 10.1073/pnas.96.14.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarthy PV, Fu M, Huang J. Developmental expression of the glial fibrillary acidic protein (GFAP) gene in mouse retina. Cell. Mol. Neurobiol. 1991;11:623–637. doi: 10.1007/BF00741450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnitzer J, Franke WW, Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J. Cell Biol. 1981;90:435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SMit JJM, Baas F, Hoogendijk JE, et al. Peripheral Neuropathy in mice transgenic for a human MDR3 P-glycoprotein mini-gene. J. Neurosci. 1996;16:6386–6393. doi: 10.1523/JNEUROSCI.16-20-06386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenwig AE. The origin of brain macrophages in traumatic lesions, Wallerian degeneration, and retro- grade degeneration. J. Neuropathol. Exp. Neurol. 1972;31(4):696–704. doi: 10.1097/00005072-197210000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Sung JH. Neuroaxonal dystrophy in mucoviscoidosis. J. Neuropathol. Exp. Neurol. 1964;23:567–583. doi: 10.1097/00005072-196410000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turbes CC. Repair, reconstruction, regeneration and rehabilitation strategies to spinal cord injury. Biomed. Sci. Instrum. 1998;34:351–356. [PubMed] [Google Scholar]
- 40.Uesaka Y, Nakamichi K, Kojima S, Ida M, Takagi A. Autosomal dominant distal myopathy with rimmed vacuoles and cytoplasmatic inclusions: report of a family. Clin. Neurol. 1997;37:1–6. [PubMed] [Google Scholar]