Abstract
Viruses have ‘studied’ immunology over millions of years of coevolution with their hosts. During this ongoing education they have developed countless mechanisms to escape from the host's immune system.To illustrate the most common strategies of viral immune escape we have focused on two murine models of persistent infection, lymphocytic choriomeningitis virus (LCMV) and murine cytomegalovirus (MCMV).
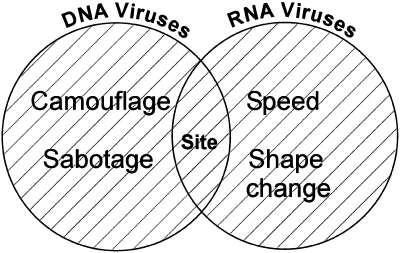
LCMV is a fast replicating small RNA virus with a genome prone to mutations. Therefore, LCMV escapes from the immune system mainly by two strategies: ‘speed’ and ‘shape change’. At the opposite extreme, MCMV is a large, complex DNA virus with a more rigid genome and thus the strategies used by LCMV are no option. However, MCMV has the coding capacity for additional genes which interfere specifically with the immune response of the host. These escape strategies have been described as ‘camouflage’ and ‘sabotage’. Using these simple concepts we describe the spectrum of viral escapology, giving credit not only to the researchers who uncovered this fascinating area of immunology but also to the viruses themselves, who still have a few lessons to teach.
Keywords: lymphocytic choriomeningitis virus, murine cytomegalovirus, virus, persistence, T lymphocyte, CD8, CD4, escape, HCV, HIV
Introduction
While the biology of viruses and that of the host's immune system are both complex, the governing principle underlying their interaction is simple – natural selection. Natural selection has led to the evolution of the pathogens we confront today, and natural selection has also shaped, over a different time scale, the human immune system (Zinkernagel 1996).
Many viral strategies for survival are phenomenally successful; most of those reading this will be infected with at least one, if not several, viruses from the herpesvirus family, commonly without clinical consequences. Dissection of the strategies used by this group of viruses has provided a detailed map of the molecular battleground on which the host-virus war is fought. These viruses persist, usually at low levels, and the biology of their persistence represents one set of linked evolutionary strategies. These are DNA-based pathogens, with large genomes by viral standards, containing hundreds of genes. Their major weapons could be described as ‘camouflage’ and ‘sabotage’, possession of highly evolved molecules, which are encoded with the incoming virus and which have evolved to disrupt conventional host defence mechanisms. The other mechanism employed by these invaders is targeting sites for replication in regions of the body perhaps less readily accessible to host defence.
In contrast, there are multiple viruses with RNA-based genomes, often much smaller, which also manage to set up persistent infection, and survive within hosts in the face of ongoing immune responses. The strategies used by this group of organisms, which have much less ‘technology’ at their disposal, are quite different. Unlike their more stable DNA counterparts, the mutability of these RNA genomes allows this group, potentially, to evolve within their host, and to set up ‘high level’ persistence. The principle strategies employed here could be described as ‘speed’ and ‘shape-change’.
This scheme of viral escapology is illustrated in Fig. 1. Naturally, as a simplified scheme, there are many viruses or strategies that are not so easily pigeonholed. For example, HIV encodes the tat gene, whose modulation of molecules at the T cell surface might be considered a ‘camouflage’ function. Alternatively, parvovirus B19 possesses a tiny DNA genome encoding only 3 genes, but may still manage to persist in the face of host immunity. There is an overlap here between mechanisms of viral persistence and immune escape. Not all viruses with known escape mechanisms (such as poxviruses) are conventionally classified as persistent. Tipping the balance in favour of the host may influence the establishment or extent or transmission of an infection, rather than always leading to long-term carriage. Nevertheless, the idea behind this review is to put the mechanisms of viral evasion of host defence into some simple categories, which would then allow individual biochemical or immunological phenomena to be placed into some overall perspective.
Figure 1.
A simple model for understanding viral persistence.
Antiviral defence
Basic mechanisms of antiviral defence
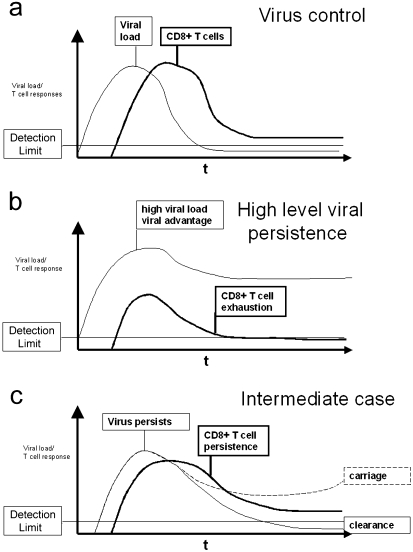
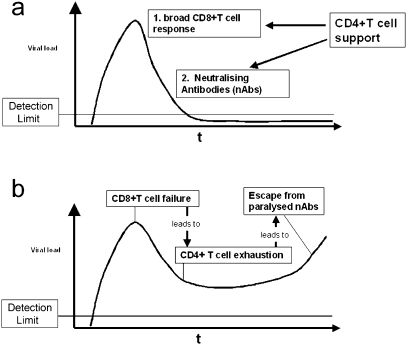
To understand immune escape, we must first outline host defence. In simple terms there are three phases of immune responses against replicating pathogens such as viruses. Firstly, immune induction, followed by an effector phase, and finally a prolonged state of memory, or, in the case of persistent viruses ‘infection/immunity’ (i.e. immune responses in a stable state maintained by ongoing infection, or low level viral replication controlled by immune responses). The dynamics of such immune control on viral load are illustrated in Figs 2(a–c); 2b highlights an example of escape leading to high level persistence.
Figure 2.
(a–c) Viral dynamics and T cell responses.Viral control or escape depends on the dynamics of the CD8+ T cells responses and the virus kinetics. Three different examples and their outcomes are illustrated in this figure. a) Early and broad effector response controls virus levels and prevents ‘escape through speed’. b) Disadvantaged effector response leads to viral escape and T cell exhaustion. c) ‘Intermediate’ effector response can lead to carriage, immunopathology or clearance.
Immune induction
Antigen presentation
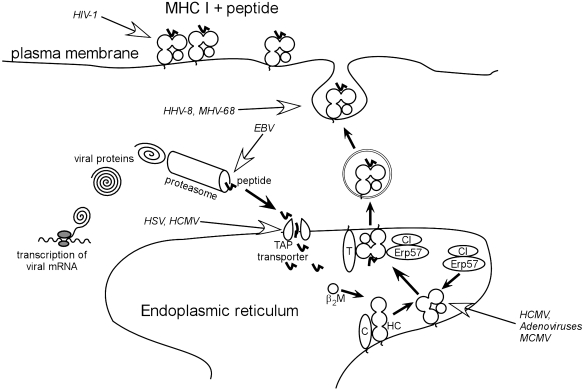
Antigen from replicating pathogens is presented to the immune system by specialized antigen presenting cells, principally dendritic cells (DCs). These may take up antigen exogenously and present it either through the Class I (Fig. 3) or Class II (Fig. 4) pathways to T cells. Infection of DCs by pathogens also leads to presentation of the same antigens, but infection of the DC is not a necessary prerequisite for antigen presentation. Since antigen presentation through the Class I pathway is a key area of interference by some viruses it is worth focusing on it in more detail (Fig. 3). In this simplified scheme, antigen is cleaved into shorter peptides, by the multicatalytic proteasome complex, transported to the TAP transporter, and then passed through to the ER. Here interactions with Class I molecules, accompanied by tapasin and other important chaperone molecules such as calreticulin and calnexin, lead to loading of peptides of the appropriate length (9–11 amino acids), and appropriate motif into stable complexes with Class I-MHC molecules and beta-2 microglobulin (Townsend & Bodmer 1989; Grandea & Van Kaer 1991). The ‘motif’ is the possession of residues by the peptides, in particular ‘anchor’ positions, which are able to interact favourably with molecules in the groove of the MHC Class I molecule alpha chain, and promote strong binding (Rammensee et al. 1995).
Figure 3.
Overview of viral interference with MHC Class I presentation. Open arrows indicate steps interfered with by viruses (‘camouflage’ strategies). Abbreviations: β2M – beta 2-microglobulin; C – calreticulin; Erp57 – thiol oxidase reductase ERp57; HC – heavy chain; T – tapasin; TAPtransporter – TAP peptide transporter associated with antigen processing; HCMV – human cytomegalovirus; HSV – herpes simplex virus; MCMV – murine cytomegalovirus; HIV-1 – human immunodeficiency virus 1; HHV-8 – human herpes virus 8; MHV-68 – murine herpes virus 68.
Figure 4.
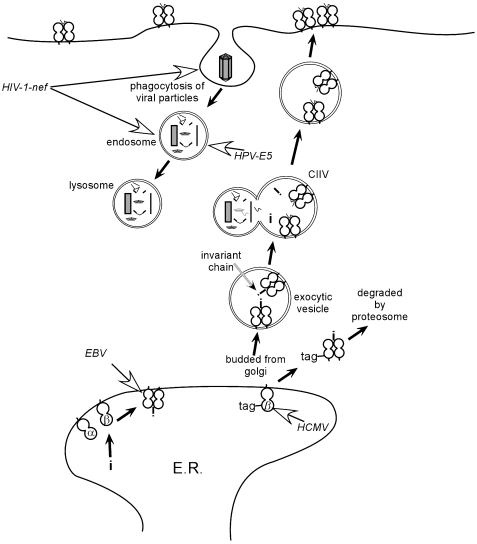
Overview of viral interference with MHC Class II presentation. Open arrows indicate steps interfered with by viruses (‘camouflage’ strategies). Abbreviations: CIIV – major histo-compatibility class II vesicle; α – alpha chain; β – beta chain; HCMV – human cytomegalovirus; EBV – Epstein‐Barr‐virus; HPV-E5 – human papilloma virus E5 protein;HIV-1-nef – human immunodeficiency virus 1, nef protein.
CD8 T cell triggering
The interaction of the CD8+ T cells with such Class I-peptide-complexes occurs via a T cell receptor (TCR). TCRs are generated with considerable diversity from combinations of host germline encoded genes. TCRs combining specific alpha and beta chains are able to bind specific combinations of host MHC Class I molecule and viral (or other) peptide. Binding is very weak by conventional antibody-antigen standards, and the contribution of CD8 to recognition of Class I is also weak, but the combination of many of these events integrated over time, with condensation of TCRs into a localized complex (‘immunological synapse’), leads to triggering of intracellular signalling (Bromley et al. 2001). Because activation of CD8+ T lymphocytes by viral antigen is a multistep process, it is clearly easy for viruses to disrupt it at many points.
CD4 T cell triggering
Class II presentation similarly utilizes peptides but these are derived not from cytoplasmic proteins via the proteasome, but via a separate pathway involving lysosomes acting on exogenous proteins. Loading of Class II complexes requires tight binding between peptides and Class II molecules, and similar T cell recognition and triggering events are thought to occur, although the key surface molecule involved in these interactions is CD4 in combination with TCR (Fig. 4).
These recognition events for naïve T cells occur on the surface of DCs, which have picked up antigen and acquired potent antigen presenting function (‘mature’ DCs). Priming, it is thought, occurs optimally in the environment of secondary lymphoid organs, where such cells home after picking up antigen. In addition to the appropriate anatomical environment, cytokine and chemokine milieu, such DCs, also express important costimulatory molecules which can provide help in triggering T cells, through, again, multiple low-affinity surface interactions (Ochsenbein et al. 1999).
B cell triggering
This also occurs optimally in secondary lymphoid tissue. B cell responses to replicating pathogens, particularly viruses with repetitive, organized antigen on their surface, may be triggered directly in the absence of T cell help to produce IgM. In certain situations, antibodies encoded by germline sequences may be sufficiently avid to provide effective antiviral activity (Kalinke et al. 1996; Bachmann et al. 1997). However conventionally, maturation of the antibody response involves a switching of antibody class from IgM to IgG, usually dependent on activity from T helper cells, and a process of ‘affinity maturation’ leads to evolution of the humoral response.
Innate responses
The above cellular subsets are important in providing sophisticated antigen-specific responses. However, these are only part of the story. Many other functions are induced during acute antiviral responses. Firstly, the interferon pathway is activated potently by double-stranded (ds) RNA. Mice deficient in interferon alpha/beta receptors are highly susceptible to viral infections, despite having an initially intact lymphocyte repertoire (van den Broek et al. 1995; Huang et al. 1993). Secondly, natural killer (NK) cells are able to respond to stimulation not specific for a particular antigen and they emerge with rapid kinetics. Finally other subsets of T cells such as gamma delta T cells and NKT cells are commonly found in some sites. Such subsets include T cells with highly restricted T cell receptors able to recognize non‐protein antigens, such as lipids, presented by nonpolymorphic MHC-like molecules, such as CD1. They appear to have particular tissue distributions, such as an enrichment in the liver and the gut, and may be involved in innate responses in such organs, although also in regulation of antigen-specific responses (Biron et al. 1999; Raulet et al. 2001).
Effector functions
Activated CD8+ T cells are able to migrate to infected tissues and recognize infected cells through the same mechanism as described above. Once triggered, CD8+ T cells perform multiple effector functions, and it is likely that the dominant function varies for different viruses and also over time. The classical function is killing, which can be mediated by release of lytic granules such as perforin, or through the Fas/Fas ligand pathway (Kagi et al. 1994; Kagi et al. 1995). Such cells also secrete cytokines, notably interferon-gamma (IFN-γ), which has important antiviral activity, as well as acting as an inflammatory mediator. Finally, such cells also secrete chemokines, such as RANTES, which have inhibitory activity against HIV (Cocchi et al. 1995), but more commonly provide signals for recruitment of other inflammatory or antiviral cells Table 1.
Table 1.
Recognition functions of the immune response in viral infection
| CD8 | Virus-infected cell; monitor intracellular pathogens |
| CD4 | APC or class II expressing virus infected cell; monitor circulating viral proteins |
| B Cell | Circulating virions or viral proteins |
| NK | Class I low cells |
| Innate responses | Pathogen-dependent ‘patterns’ as opposed to specific antigens |
CD4+ T lymphocytes play a central role in initiating, coordinating and maintaining antiviral immune responses. CD4+ T lymphocytes may provide many of the same effector functions as CD8+ T lymphocytes, particularly release of antiviral cytokines. In addition, CD4 cells may ‘condition’ DCs for enhanced antigen presentation via interactions through CD40-CD40L, thus providing indirect help for CD8 T cells, as well as release of supportive cytokines such as IL-2 (Ridge et al. 1998; Schoenberger et al. 1998). Th2 type CD4 + lymphocytes are thought to modulate immune responses by secretion of anti-inflammatory cytokines such as IL-10, although the distinctions between different subsets are not always clear-cut in human disease.
As far as B cells are concerned, the principal activity in this virus-centred scheme is mediated via the action of neutralizing antibodies, which inhibit infection of target cells. The action of neutralization may be simple, masking effects on binding molecules, but in other cases may involve opsonization of complement, Fc receptor binding, etc. Antibodies to internal or nonstructural viral components would be considered non-neutralizing (Zinkernagel 1996).
NK cells possess inhibitory receptors, which are triggered by engagement of MHC class I molecules on the surface of target cells. Cells low in MHC class I are NK targets. There are also activation signals involved, although the exact nature of these is not as well worked out as for conventional CD8+ T lymphocytes (but will have been explored fully by viruses).Table 2
Table 2.
Effector functions of the immune response during viral infection
| CD8 | Kill virus infected cells (cytotoxicity) |
| Induce apoptosis | |
| Secrete inflammatory cytokines | |
| Recruit other cells via chemokines | |
| CD4 | Co-ordination of immune responses |
| Induction and maintenance of CD8 responses | |
| Induction and maintenance of B cell responses | |
| Potentially, killing of virus infected cells | |
| Innate responses | Suppression of viral replication |
| Secretion of inflammatory cytokines | |
| Lysis of viral infected cells |
Memory
Activation, followed by effector function, is followed usually by a prolonged phase described as memory, or alternatively ‘infection-immunity’ if the pathogen still persists. Very large numbers of T cells may be induced during the acute phases of viral infection, but after effector function is displayed, the numbers dwindle rapidly, partly because of a reduced drive to expand by antigen, and partly due to ‘activation-induced’ cell death. The total number of cells left, and their function, are matters of considerable current interest and debate amongst immunologists, since antigen is not absolutely required to maintain T cell memory, but the functional state of the memory depends on the extent to which it is continuously exposed to antigen (Ahmed & Gray 1996; Zinkernagel et al. 1996). For most of the viruses we are considering, this is not an issue, since antigen will always be present to some extent.
One important determinant of the number of T cells left in the memory phase is thought to be the initial burst size (Hou et al. 1994). Viral escape mechanisms acting early to inhibit presentation, and therefore expansion, of virus-specific T cells could have a large downstream effect on the total number of cells in the long term. This issue is actually quite complicated, since on the other hand, impaired effector function leads to more viral replication and thus a stronger antigenic drive. The simple observation that herpesviruses, which contain numerous camouflage genes, nevertheless invoke and sustain the most vigorous acute and chronic T cell responses ever described, suggests that escape does not necessarily imply weaker host defence (Callan et al. 1998; Gillespie et al. 2000; Lechner et al. 2001).
Example 1: LCMV
To illustrate mechanisms of viral persistence in vivo we have chosen 2 murine models, the first of which is Lymphocytic Choriomeningitis Virus (LCMV). This virus represents, in the scheme outlined above, a small RNA based mutable virus capable of low level or high-level persistence.
The closest human homologues of LCMV are arenaviruses, which tend to cause acute haemorrhagic disease, rather than persistence. However in the mouse, its natural host, it is relatively noncytopathic, and the various high level infections set up have been compared to human infection by HIV, HBV or HCV (Klenerman & Zinkernagel 1997). More recently established is the persistence of this virus at a low level over long periods. The biology of this and the mechanisms of immune evasion involved are much less well studied (Ciurea et al. 1999). The immune factors which control LCMV infection, and which influence at what level the virus persists have been established (Figs 5a, b). These are as follows:
Figure 5.
(a–b) Viral dynamics and the immune response (humoral and cellular). a) Viral kinetics under strong cellular and antibody responses – viral control. Early efficient cellular response (CTL with T cell help) followed by neutralizing antibody response prevents mutational escape due to low viral replication rate. b) Viral kinetics during escape from sequential immune responses. A chain reaction causing a failure of all three arms of the immune system leads to mutational escape of the virus.
CD8+ T lymphocytes provide the major early defence against infection (with very large expansions peaking at about 1 week after infection) (Gallimore et al. 1998).
CD8+ T lymphocytes also cause the early immunopathology associated with infection (Althage et al. 1992).
Perforin mediated killing is needed to clear infection, although IFN-γ plays an important role (Kagi et al. 1995).
In the long term (i.e. after 6–8 weeks), CD8+ T cells cannot act without support from CD4+ T cells and neutralizing antibody-producing B cells. If these responses are not present, the virus re-emerges (Planz et al. 1997).
Even when the virus is cleared from the blood, in the face of normal memory responses, viral genes and proteins are still often detectable in organs at very low level (Ciurea et al. 1999).
Under circumstances where the initial CD8 + response is incomplete, high level carriage ensues, with disappearance of the T cells (exhaustion or deletion) (Moskophidis et al. 1993).
The simple lessons from this model and those like it are that the kinetics and distribution of the viral replication and host response are major factors in determining outcome. Also host defence must be considered in an holistic way – different responses play different roles at different times, the overall balance of which will also affect outcome.
Speed
LCMV demonstrates one paradigm of the RNA viral use of speed to escape from host defence. This strategy is only really sustainable by relatively noncytolytic viruses – since persistence of highly lytic viruses, perhaps with the exception of lactate-dehydrogenase-virus (LDV) (van den Broek et al. 1997), a virus with highly restricted host cell range, will lead to host death. The development of high-level persistence by LCMV has been well explored using different strain/mouse combinations (Moskophidis et al. 1994). Although the mechanism of T cell exhaustion is not defined, the phenomenon appears to develop under conditions where the virus is present in high load, and in a widespread distribution. Cell tropism may also affect the induction of exhaustion. Some viral strains do not induce exhaustion even if given intravenously in high dose.
Features that impair the maintenance of host CD8 + responses promote escape through speed. These include lack of CD4 T cells (Battegay et al. 1994); interestingly, lack of CD4 cells under some circumstances does not lead to complete exhaustion, but rather, maintenance of CD8+ T lymphocytes with reduced effector function (Zajac et al. 1998). It is likely that exposure of CD8+ T lymphocytes to high levels of antigen in circumstances where presentation is not on specialized DCs (i.e. in nonlymphoid organs) and without CD4 help, promotes antigen-induced cell death. Under similar conditions of prolonged antigenic exposure, CD4 exhaustion may also be observed, although the kinetics of induction are somewhat slower, perhaps because presentation is limited to Class II bearing cells (Oxenius et al. 1998; Ciurea et al. 2001).
Escape through speed leads to high level antigen persistence in the mouse – thus this model is relevant only to a few human viruses such as HIV, HBV and HCV. There is some evidence for exhaustion in all of these infections.
In later stage HIV, HIV-specific CTL precursors disappear in preference to those specific for other infections (Carmichael et al. 1993). An inverse correlation has been observed between the numbers of HIV-specific CTL and viral load, which may indicate that high viral loads lead to suppression of such CTL (although the interpretation of this is complex) (Ogg et al. 1998). Also, early large expansions of CD8+ T lymphocytes bearing particularly restricted Vβ chains (i.e. of restricted specificity) has been associated with rapid progression, perhaps through a similar mechanism of activation-induced cell death (Pantaleo et al. 1994). On the other hand, T cells may persist, indeed individual clones may persist for many years in the face of continued viral replication (Kalams & Walker 1995). Also large expansions of activated T cells with maintained effector function are commonly seen in chronic HIV (Goulder et al. 2001), so if exhaustion occurs, it is not commonly the very rapid picture seen in experimental models. It is likely that it is one part of the ongoing dynamic interaction between host and virus.
In HBV and HCV, in contrast, high levels of virus are commonly associated with low levels of specific CD8+ T lymphocytes, and also low levels of specific CD4+ T lymphocytes (Guidotti et al. 1999; Lechner et al. 2000a Lechner et al. 2000a, b; Takaki et al. 2000). Evidence that high viral loads are associated with suppression of CD4+ T cells in HBV comes from studies where treatment with the antiviral drug lamivudine leads to emergence of specific CD4+ T cells, with similar data from HCV (although the treatment is more complex) (Ferrari et al. 1998; Cramp et al. 2000). In acute disease, CD8+ T cell responses are seen early, but may not be sustained, particularly in chronic HCV infection, as opposed to those who clear virus (Lechner et al. 2000a, b). Thus exhaustion of responses appears to be more likely in these infections.
The role of the liver as an immune modulator is well known from transplant studies, and further studies of the role of the intrahepatic environment per se as an ‘exhaustogenic’ site are warranted. There is emerging evidence that activated T cells go to the liver at the time of down-regulation of acute responses, and that apoptosis occurs in this environment (Crispe et al. 2000). Thus this mechanism of immune escape may overlap with ‘site’.
Shape-changing
Mutation of viral species within individuals infected by RNA-based viruses is now held up as a paradigm of Darwinian evolution (Jones 2000). The role of the immune response in natural selection of ‘escape mutants’ has, however, been contentious, although consistent data are now emerging. The LCMV model paved the way for understanding of the role of T cells in this selection. Wild-type mice of various strains do not display immune selection and escape. However, a mouse in which the majority of T cells were directed toward a single epitope through insertion of TCR transgenes led to rapid escape under certain conditions (Pircher et al. 1990; Klenerman & Zinkernagel 1998). If the levels of infection were low, virus infection was rapidly cleared – as the dose of infection was increased, selection occurred rapidly leading to the emergence of viruses which were not recognized by the host TCR. This indicates that a high viral load and a strong/focused selective force are required to see escape under these artificial conditions. Similar effects were observed in vitro (Aebischer et al. 1991).
Infection of normal mice with such wild-type virus may influence subsequent immune responses to variant viruses, a phenomenon described as ‘original antigenic sin’ (Klenerman & Zinkernagel 1998). This may be due to partial cross-reactivity between epitopes, which act as ‘altered peptide ligands’ (APL) and partially trigger T cell responses (Klenerman et al. 1995). This may play some role in the ability of hosts to mount sequential immune responses against sequentially emerging mutants (see below).
Escape from antibodies may also occur, as can be demonstrated using a similar transgenic strategy. Mice transgenic for only the heavy chain of a neutralizing antibody directed at LCMV glycoprotein generate high levels of antibodies very rapidly after infection. If doses of the infecting virus are high enough to lead to T cell exhaustion, antibody then becomes the primary immune effector, and antibody escape is rapid (Seiler et al. 2000). Interestingly, in this system, modest effects of an antiviral drug act synergystically with the antibody to prevent escape and lead to resolution of infection (Seiler et al. 2000). More recently, escape from polyclonal antibody has been observed. In experiments where CD8+ T lymphocytes are not present, once again high levels of virus ensue, and antibody becomes the dominant antiviral effector – and selector. The emergence of neutralization resistant virus is somewhat slower than for CD8 T cell mediated selection (10 weeks v 2 weeks), but is inevitable. In this model, escape occurs only once – after this the CD4 responses are exhausted and the new virus, although immunogenic in new hosts, fails to elicit new antibody responses in the infected host (Ciurea et al. 2001). There is thus a ‘domino’ effect – failure of or escape from one arm of the immune response can lead on to failure or escape from other arms (Fig 5b).
How does this relate to human infection? Mutational escape of HIV from T cells (both CD8 and CD4) has been observed, in chronic infection (Phillips et al. 1991; Goulder et al. 1997; Harcourt et al. 1998) and, more rarely, during acute infection (Borrow et al. 1997; Price et al. 1997). In some cases, escape has been accompanied by clinical and virological deterioration, and this is confirmed by work in the SIV model (Dzuris et al. 2000; Vogel et al. 2001). Exactly what sort of immune responses lead to escape is by no means clear as the phenomenon is not universal. It is likely that focused responses are the most efficient at immune selection, but outside monkey models, information as to the breadth of the response, and, most importantly, the sequence of the infecting strain, is not always available.
Original sin for T cells has also been observed in HIV (McAdam et al. 1995; Klenerman et al. 1998), and mutants persist which are immunogenic, but which do not elicit new CD8 T lymphocyte responses. Also, APL may affect T cell recognition in ‘antagonist’ assays, whereby low levels of variants may interfere with recognition of wild-type peptide (Klenerman et al. 1994). How this might work in vivo has not been established, although it also occurs in HBV (Bertoletti et al. 1994).
Mutational escape has also been observed in HCV and HBV, but may occur less commonly than in HIV (Chang et al. 1997). Again, mutation leading to loss of T cell recognition has been observed in acute disease and in chronic disease (Chang et al. 1997), but clinical deterioration as a result of escape is not clearly reported. Part of the difficulty may be that of identifying the immunodominant response in any one individual at any one time. It should be pointed out that HBV is a DNA virus, but mutation may occur during the RNA intermediate phase, and particularly since replication rates are high. Drug resistance mutants, for example, emerge rapidly (Pichoud et al. 2000). Many more data are required before we can assess the role of shape-changing v speed or even camouflage in persistence of these infections.
Given the highly active and sometimes highly focused CD8+ T lymphocyte responses seen in acute HCV (Lechner et al. 2000c), and its propensity to persist, one might predict that escape mutation might occur most readily at this point; (this might in fact be the case for antibody responses in this infection, Farci et al. 2000). Such issues are not entirely academic as they have important impact on how one might design vaccines – if escape occurs very readily, strategies targeting multiple epitopes and multiple variants are essential.
The model of LCMV, although excellent for analysing factors which affect host-virus balance in vivo, is a poor one for understanding ‘camouflage’ based escape strategies. These have been primarily identified in low level persistence of larger more stable viruses. In the next section we describe a range of mechanisms which could interfere with host defence – induction, effector function or co-ordination. A better model for understanding the basis of this is murine cytomegalovirus (MCMV).
Example 2: MCMV
Camouflage
MCMV is a cytopathic β-herpesvirus with a large double-stranded DNA genome of about 230 kb to encode an estimate of 170–200 viral proteins, all of which might be potential antigenic targets for the host's immune system. Replication is a rather slow (24–36 h) multistep process subdivided into immediate early (IE), early (E) and late (L) phase of gene expression and therefore ‘speed’ is no option for immune escape of CMV. Nevertheless, lifelong persistent infection without overt disease is the usual outcome of CMV-infection indicating the high degree of adaptation of the virus to the immune system of the host and vice versa due to millions of years of coevolution. Only in the rare event of immunosuppression is serious harm caused to the host. The size of the CMV genome provides the coding capacity for many proteins capable of modulating the host's immune response, instrumental in establishing this subtle balance (Mocarski 1996).
The major strategy of MCMV to persist is to hide from the immune system by latency. After productive primary infection has eventually been cleared by the immune system the viral genome is not eliminated but persists as episomal DNA. In latency, viral proteins, which are the major target for the immune system, are not produced (or only to a very limited extent). From latent genomes productive replication and virus shedding can be reinitiated to allow virus transmission to a new host (Yu et al. 1995; Kurz & Reddehase 1999).Table 3
Table 3.
Characteristics of LCMV escape
| Camouflage | Not clearly important |
| Site | May persist in kidney |
| Shape changing | If CD8 + responses are highly focused |
| Escape from B cells/CD4 cells if CTL fail | |
| Speed | Leads to CTL exhaustion and high-level carriage |
Primary MCMV-infection is controlled mainly by CD8+ T cells and NK cells, but complete termination of productive MCMV replication in salivary glands also requires CD4+ T cells (Reddehase et al. 1985; Shellam et al. 1981; Reddehase et al. 1987; Jonjic et al. 1989). Remarkably, not only resolution of productive infection but also maintenance of latency seems to be under immune control. Again CD8+ T cells have been shown to play a major role but CD4+ T cells, NK cells and antibodies contribute to the maintenance of MCMV-latency (Jonjic et al. 1994; Polic et al. 1998).
Escape from CD8+ T cell control
Since CD8+ T cells seem to be major players in MCMV-control, it is not surprising that potent immune evasive mechanisms are directed against activation of CD8+ T cells. To date mainly mechanisms directed against the class I antigen presentation pathway have been identified – a set of mechanisms aimed at camouflage (Hengel et al. 1999). MHC I down-regulation occurs in vitro within a few hours after infection when E-genes of the virus start to be expressed. During the E-phase of viral replication m152/gp37/40 expression leads to retention of newly formed MHC-peptide complexes in the ER-Golgi intermediate compartment (ERGIC) preventing them from reaching the cell surface (Ziegler et al. 1997). Deletion of m152 from the viral genome had no effect on virus replication in vitro, whereas replication was significantly reduced in vivo due to enhanced virus clearance by CD8+ T cells demonstrating the effectiveness of this camouflage mechanism in the natural host (Krmpotic et al. 1999).
In addition, m06/gp48, which is expressed later in the E-phase than m152/gp37/40 and during the L-phase of MCMV replication, has been shown to bind MHC class I/β2m in the ER and after transfer through the Golgi the complex is redirected to the endosomal/lysosomal compartment for degradation leading to further reduction of MHC I expression on the surface of MCMV-infected cells and to reduced surveillance by CD8+ T cells (Reusch et al. 1999).
Escape from NK cells
The down-regulation of MHC I on the cell surface of MCMV infected cells should make these cells particularly vulnerable for NK cell mediated effector mechanisms recognizing the ‘missing self’. Again, the virus has found appropriate answers to this challenge during evolution. m144, a MCMV-encoded homologue of mouse MHC I, seems to serve as a decoy receptor for NK cells substituting the down-regulated MHC I molecule. Although direct evidence of m144 expression on the cell surface of MCMV–infected cells and interaction of m144 with inhibitory receptors on NK cells is still lacking, m144-deletion mutants were shown to replicate significantly less than wild type virus during the early stages of infection. This is due to enhanced NK cell control. Depletion of NK cells abrogated the attenuated phenotype of the mutant virus in vivo and, in addition, m144 transfected RMA-S cells (MHC I deficient due to TAP-deficiency) were resistant to lysis by activated NK cells (Farrell et al. 1997; Cretney et al. 1999; Farrell et al. 1999).
Another MCMV protein, m04/gp34, which is expressed late in the E-phase of viral replication, was shown to bind to certain MHC I molecules in the ER. Thereafter, the complex is transported to the cell surface bypassing the effect of m152/gp37/40. It is speculated that the complexed MHC I molecules would thereby silence the host's NK cell response (Kleijnen et al. 1997).
Escape from CD4+ T cells
Although CD4+ T cells are dispensable for resolution of systemic primary MCMV infection, they seem to have a unique role in the clearance of productive virus infection from the salivary glands and therefore they may limit the spread of the virus from host to host (Jonjic et al. 1989; Lucin et al. 1992). Recently, several mechanisms of MCMV that seem to interfere with activation of CD4+ T cells by macrophages have been identified. Infection of macrophages with MCMV leads to early production of IL-10 which down-regulates MHC class II expression in a paracrine fashion (Redpath et al. 1999). In addition, MCMV counteracts IFNγ-induced up-regulation of MHC class II in macrophages (Heise et al. 1998a; Heise et al. 1998b).
Overall, MCMV seems to be a master in the art of biological camouflage to ensure persistent infection. However, as a cytopathic organism complete avoidance of host defence would lead to continuous replication with significant tissue destruction and this would not be beneficial for its own survival due to host mortality. Therefore, despite a whole range of immune evasive mechanisms the virus seems to allow an amount of immune control, which is optimal for its own survival.Table 4
Table 4.
Escape mechanisms
| Escape mechanisms | Genes responsible MCMV | HCMV | Other examples |
|---|---|---|---|
| Restricted gene expression in latency | |||
| Infection occurs at sites that are difficult to access by the immune response | |||
| Escape from T cell recognition | |||
| CD8+ T cells: interference with MHC | m152 | US2, US3, | EBV: EBNA1 |
| Class I Ag presentation pathway | m06 | US6, US11 | AV: E3/19K |
| HSV: ICP47 | |||
| CD4+ T cells: interference with MHC | m144 | US2 | EBV: BZLF2 |
| Class II Ag presentation | |||
| Escape from NK cell recognition | |||
| Expression of a decoy Class I molecule | m04 | ||
| Controlled expression of defined peptide/Class I complexes | UL40 | ||
| Up-regulation of HLA-E | |||
| Interference with the expression and | m129/ | UL144 | |
| Function of antiviral cytokines and cytokine receptors | m131 | US27,US28 | |
| TNF-R homology | UL146 | ||
| Viral chemokine receptors | |||
| CXC chemokine agonist | UL111a | ||
| CC chemokine agonist | |||
| viral IL-10 homologue | EBV: BCRF1 | ||
AV: Adenovirus; EBV: Epstein-Barr-Virus; HCMV: Human cytomegalovirus; HSV: Herpes simplex virus; MCMV: Murine cytomegalovirus
Other examples of camouflage
There are numerous other examples of viral interference with MHC Class I presentation to CD8 lymphocytes (Lalani et al. 2000). The adenovirus early protein E3/19K binds directly to MHC class I molecules, inhibiting proper glycosylation and processing of the MHC Class I molecule, eventually leading to its decreased expression on the cell surface (Beier et al. 1994). Interestingly, this same molecule also binds to tapasin, and may have two sites of action to prevent Class I peptide expression (Bennett et al. 1999).
The herpesviridae utilize a range of different viral proteins to interfere with multiple steps in Class I presentation in order to escape T cell detection (further evidence that CD8+ T lymphocytes are major antiviral effectors for this group). HCMV has developed two major mechanisms to evade MHC class I dependent immune responses, using several genes in the unique short (US) region that is nonessential for in vitro propagation of the virus. From this gene family, four glycoproteins US2, US3, US6 and US11 are known to down-regulate MHC class I expression. US2 and US11 interfere with the MHC class I pathway immediately after translocation of the heavy chain into the lumen of the ER (Jones et al. 1996; Jones & Sun 1997). They relocate the glycosylated heavy chain by an unknown mechanism into the cytosol and consequently cause its proteasomal degradation. US3 uses a different strategy interfering with the MHC class I assembly pathway, perhaps through an indirect effect on transport of MHC class I molecules (Colberg–Poley 1996; Jones et al. 1996). Finally, US6 targets a different checkpoint in the MHC class I assembly pathway, namely the TAP transport machinery. Biochemical studies revealed that US6 is a glycoprotein that resides in the ER (Ahn et al. 1997). It inhibits peptide transport through the TAP pore during late stages of infection through the inhibition of ATP binding to TAP1 (Hewitt et al. 2001).
Herpes simplex virus (HSV) inhibits TAP transport using the protein ICP-47. In contrast to US6, ICP-47 affects peptide binding to TAP (Hill et al. 1995; Goldsmith et al. 1998), since it is recognized by TAP in a manner similar to peptides. Once bound, ICP-47 prevents peptide binding further by inducing conformational changes in the TAP heterodimer (Tomazin et al. 1996). MHC class I molecules, which are not loaded with translocated peptide, are retained in a tapasin-dependent manner in the ER lumen. These are at later stages targets for cytosolic relocation and proteasomal degradation.
The above molecules illustrate examples of modification of maturation, assembly and export of MHC-peptide complexes (Fig. 3). Other herpesviruses employ strategies to interfere with antigen processing at an earlier stage. EBV nuclear antigen (EBNA 1) is the dominant antigen expressed in latent EBV infection, yet is poorly processed when expressed intracellularly (Levitsky et al. 1997). This is due to its C-terminus containing a long glycine-alanine repeat, which renders the protein indigestible to proteasomes (Levitskaya et al. 1995). Interestingly, although these were missed in earlier studies, responses to this protein do occur, but in this case through cross-presentation (i.e. uptake by an uninfected DC and presentation through the Class I pathway) (Ferlazzo et al. 2000; Subklewe et al. 2001).
As described above many viruses down-regulate MHC class I surface expression and therefore become a target for NK cells. To escape CTL recognition and also circumvent NK detection, viruses have responded with exquisite subtlety. NK cells are normally under constant repression induced by NK inhibitory receptors (KIR) including those specific for HLA-C and HLA-E (Lanier 2001). When these receptors bind their ligand they transmit inhibitory signals which prevent lysis of the cell. In contrast, the majority of CTL recognition of peptides occur in the context of HLA-A and HLA-B proteins (Littaua et al. 1991). HIV-1, for example selectively down-regulates HLA-B and HLA-A molecules which makes it invisible to CTL, but does not interfere significantly with HLA-C and HLA-E surface expression thus appearing to be unaffected when subjected to NK cell scrutiny (Cohen et al. 1999). Using a slightly different approach, HCMV encodes for UL40 protein that provides a peptide selectively required for HLA-E maturation and up-regulation. Infected cells preferentially express this Class I molecule and again appear ‘normal’ to NK cells.
Most viral escape mechanisms affecting Class II antigen presentation are indirect, affecting the responsiveness of the cell to exogenous factors which would lead to increased Class II expression rather than perturbing the intracellular pathways of synthesis or antigen loading. These mechanisms are discussed within the following virokines section. There are however, a number of viruses that may directly affect MHC class II expression (Fig. 4). One such example is EBV, which encodes a protein, BZLF2, that interferes directly with MHC class II expression. BZLF2 is a type II glycoprotein that is expressed at late timepoints of infection and binds to the HLA-DR heterodimer leading to its retention in the ER. Furthermore it has been shown that BZLF2 can be also transported to the cell surface when coexpressed with other EBV proteins, where it might facilitate viral infection of MHC class II positive cells (Spriggs et al. 1996). Another example is the US2 protein encoded by HCMV that targets DR and DM β-chains for proteasomal degradation (Tomazin et al. 1999). The HIV-1 regulatory protein nef has been shown to have a direct effect on CD4 cells. It becomes expressed on the surface of infected cells (Fujii et al. 1996) and leads to an increased susceptibility to apoptosis due to the reduction in the antiapoptotic proteins Bcl‐2 and BCL-X (Rasola et al. 2001). Interestingly its presence on the surface of CD4 cells may also be responsible for viral persistence within long lived productively infected CD4 cells, as interactions with membrane TNF expressing macrophages reverses the nef triggered apoptosis of the CD4 cells (Mahlknecht et al. 2000).
Sabotage of immune responses – interference with cytokines and chemokines
MCMV encodes not only numerous host-derived genes, which it uses for camouflage, but also others utilized for sabotage. By sabotage in this context we imply synthesis of molecules designed to disrupt or manipulate host inflammatory/immune responses. This differs from camouflage, which is mainly a strategy concerned with invisibility. The prime targets for sabotage strategies are chemokines and cytokines, small secreted molecules that have potent effects on the activity of cells close by. Cells expressing specific receptors can be stimulated to drastically change their behaviour and in the case of chemoattractant cytokines stimulate migration towards their source. Given their central role in coordinating the complex network of cells involved in inflammatory responses to infectious agents, it is not surprising that many viruses have developed techniques to prevent and interfere with the biological activities of cytokines. We are currently aware of three different ways viruses twist immune responses to their advantage using the language of cytokines.
1) They may encode secreted cytokine homologues (vCks) or virokines that can act in an agonistic or antagonistic manner;
2) They may produce soluble chemokine scavengers known as cytokine binding proteins (vCkBPs) that sequester free chemokines;
3) They may produce dummy or virally encoded cell-surface cytokine receptor homologues (vCkRs) which alter cell responsiveness to a cytokine. These receptors are known as viroceptors.
Secreted cytokine homologues (vCks)
Several herpesviruses and poxviruses encode for vCks that have a striking homology with open reading frames (ORF) of human cytokines. Cytokines can be divided, in general into two functional groups, those that support inflammation and those that help the immune system to return into a quiescent state.
Proinflammatory cytokines, e.g. interleukin 1 (IL1) and tumour necrosis factor alpha (TNFα) support leucocyte activation and recruitment. Anti-inflammatory cytokines such as interleukin 10 (IL10) and transforming growth factor beta (TGFβ) may inhibit major tissue damage. In the following paragraphs we will apply this functional division to the virally secreted cytokine homologues, aka virokines.
Proinflammatory vCks
Herpesviruses express functional agonistic virokines for recruiting susceptible target cells for infection. During the course of infection, murine cytomegalovirus (MCMV) expresses 2 soluble virokines called MCK-1 and 2 that act as CC chemoattractant cytokines, i.e. ligands for cells such as monocytes and macrophages (Saederup et al. 1999). MCMV expressing mutant MCK-1 or MCK2 resulted in a reduced inflammatory response and reduced monocyte-associated viremia. (Kapasi & Rice 1988; Fleming 1999; Saederup et al. 1999). Thus, the expression of these chemokines attracts cells that promote viral replication and dissemination. A similar example maybe the action of vCXC/UL146, a CXC chemokine agonist, in HCMV infection, which appears to recruit neutrophils to the site of infection as opposed to monocytes (Lalani et al. 2000; Penfold et al. 1999).
HIV‐1 may also take advantage of the host defence system in a similar fashion. HIV-1 tat protein shows partial similarity with monocyte chemotactic protein (MCP), a potent chemoattractant for monocytes (Albini et al. 1998). Interestingly M-trophic HIV-1 infected cells release gp120 that acts as a chemoattractant for uninfected DCs (Lin et al. 2000). These few examples show how viruses are able to regulate leucocyte trafficking to the site of infection and therefore enhance the density of possible target cells for viral spread as well as potentially modulating the inflammatory response.
Anti-inflammatory vCks
Analysis of the sequences of many herpesvirus and poxvirus genomes has also revealed viral open reading frames that encode for anti-inflammatory virokines. One clear example is vMCC-1, a virokine expressed during Molluscum contagiosum virus (MCV) infection. MCV belongs to the family of poxviruses that induce benign skin papules in humans. An exacerbation of the disease is often seen in immunocompromised patients and children. VMCC-I shows structural homology to CC chemokines but contains a N-terminal amino acid deletion at a site that is crucial for receptor activation. It exhibits highly specific binding to the CC chemokine receptor CCR8 (Luttichau et al. 2000) and therefore inhibits receptor signalling. In vitro studies using a recombinant vMCC-I protein indicate interference with the response of inflammatory cells to chemokines without being chemoattractant itself. Consistent with this, MCV lesions are typically devoid of infiltration of leucocytes (Gottlieb & Myskowski 1994).
So far four viral IL10 homologues have been identified. IL10, a classical anti-inflammatory cytokine, has been shown recently to convert proinflammatory signalling receptors to anti-inflammatory decoy receptors and therefore functions as a regulatory cytokine (Sozzani et al. 1998). HCMV encodes for a biological active IL10 (UL111a) which shows low sequence similarity to other vIL10 molecules (Kotenko et al. 2000). The BCRF1 gene of EBV exhibits 78% similarity with human IL10 (Hsu et al. 1990). This is potentially a very potent means of viral sabotage but in vivo evidence for its role is still sparse.
Cytokine binding proteins (vCkBPs)
There are other virally encoded proteins secreted in order to down modulate host immune responses. Viral infection of mammalian cells is accompanied by the induction of interferons, along with other cytokines (IL1, TNFα, IL6, IL8). Large DNA viruses often encode proteins whose function is to inactivate released cytokines or chemokines. The Molluscum contagiosum virus (MCV) described above encodes 2 genes, MC53L and MC54L, which have high homology with human IL-18 binding proteins, and which bind IL18 in vitro (Xiang & Moss 1999). Other viruses such as the ectromelia and cowxpox viruses also encode IL18 binding proteins (Calderara et al. 2001). All of these potentially interfere with a critical action of IL18 in pro-inflammatory immune responses.
Ironically there are many examples of host-encoded soluble cytokine receptors, products of alternate splicing (Mosley et al. 1989) or produced by cleavage from the cell surface (Nophar et al. 1990), whose purpose is believed to down‐regulate an active immune response as an infection is resolved or to extend the half life of a cytokine (Mohler et al. 1993). Viruses may well have simply exploited these mechanisms for their own advantage.
Conclusions
In this review we have explored the mechanisms of viral persistence in the face of host immunity based on a simple model. Some viruses follow the LCMV pattern and may escape through a combination of speed and shape-changing, whilst others may follow the MCMV/herpes family pattern and escape through camouflage or sabotage. The former is an attractive route for smaller, mutable genomes, while the latter is clearly exemplified by larger pathogens, which have stolen cDNAs from their hosts in many cases. (Both sets of viruses may also use ‘site’ to evade, persist and spread; Fig. 1). The method of acquisition of these cDNAs in human pathogens is not always clear, but generally, endogenous reverse transcriptases may play a role in this process. One might predict that the even larger pathogens (intracellular bacteria, mycobacteria and yeasts) will be found to exploit similar stealth strategies, although the evolution of their genes may be different.
One of the main issues in this area is defining which of the phenomena are biologically relevant. This relies on an understanding not just of the biochemical processes involved in antigen processing or T cell recognition, but also on the physiology and anatomy of complex host immune responses. It is very easy to identify in vitro phenomena that influence T cell recognition, but often it is very difficult to demonstrate that such phenomena play an important role in vivo. Partly this may be because some effects are simply quantitative advantages in viral survival, which only become manifest over long periods in the host. This differs from, for example, drug escape strategies, which result from very intense selective pressures. The effect of the local environment, e.g. within salivary glands on the fine balance between host and pathogen requires more investigation.
More work needs to be done using ex vivo immune responses in human disease, and relevant in vivo animal models to define the relevance of viral escape strategies and how they contribute to viral persistence. We can feel confident, however, that such work will reveal not only details of any given viral life cycle, but the biologically important functions of the antiviral immune response.
Acknowledgments
This work was sponsored by the EU (QLK2-CT-1999–00356), the Swiss Foundation for Biomedical Research (SSMBS) and the Wellcome Trust. We are grateful to Simone Korten, Annette Oxenius and Neil Young for their helpful comments and suggestions. We also thank Rodney Phillips for the support in the lab.
References
- Aebischer T, Moskophidis D, Rohrer UH, Zinkernagel RM, Hengartner H. In vitro selection of lymphocytic chorio-meningitis virus escape mutants by cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA. 1991;88:11047–11051. doi: 10.1073/pnas.88.24.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Ahn K, Gruhler A, Galocha B, Jones TR, Wiertz EJ, Ploegh HL, et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- Albini A, Ferrini S, Benelli R, et al. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althage A, Odermatt B, Moskophidis D, et al. Immunosuppression by LCMV infection: competent effector T and B cells but impaired antigen presentation. Eur J. Immunol. 1992;22:1803–1812. doi: 10.1002/eji.1830220720. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Kalinke U, Althage A, et al. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier DC, Cox JH, Vining DR, Cresswell P, Engelhard VH. Association of human class I MHC alleles with the adenovirus E3/19K protein. J. Immunol. 1994;152:3862–3872. [PubMed] [Google Scholar]
- Bennett EM, Bennink JR, Yewdell JW, Brodsky FM. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J. Immunol. 1999;162:5049–5052. [PubMed] [Google Scholar]
- Bertoletti A, Sette A, Chisari FV, et al. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Wei X, et al. Antiviral pressure exerted by HIV-1 specific CTL during primary infection demonstrated by rapid selection of CTL escape virus. Nature Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, et al. The immunological synapse. Annu. Rev. Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Calderara S, Xiang Y, Moss B. Orthopoxvirus IL-18 binding proteins: affinities and antagonist activities. Virology. 2001;279:22–26. doi: 10.1006/viro.2000.0689. 10.1006/viro.2000.0689. [DOI] [PubMed] [Google Scholar]
- Callan MF, Annels N, Steven N, et al. T cell selection during the evolution of CD8+ T cell memory in vivo. Eur J. Immunol. 1998;28:4382–4390. doi: 10.1002/(SICI)1521-4141(199812)28:12<4382::AID-IMMU4382>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1) -specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J. Exp. Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KM, Rehermann B, McHutchison JG, et al. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J. Clin. Invest. 1997;100:2376–2385. doi: 10.1172/JCI119778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea A, Hunziker L, Klenerman P, Hengartner H, Zinkernagel RM. Impairment of CD4 (+) T Cell Responses during Chronic Virus Infection Prevents Neutralizing Antibody Responses against Virus Escape Mutants. J. Exp. Med. 2001;193:297–306. doi: 10.1084/jem.193.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea A, Klenerman P, Hunziger L, Horvath E, Hengartner H, Zinkernagel R. Low level persistence of LCMV in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 1999;96:11964–11969. doi: 10.1073/pnas.96.21.11964. 10.1073/pnas.96.21.11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Devico AL, Garzino DA, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RTDMD, Mandelboim OBKC, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley AM. Functional roles of immediate early proteins encoded by the human cytomegalovirus UL36-38, UL115-119, TRS1/IRS1 and US3 loci. Intervirology. 1996;39:350–360. doi: 10.1159/000150506. [DOI] [PubMed] [Google Scholar]
- Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov NV. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–355. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- Cretney E, Degli-Esposti MA, Densley EH, Farrell HE, Davis-Poynter NJ, Smyth MJ. m144, a murine cytomegalovirus (MCMV) -encoded major histocompatibility complex class I homologue, confers tumor resistance to natural killer cell-mediated rejection. J. Exp. Med. 1999;190:435–444. doi: 10.1084/jem.190.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol. Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- Dzuris JL, Sidney J, Appella E, Chesnut RW, Watkins DI, Sette A. Conserved MHC class I peptide binding motif between humans and rhesus macaques. J. Immunol. 2000;164:283–291. doi: 10.4049/jimmunol.164.1.283. [DOI] [PubMed] [Google Scholar]
- Farci P, Shimoda A, Coiana A, et al. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- Farrell HE, Degli-Esposti MA, Davis-Poynter NJ. Cytomegalovirus evasion of natural killer cell responses. Immunol. Rev. 1999;168:187–197. doi: 10.1111/j.1600-065x.1999.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Farrell HE, Vally H, Lynch DM, et al. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature. 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Semino C, Spaggiari GM, Meta M, Mingari MC, Melioli G. Dendritic cells efficiently cross-prime HLA class I-restricted cytolytic T lymphocytes when pulsed with both apoptotic and necrotic cells but not with soluble cell-derived lysates. Int. Immunol. 2000;12:1741–1747. doi: 10.1093/intimm/12.12.1741. 10.1093/intimm/12.12.1741. [DOI] [PubMed] [Google Scholar]
- Ferrari C, Penna A, Bertoletti A, et al. Antiviral cell-mediated immune responses during hepatitis B and hepatitis C virus infections. Recent Results Cancer Res. 1998;154:330–336. doi: 10.1007/978-3-642-46870-4_24. [DOI] [PubMed] [Google Scholar]
- Fleming P. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J. Virol. 1999;73:6800–6809. doi: 10.1128/jvi.73.8.6800-6809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Otake K, Tashiro M, Adachi A. Human immunodeficiency virus type 1 Nef protein on the cell surface is cytocidal for human CD4+ T cells. FEBS Lett. 1996;393:105–108. doi: 10.1016/0014-5793(96)00862-9. 10.1016/0014-5793(96)00862-9. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Glitheroe A, Godkin A, et al. Induction and exhaustion of LCMV-specific CTL visualised using soluble tetrameric MHC-peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific. J. Virol. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith K, Chen W, Johnson DC, Hendricks RL. Infected cell protein (ICP) 47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J. Exp. Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb SL, Myskowski PL. Molluscum contagiosum. Int. J. Dermatol. 1994;33:453–461. doi: 10.1111/j.1365-4362.1994.tb02853.x. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Altfeld MA, Rosenberg ES, et al. Substantial differences in specificity of HIV-specific cytotoxic t cells in acute and chronic HIV infection. J. Exp. Med. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder P, Phillips R, Colbert R, et al. Late escape from an immunodominant CTL response associated with progression to AIDS. Nature Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- Grandea AG, III, Van Kaer L. Tapasin: an ER charperone that controls MHC class I assembly with peptide. Trends Immunol. 1991;22:194–199. doi: 10.1016/s1471-4906(01)01861-0. [DOI] [PubMed] [Google Scholar]
- Guidotti L, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- Harcourt GC, Garrard S, Davenport MP, Edwards A, Phillips RE. HIV-1 variation diminishes CD4 T lymphocyte recognition. J. Exp. Med. 1998;188:1785–1793. doi: 10.1084/jem.188.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise MT, Connick M, Virgin HW. Murine cytomegalovirus inhibits interferon gamma-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II-associated genes. J. Exp. Med. 1998a;187:1037–1046. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise MT, Pollock JL, O'Guin A, Barkon ML, Bormley S, Virgin HW. Murine cytomegalovirus infection inhibits IFN gamma-induced MHC class II expression on macrophages: the role of type I interferon. Virology. 1998b;241:331–344. doi: 10.1006/viro.1997.8969. [DOI] [PubMed] [Google Scholar]
- Hengel H, Reusch U, Gutermann A, et al. Cytomegaloviral control of MHC class I function in the mouse. Immunol. Rev. 1999;168:167–176. doi: 10.1111/j.1600-065x.1999.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Hewitt EW, Gupta SS, Lehner PJ. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. EMBO J. 2001;20:387–396. doi: 10.1093/emboj/20.3.387. 10.1093/emboj/20.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Jugovic P, York I, et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- Hsu DH, de Waal Malefyt R, Fiorentino DF, et al. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Jones S. Almost Like a Whale. London: Anchor (Transworld Publishers); 2000. [Google Scholar]
- Jones TR, Sun L. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 1997;71:2970–2979. doi: 10.1128/jvi.71.4.2970-2979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 1989;169:1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski UH. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J. Exp. Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kagi D, Seiler P, Pavlovic J, et al. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur. J. Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Kalams SA, Walker BD. Cytotoxic T lymphocytes and HIV-1 related neurologic disorders. Curr. Top Microbiol. Immunol. 1995;202:79–88. doi: 10.1007/978-3-642-79657-9_6. [DOI] [PubMed] [Google Scholar]
- Kalinke U, Bucher EM, Ernst B, et al. The role of somatic mutation in the generation of the protective humoral immune response against vesicular stomatitis virus. Immunity. 1996;5:639–652. doi: 10.1016/s1074-7613(00)80277-0. [DOI] [PubMed] [Google Scholar]
- Kapasi K, Rice GP. Cytomegalovirus infection of peripheral blood mononuclear cells: effects on interleukin-1 and – 2 production and responsiveness. J. Virol. 1988;62:3603–3607. doi: 10.1128/jvi.62.10.3603-3607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen MF, Huppa JB, Lucin P. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 1997;16:685–694. doi: 10.1093/emboj/16.4.685. 10.1093/emboj/16.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Meier UC, Phillips RE, McMichael AJ. The effects of natural altered peptide ligands on the whole blood cytotoxic T lymphocyte response to human immunodeficiency virus. European J. Immunol. 1995;25:1927–1931. doi: 10.1002/eji.1830250720. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Rowland-Jones S, McAdam S, et al. Naturally ocurring HIV-1 gag variants antagonise cytotoxic T-cell activity. Nature. 1994;369:403–406. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Zinkernagel R. What can we learn about HIV infection from a study of LCMV? Immunol. Rev. 1997;159:5–16. doi: 10.1111/j.1600-065x.1997.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Zinkernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:482–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc. Natl. Acad. Sci. USA. 2000;97:1695–1700. doi: 10.1073/pnas.97.4.1695. 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotic A, Messerle M, Crnkovic-Mertens I, Polic B, Jonjic S, Koszinowski UH. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J. Exp. Med. 1999;190:1285–1296. doi: 10.1084/jem.190.9.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz SK, Reddehase MJ. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J. Virol. 1999;73:8612–8622. doi: 10.1128/jvi.73.10.8612-8622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani AS, Barrett JW, McFadden G. Modulating chemokines: more lessons from viruses. Immunol. Today Rev. 2000;21:100–106. doi: 10.1016/s0167-5699(99)01556-x. [DOI] [PubMed] [Google Scholar]
- Lanier LL. On guard – activating NK cell receptors. Nat Immunol. 2001;2:23–27. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]
- Lechner F, Gruener NH, Urbani S, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J. Immunol. 2000a;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lechner F, Sullivan J, Spiegel H, et al. Why do cytotoxic T lymphocytes fail to eliminate HCV? lessons from studies using MHC class I tetramers. Phil. Trans. R. Soc. Lond. B. 2000b;355:1085–1092. doi: 10.1098/rstb.2000.0646. 10.1098/rstb.2000.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 2000c;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner F, Vargas Cuero AL, Kantzanou M, Klenerman P. Studies of human antiviral CD8+ lymphocytes using class I peptide tetramers. Rev. Med. Virol. 2001;11:11–22. doi: 10.1002/rmv.295. 10.1002/rmv.295. [DOI] [PubMed] [Google Scholar]
- Levitskaya J, Coram M, Levitsky V, et al. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- Levitsky V, Zhang QJ, Levitskaya J, Kurilla MG, Masucci MG. Natural variants of the immunodominant HLA A11-restricted CTL epitope of the EBV nuclear antigen-4 are nonimmunogenic due to intracellular dissociation from MHC class I: peptide complexes. J. Immunol. 1997;159:5383–5390. [PubMed] [Google Scholar]
- Lin CL, Sewell AK, Gao GF, Whelan KT, Phillips RE, Austyn JM. Macrophage-tropic HIV Induces and Exploits Dendritic Cell Chemotaxis. J. Exp. Med. 2000;192:587–594. doi: 10.1084/jem.192.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littaua RA, Oldstone MB, Takeda A, et al. An HLA-C-restricted CD8+ cytotoxic T-lymphocyte clone recognizes a highly conserved epitope on human immunodeficiency virus type 1 gag. J. Virol. 1991;65:4051–4056. doi: 10.1128/jvi.65.8.4051-4056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski UH. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttichau HR, Stine J, Boesen TP, et al. A highly selective CC chemokine receptor (CCR) 8 antagonist encoded by the poxvirus molluscum contagiosum. J. Exp. Med. 2000;191:171–180. doi: 10.1084/jem.191.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht U, Deng C, Lu MC, et al. Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence. J. Immunol. 2000;165:6437–6446. doi: 10.4049/jimmunol.165.11.6437. [DOI] [PubMed] [Google Scholar]
- McAdam SN, Klenerman P, Tussey L, et al. Immunogenic HIV variants that bind to HLA-B8 but fail to stimulate CTL responses. J. Immunol. 1995;155:2729–2736. [PubMed] [Google Scholar]
- Mocarski ES. Cytomegaloviruses and Their Replication. 3. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- Mohler KM, Torrance DS, Smith CA, et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J. Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Hengartner H, Zinkernagel RM. MHC class I and non-MHC-linked capacity for generating an anti-viral CTL response determines susceptibility to CTL exhaustion and establishment of virus persistence in mice. J. Immunol. 1994;152:4976–4983. [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Mosley B, Beckmann MP, March CJ, et al. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989;59:335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Nophar Y, Kemper O, Brakebusch C, et al. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R., cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J. 1990;9:3269–3278. doi: 10.1002/j.1460-2075.1990.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein A, Klenerman P, Ludewig B, Pericin M, Hengartner H, Zinkernagel R. Immune surveillance against a solid tumor fails due to immunological ignorance. Proc. Natl. Acad. Sci. USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg G, Jin X, Bonhoeffer S, et al. Quantitation of HIV-1 specific CTL and plasma load of HIV-1. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Demarest JF, Soudeyns H, et al. Major expansion of CD8+ T cells with a predominant Vb usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- Penfold ME, Dairaghi DJ, Duke GM, et al. Cytomegalovirus encodes a potent alpha chemokine. Proc. Natl. Acad. Sci. USA. 1999;96:9839–9844. doi: 10.1073/pnas.96.17.9839. 10.1073/pnas.96.17.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RE, Rowland-Jones SL, Nixon DF, et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- Pichoud C, Berby F, Stuyver L, Petit MA, Trepo C, Zoulim F. Persistence of viral replication after anti-HBe seroconversion during antiviral therapy for chronic hepatitis B. J. Hepatol. 2000;32:307–316. doi: 10.1016/s0168-8278(00)80077-x. [DOI] [PubMed] [Google Scholar]
- Pircher H, Moskophidis D, Rohrer U, Burki K, Hengartner H, Zinkernagel RM. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Planz O, Ehl S, Furrer E, et al. A critical role for neutralising antibody-producing B cells, CD4+ cells and interferons in persistent infections of mice with LCMV: implications for adoptive immunotherapy of virus carriers. Proc. Natl. Acad. Sci. USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polic B, Hengel H, Krmpotic A, et al. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Goulder PJ, Klenerman P, et al. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee HG, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- Rasola A, Gramaglia D, Boccaccio C, Comoglio PM. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 2001;166:81–88. doi: 10.4049/jimmunol.166.1.81. [DOI] [PubMed] [Google Scholar]
- Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu. Rev. Immunol. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J. Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath S, Angulo A, Gascoigne NR, Ghazal P. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 1999;162:6701–6707. [PubMed] [Google Scholar]
- Reusch U, Muranyi W, Lucin P, Burgert HG, Hengel H, Koszinowski UH. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999;18:1081–1091. doi: 10.1093/emboj/18.4.1081. 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Saederup N, Lin YC, Dairaghi DJ, Schall TJ, Mocarski ES. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc. Natl. Acad. Sci. USA. 1999;96:10881–10886. doi: 10.1073/pnas.96.19.10881. 10.1073/pnas.96.19.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Seiler P, Senn B, Klenerman P, Kalinke U, Hengartner H, Zinkernagel R. Additive effect of neutralizing antibody and antiviral drug treatment in preventing virus escape and persistence. J. Virol. 2000;74:5896–5901. doi: 10.1128/jvi.74.13.5896-5901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellam GR, Allan JE, Papadimitriou JM, Bancroft GJ. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc. Natl. Acad. Sci. USA. 1981;78:5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S, Bonecchi R, D'Amico G, et al. Old and new chemokines. Pharmacological regulation of chemokine production and receptor expression: mini-review. J. Chemother. 1998;10:142–145. doi: 10.1179/joc.1998.10.2.142. [DOI] [PubMed] [Google Scholar]
- Spriggs MK, Armitage RJ, Comeau MR, et al. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR beta chain and inhibits antigen presentation. J. Virol. 1996;70:5557–5563. doi: 10.1128/jvi.70.8.5557-5563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subklewe M, Paludan C, Tsang M, Mahnke K, Steinman R, Munz C. Dendritic cells cross-present latency gene products from Epstein-Barr virus-transformed b cells and expand tumor-reactive CD8(+) killer T cells. J. Exp. Med. 2001;193:405–412. doi: 10.1084/jem.193.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki A, Wiese M, Maertens G, et al. Cellular immune response persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nature Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- Tomazin R, Boname J, Hegde NR, et al. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- Tomazin R, Hill AB, Jugovic P, et al. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- Townsend A, Bodmer H. Antigen recognition by class I -restricted cytotoxic T lymphocytes. Ann. Rev. Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MF, Sporri R, Even C, et al. Lactate dehydrogenase-elevating virus (LDV): lifelong coexistence of virus and LDV-specific immunity. J. Immunol. 1997;159:1585–1588. [PubMed] [Google Scholar]
- Vogel TU, Allen TM, Altman JD, Watkins DI. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 2001;75:2458–2461. doi: 10.1128/JVI.75.5.2458-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Moss B. IL-18 binding and inhibition of interferon gamma induction by human poxvirus-encoded proteins. Proc. Natl. Acad. Sci. USA. 1999;96:11537–11542. doi: 10.1073/pnas.96.20.11537. 10.1073/pnas.96.20.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Henry SC, Xu F, Hamilton JD. Expression of a murine cytomegalovirus early-late protein in ‘latently’ infected mice. J. Infect Dis. 1995;172:371–379. doi: 10.1093/infdis/172.2.371. [DOI] [PubMed] [Google Scholar]
- Zajac A, Blattman J, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H, Thale R, Lucin P, et al. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 1997;6:57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R, Bachmann M, Oehen S, Kundig T, Pircher H, Hengartner H. On Immunological Memory. Ann. Rev. Immunol. 1996;14:333–68. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]