Abstract
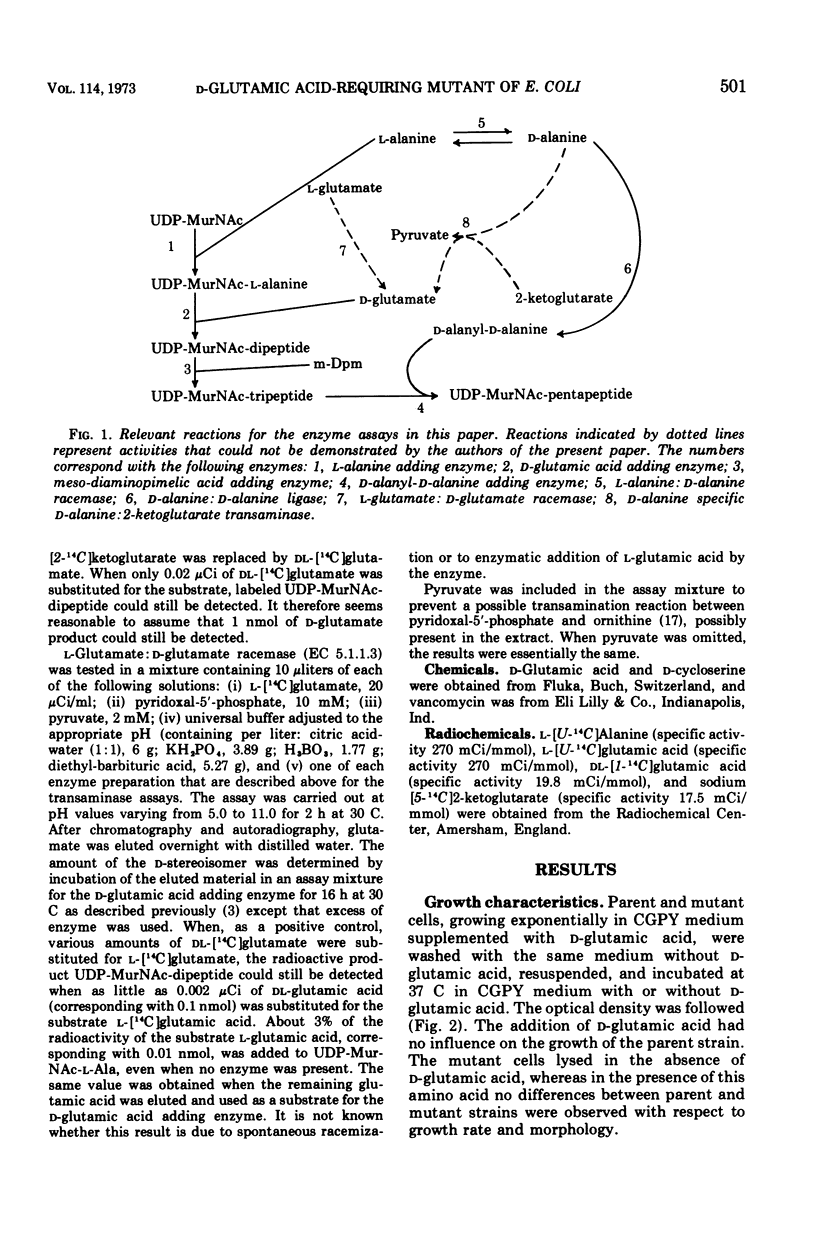
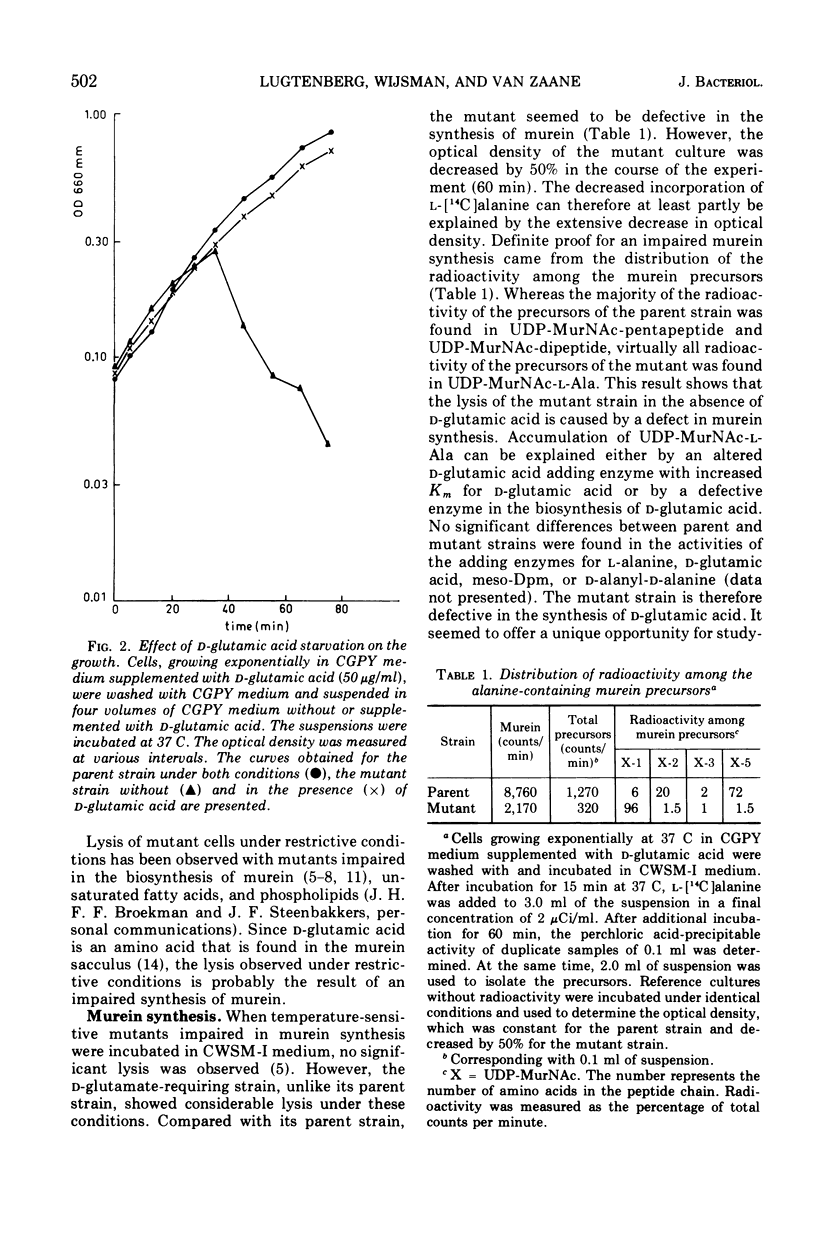
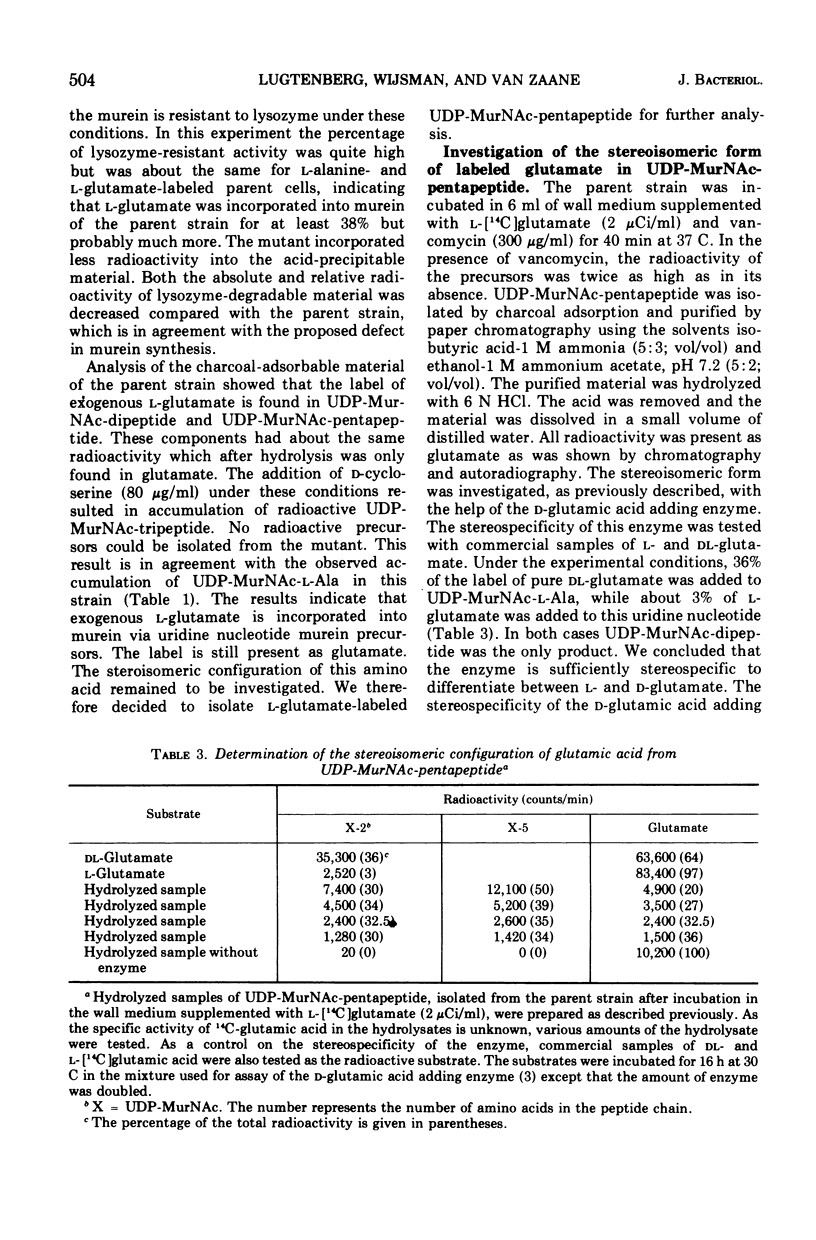
Some properties of a d-glutamic acid auxotroph of Escherichia coli B were studied. The mutant cells lysed in the absence of d-glutamic acid. Murein synthesis was impaired, accompanied by accumulation of uridine-5′-diphosphate-N-acetyl-muramyl-l-alanine (UDP-MurNac-l-Ala), as was shown by incubation of the mutant cells in a cell wall medium containing l-[14C]alanine. After incubation of the parental strain in a cell wall medium containing l-[14C]glutamic acid, the acid-precipitable radioactivity was lysozyme degradable to a large extent. Radioactive UDP-MurNac-pentapeptide was isolated from the l-[14C]glutamic acid-labeled parental cells. After hydrolysis, the label was exclusively present in glutamic acid, the majority of which had the stereo-isomeric d-configuration. Compared to the parent the mutant incorporated less l-[14C]glutamic acid from the wall medium into acid-precipitable material. Lysozyme degraded a smaller percentage of the acid-precipitable material of the mutant than of that of the parent. No radioactive uridine nucleotide precursors could be isolated from the mutant under these conditions. Attempts to identify the enzymatic defect in this mutant were not successful. The activity of UDP-MurNac-l-Ala:d-glutamic acid ligase (ADP; EC 6.3.2.9) (d-glutamic acid adding enzyme) is not affected by the mutation. Possible pathways for d-glutamic acid biosynthesis in E. coli B are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COTA-ROBLES E. H., DUNCAN P. H. The effect of D-glutamic acid upon spheroplast formation in Escherichia coli B. Exp Cell Res. 1962 Nov;28:342–349. doi: 10.1016/0014-4827(62)90288-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J. Studies on Escherichia coli enzymes involved in the synthesis of uridine diphosphate-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1972 Apr;110(1):26–34. doi: 10.1128/jb.110.1.26-34.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., de Haan P. G. A simple method for following the fate of alanine-containing components in murein synthesis in Escherichia coli. Antonie Van Leeuwenhoek. 1971;37(4):537–552. doi: 10.1007/BF02218524. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutant of Escherichia coli K-12 with an impaired D-alanine:D-alanine ligase. J Bacteriol. 1973 Jan;113(1):96–104. doi: 10.1128/jb.113.1.96-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activities of the L-alanine adding enzyme and the D-alanyl-D-alanine adding enzyme. J Bacteriol. 1972 Apr;110(1):35–40. doi: 10.1128/jb.110.1.35-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activity of the diaminopimelic acid adding enzyme. J Bacteriol. 1972 Apr;110(1):41–46. doi: 10.1128/jb.110.1.41-46.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A., van Bellegem T. H. In vivo and in vitro action of new antibiotics interfering with the utilization of N-acetyl-glucosamine-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1971 Oct;108(1):20–29. doi: 10.1128/jb.108.1.20-29.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Carrion M., Jenkins W. T. D-Alanine-D-glutamate transaminase. I. Purification and characterization. J Biol Chem. 1965 Sep;240(9):3538–3546. [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- NATHENSON S. G., STROMINGER J. L., ITO E. ENZYMATIC SYNTHESIS OF THE PEPTIDE IN BACTERIAL URIDINE NUCLEOTIDES. IV. PURIFICATION AND PROPERTIES OF D-GLUTAMIC ACID-ADDING ENZYME. J Biol Chem. 1964 Jun;239:1773–1776. [PubMed] [Google Scholar]
- Venkateswaran P. S., Wu H. C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wijsman H. J. A genetic map of several mutations affecting the mucopeptide layer of Escherichia coli. Genet Res. 1972 Aug;20(1):65–74. doi: 10.1017/s0016672300013598. [DOI] [PubMed] [Google Scholar]
- Wijsman H. J. The characterization of an alanine racemase mutant of Escherichia coli. Genet Res. 1972 Dec;20(3):269–277. doi: 10.1017/s001667230001380x. [DOI] [PubMed] [Google Scholar]
- Yorifuji T., Misono H., Soda K. Arginine racemase of Pseudomonas graveolens. II. Racemization and transamination of ornithine catalyzed by arginine racemase. J Biol Chem. 1971 Aug 25;246(16):5093–5101. [PubMed] [Google Scholar]


