Abstract
Individuals differ in their social status and societies in the extent of social status differences among their members. There is great interest in understanding the key factors that contribute to the establishment of social dominance structures. Given that stress can affect behavior and cognition, we hypothesized that, given equal opportunities to become either dominant or submissive, stress experienced by one of the individuals during their first encounter would determine the long-term establishment of a social hierarchy by acting as a two-stage rocket: (1) by influencing the rank achieved after a social encounter and (2) by facilitating and/or promoting a long-term memory for the specific hierarchy. Using a novel model for the assessment of long-term dominance hierarchies in rats, we present here the first evidence supporting such hypothesis. In control conditions, the social rank established through a first interaction and food competition test between two male rats is not maintained when animals are confronted 1 week later. However, if one of the rats is stressed just before their first encounter, the dominance hierarchy developed on day 1 is still clearly observed 1 week later, with the stressed animal becoming submissive (i.e., looser in competition tests) in both social interactions. Our findings also allow us to propose that stress potentiates a hierarchy-linked recognition memory between “specific” individuals through mechanisms that involve de novo protein synthesis. These results implicate stress among the key mechanisms contributing to create social imbalance and highlight memory mechanisms as key mediators of stress-induced long-term establishment of social rank.
Keywords: social recognition, learning, memory, protein synthesis, social status, dominance hierarchy, rat
Introduction
Individuals differ in their social status and societies in the extent of social status differences among their members. Strong evidence indicates that peer status in humans is related to both general and mental health: the lower the status position, the more common health problems are. In different animal species, rank has also been shown to influence physiology, behavior, the ability to produce offspring, and health (Bartolomucci, 2005, 2007; Beehner et al., 2005; Clutton-Brock et al., 2006; Raleigh et al., 1991; Sapolsky, 2005; Sapolsky and Share, 1994; Tamashiro et al., 2005). Moreover, at the population level, societies with large disparities in the (socioeconomic) status of their individuals (i.e., strongly hierarchical societies) appear to have more malaise and shorter longevity than more egalitarian societies, that tend to be healthier (Ötsberg, 2003; Wilkinson, 1999; but see Wilkinson and Pickett, 2006).Although the organization of individuals in dominance hierarchies (arrangement of group members into a priority order to get access to available resources) occurs readily in many species both in nature and under experimental conditions (from some insects and crustaceans, to different fish, birds, and mammals including humans), the determinants are not fully understood. Frequently, social hierarchies are stable over long periods of time and the insertion of new individuals falls into pre-determined positions. However, there are also many circumstances in which individuals meet for the first time without a pre-established hierarchical order but, eventually, a dominance order is achieved. In the wild, animals are frequently exposed to encounters with new conspecifics (Broom, 2002). Likewise, examples of situations in which humans join new groups of people are multiple, including children starting at school or adults joining a new workplace or a leisure group. Given that once established, hierarchies tend to be quite stable (Broom, 2002), the outcome of an initial encounter between two or more individuals can have important consequences for future behavioral interactions and fitness.
Classically, the factors recognized to influence the organization of individuals that meet for the first time in a dominance structure are classified into two categories: the so called (i) “intrinsic” factors or traits, such as sex, age, and in animals notably size, that predispose individuals into “pre-determined” positions, and (ii) “extrinsic” factors, which generally refer to animals' former social experience. In many species, the hierarchy is established through aggressive contest. Whereas the impact of attributes from the former category, such as body weight or size in the outcome of aggressive encounters seems to be straightforward and does not receive much attention, the importance of previous experience, as manifested by winner, loser and bystander effects concentrates a large number of both experimental and modeling studies. However, dominance hierarchy formation appears to be a much richer and more complex phenomenon than previously thought (Chase et al., 2002) which lead us to hypothesize that other factors, such as stress, for example- acting at the time of the first encounter/s might have an important impact on determining the establishment of a hierarchy.
Although stress is recognized to be both an important “negative” consequence of inequality in social interactions and a main mediator of the wide range of health problems derived from social inequity (Sapolsky, 2005), the contribution of stress to create social imbalance is surprisingly largely overlooked in current models of dominance hierarchy formation. This occurs despite some suggestions that stress exposure can influence dominance–submissive relationships (Gray, 1987; Mikics et al., 2004). Since stress is a potent modulator of cognitive function and memory mechanisms (de Kloet et al., 1999; Joels, 2006; Kim and Diamond, 2002; McGaugh and Roozendaal, 2002; Roozendaal, 1999; Roozendaal et al., 2006; Sandi, 1998, 2004; Shors, 2006), we reasoned that stress can have important and long-lasting influences on social relationships by amplifying the memory for the social status that is established after a first encounter between conspecifics. Therefore, we hypothesized that stress can affect the formation of uneven societies by acting as a two-stage rocket: (1) by affecting the outcome of social interactions and (2) by promoting a long-term memory about the achieved status within a specific social framework. Specifically, here we tested the hypothesis that, given equal circumstances and opportunities to become either dominant or submissive (which would require confronting individuals equivalent for key “intrinsic” and “extrinsic” factors; see above), stress experienced by one of the individuals at the moment of a first social encounter would determine the long-term establishment of a social hierarchy by (1) influencing the rank achieved after a social encounter and (2) promoting a long-term memory for the specific hierarchy.
To test these hypotheses, we set up an experimental procedure in which pairs of male Wistar rats, matched for both their body weight and anxiety-like behavior in an elevated plus maze (EPM) are firstly confronted on day 1 and then again 1 week later (day 8). Under control conditions, the dominance–submission hierarchy that becomes evident during interaction on day 1 is not maintained in a water competition test (WCT) performed 1 week later. However, if one of the rats in the pair is submitted to stress (a contextual fear conditioning session) just before being exposed to the first social encounter, the dominance hierarchy developed on day 1 (the stressed animal becomes the submissive) is still clearly observed when the same animals are submitted to a WCT 1 week later. We also show that this effect of stress is (i) specific in potentiating a hierarchy-linked recognition memory for “a particular individual,” and (ii) involves de novo protein synthesis during the consolidation period occurring after the first social encounter.
Materials and Methods
Subjects
Male Wistar rats (Charles River Laboratories, Lyon, France), weighing 350 g at the beginning of the experiments, were housed either isolated or in groups of 3 per cage (c.f. specific experiment). They were maintained under light (12 hours light/dark cycle; lights on at 7:00 AM) and temperature (22 ± 2 °C) controlled conditions. Food and water were available ad libitum. Animals were weighed weekly. Animal care procedures were approved through a license issued by the Cantonal Veterinary Authorities (Vaud, Switzerland).
All experiments were conducted between 9:00 and 14:00 h, except the “WCT” that started in the afternoon, after a brief water deprivation period of 6 hours (from 8:00 to 14:00 hours) and always finished to before the dark cycle started. Rats were handled and marked during the 3 days before social interactions started. “Color-coded” fur-marks positioned in different body parts (head, shoulder, or back) helped identifying animals during social interactions. These marks had to be re-applied the days before the competition tests took place.
EPM
Anxiety-related behavior was evaluated using the plus-maze test (Herrero et al., 2006). The plus-maze consists of two opposing open arms (45 × 10 cm2) and two enclosed arms (45 × 10 × 50 cm3) that extend from a central platform (10 × 10 cm2), elevated 65 cm above the floor. The rats were placed individually on the central platform facing the same enclosed arm and were allowed to freely explore the maze for 5 minutes. The behavior of each rat was monitored using a video camera, and the movements of the rats were automatically registered and analyzed with a computerized tracking system (Ethovision 3.1.16, Noldus IT, The Netherlands). The time spent in the open and closed arms, as well as the number of times the animal entered each type of arm and the latency before entering an open arm were recorded.
Allocation of animals to dyads and experimental conditions
To distribute animals in pairs (or dyads) for the social interaction procedures, they were matched in each experiment according to their body weight, anxiety level (as defined by their percent time spent in the open arms in the EPM), and average number of chocopop flakes consumed during the habituation phase. In one experiment in which rats were housed in groups of 3, their rank status in their respective homecages was also considered for the matching. In such experiment, triads were tested twice for their dominancy in the homecage during the week before the actual experiment started. On two different days (with a 72 hours interval), group-caged rats were water-deprived for 6 hours (9:00 till 15:00 hours) and subsequently submitted (all three animals simultaneously) to a WCT in the same homecage and colony room (hence, this test clearly differs from the WCT used to evaluate memory for the social hierarchy). Rats with the highest ratio in time devoted to water consumption were identified as alpha, those with the lowest ratio as omega, and the rats in between both types as beta. Subsequent confrontations to evaluate the impact of stress on social memory were done between unfamiliar animals with equivalent hierarchy status in the homecage (i.e., alpha male vs. alpha male, etc). Once the social interaction experiments started on day 1 with the first social encounter followed by the food competition test, all animals were from then on kept isolated, with all experimental procedures being the same as in previous experiments.
Therefore, animals in each pair were considered “equivalent” in their probability to become either dominant or subordinate when mutually submitted to an encounter. Depending on the goal of each experiment, dyads were randomly assigned to control (non-stress pairs; Pns) or stress (stress pairs; Ps) conditions. In the Pns condition, rats were directly confronted without any prior manipulation: none of the rats (Rns) in the pair were stressed. In the Ps condition, one rat (Rs) in each pair was exposed to stress immediately before the first social encounter, whereas the other rat (Rns) in the pair was not stressed.
Stress delivery – the contextual fear conditioning task
A key experimental manipulation in this study is the administration of stress to rats allocated to the “stress” condition (i.e., Rs rats from the Ps dyad). This consisted on the administration of 3 electric footshocks in a confined chamber, using a protocol that is classically used to induce contextual fear conditioning in rodents. We selected this procedure because it provides the possibility of testing potential effects of treatments (such as the pharmacological approach used in the current study) on the the memory of the stressor itself (for example, by measuring the behavioral responses to the exposure to the context in subsequent tests for assessing the stability of the contextual fear memory).
More precisely, stress was delivered in a rodent observation chamber (30 × 37 × 25 cm3; Panlab, Spain) that was positioned inside a sound-attenuating chamber, illuminated by a 20 W bulb. Ventilation fans provided a background noise of 68 dB. Rats in the “stress” condition were individually placed in this chamber that was constructed of black stainless steel walls of smooth texture, with ceiling and door made of Plexiglas, and the floor consisting of 20 steel rods wired to a shock source and solid-state scrambler for the delivery of footshock. After 3 minutes, the rats received three 1-second footshocks of 1 mA intensity. The inter-shock interval was 60 seconds, and the rats were removed from the chambers 30 s after the final shock presentation (thus, a stress/conditioning session lasted approximately 330 seconds). Each observation cage was cleaned with a 1% acetic acid solution before and after each session. Immediately after the stress session, rats were transported to an adjacent room for the “Food Competition Test.”
As indicated above, animals were tested for their contextual fear memories after all social interaction procedures had been performed (i.e., this retrieval test was generally carried out 8 days after conditioning). Rats were placed back into the chamber where they had previously received shock (this time in the absence of shock) for an 8-minutes context test. A video-camera recorded their behavior. Subsequently, the time spent by each rat either freezing or active was scored blindly by an experimenter assisted by a computer program (The Observer 5.0.25, Noldus, 2003). Freezing was defined as behavioral immobility except for movement needed for respiration.
Testing for generalization of freezing behavior was performed 2 hours after the Fear Conditioning Test. Rats were placed for 5 minutes into a new chamber (context B; 30 × 22 × 30 cm3) with carton walls and a black plastic floor, and their behavior was recorded and analyzed as described above.
Food competition test: establishment of a social hierarchy
In order to habituate animals to the rewarding food used in the “Food Competition Test” (FCT), they received eight Chocopop flakes (Kellogg's, Switzerland) in their homecages daily, during three consecutive days. Following a period of 2 resting days during which they were left undisturbed, every rat was individually habituated on the three following days to both the “food competition box” and to the room (where the box was located) where the social interaction test would later take place. During these habituation procedures, each rat was located in the testing room, firstly in a new homecage (cleaned sawdust; no food or water) for 20 minutes and then, on the following 10 minutes, in the food competition box. This box (60 × 40 × 40 cm3) has a feeder in the middle of one of the walls. During each habituation day, the feeder contained eight Chocopop flakes and the number of flakes consumed by each rat was recorded.
On the day following this habituation period, pairs of animals unfamiliar to each other were confronted for the first time. On a first phase (pre-FCT), each pair of rats was submitted to a 20-minute interaction in a new homecage located in the testing room. The second phase started immediately afterwards and was the FCT proper. This consisted of a 10-minute interaction in the food competition box that for each pair had eight Chocopop flakes in the feeder.
Each encounter (both, during the pre-FCT and the FCT sessions) was video-recorded and scored using “The Observer 5.0.25” (Noldus, 2003) software for collection and analysis of observational data. Observations were carried out blindly by two independent and intensively trained observers who showed a degree of agreement above 93% in their respective scores. During the encounters, the following behavioral categories were monitored: (i) “offensive behavior,” including total frequencies of the following behaviors: attacks, chasing, bites, “knocking-down” (behavior in which the animal walks or stands on top of its opponent, which is usually laying down on its back), displacements (one rat pushes and takes the place of the other) and (ii) “defensive behavior,” including a characteristic freezing for this interactions (i.e., immobility with the head orientated towards the opponent). The status in social hierarchy (dominance–subordination relationship) of rats in each pair was established on the basis of their respective percentage of offensive and defensive behaviors. Additionally, other behaviors were also scored in the FCT: total flakes consumed and frequency of passes over the feeder. For statistical analyses, the behavioral observation period of 20 minutes social interaction before FCT was split up in four periods of 5 min.
After these consecutive encounters on day 1, rats were returned to their homecages and remained undisturbed until they were submitted to the WCT that took place 7 days afterwards (day 8).
WCT: assessment of long-term maintenance of a social hierarchy
In order to assess whether animals keep a “long-term memory” for the hierarchy established with a particular individual during the first encounter, hierarchical behavior within each of the pairs was again evaluated 7 days after the FCT. The evaluation was performed by means of a “WCT.” The main reason to change the modality of reward on the competition test from “food” to “water” was to avoid the re-establishment of a hierarchy on this second test based on a memory for the “test” and/or “reward modality,” given that we were interested in specifically testing memory for the “individual.”
On the day of the WCT, rats were deprived of water during the 6 hours prior the test. Then, each pair of rats was confronted in a new homecage in the testing room and allowed to interact briefly. After 2 minutes, a 10-minute WCT was given by placing a bottle of water in the feeder holder. Behavior was video-recorded and scored blindly using “The Observer 5.0.25” (Noldus, 2003) software (see FCT above). The main readouts of this test were the frequency, latency, and duration of water consumption for each rat within each dyad. A large literature illustrates that the animal in the dyad that drinks more is the dominant rat; the other one being consequently the submissive (Baenninger, 1970; Drew and Dickey, 1977; Lucion and Vogel, 1994). In addition, we also evaluated the same behaviors described above for the FCT (except those specifically related to pellets eaten and passes over the feeder).
Protein synthesis inhibition
In the last experiment, we aimed to inhibit protein synthesis inhibition to test its impact on the establishment of social memory. For this purpose, we used the protein synthesis inhibitor anisomycin (Sigma Chemical Co., Switzerland). Anisomycin was dissolved in saline by adding 1 N HCl. The pH was adjusted to 7.4 by adding NaOH. Rats were injected immediately after completion of the FCT either with the anisomycin (150 mg/kg of body weight, i.p.) or with vehicle. This dose of anisomycin was selected based on previous studies that showed its effectiveness at inhibiting approximately 90% of cerebral protein synthesis 15–45 minutes after injection (Davis and Squire, 1984) and at impairing different types of memory, including emotional memory (Bourtchouladze et al., 1998) and social recognition for a conspecific juvenile (Kogan et al., 2000).
Statistics
Since hierarchy-related differences in social behavior between animals in each pair take some time to develop, dominant–submissive behaviors of the first interaction in the new homecage were computed in four blocks of either 5 or 10 minutes. Dominance level values were calculated as the ratio between contestants' scoring in each confronted pair on (i) frequency of passes over feeding during the FCT (day 1; see Results section for the validation of this performance measure as an index) and (ii) percent time consuming water during the WCT (day 8). Within-group comparisons over time on behavioral data were analyzed with paired non-parametric statistics, the Wilcoxon signed ranks test. Hierarchy status achieved in each competition test and comparisons of hierarchy status between different tests and experimental conditions were analyzed with Chi-square analyses (Pearson chi-square and Fisher's exact test, two-tailed). All Data are expressed as mean ± standard error of the mean (SEM). Statistical significance (two-tailed) was set at p ≤ 0.05. The computer software SPSS® was used for all statistical analyses.
Results
Validation of hierarchy measures
Firstly, we aimed to identify and validate an easily measurable parameter of rats' behavior during the FCT, which would reflect the hierarchy status of the animals throughout the two social encounter tests on day 1 (i.e., interaction in a new homecage and FCT). Among various correlations observed between performance parameters obtained in the first and second social encounters on day 1 (data not shown), we found an interesting correlation that served for the purposes of this analysis: a positive correlation (n = 30; r = 0.66, p < 0.01) between an integrated measure of dominance behavior (i.e., the “percentage of displacement and knocking-down”) during the block interval 10–20 minutes of the first social interaction and the “percentage of passes over feeder.” Offensive behavior showed a positive correlation with the “percentage of Chocopop flakes consumed” (r = 0.35, p < 0.05). Furthermore, we found a relevant correlation between “percentage of offensive behavior” and “percentage of passes over feeder” (r = 0.52, p < 0.003). The higher the percentage of offensive behavior shown by animals in the first encounter, the higher the percentage of passes over feeder they displayed on the FCT. This correlation indicates that the percentage of times animals in each pair pass over the feeder in the FCT represents a good index of the role each animal develops in the social hierarchy established on day 1. Although the parameter related to the number of pellets consumed also showed relevant correlations with other behavioral indexes of animals' dominancy during physical interactions, the use of this parameter as an index of dominancy was avoided given that the goal of the study was to assess stress effects and stress is well known to affect feeding behavior. Hence, the “percentage of passes over feeder” (or marking) was the behavioral variable selected for further validation.
Passes over the feeder is a behavioral pattern that belong to what is known as “marking” behavior. Marking is observed in many mammals. Rats use anogenital drag to deposit an odor whose scent conveys substantial information about the marking animal. Aggressive males “mark” more than subordinate males, and females prefer the odors of aggressive males. Scent-marking is particularly associated with dominance in male–male interactions in many rodent species (Albers and Ferris, 1986; Bamshad and Albers, 1996; Ferris et al., 1985, 1988, 1993). Furthermore, rats injected with testosterone showed a significant increase in scent-marking and aggression in the opponent's homecage, while administration of an androgen receptor blocker significantly inhibited scent marking (Vagell and McGinnis, 1998).
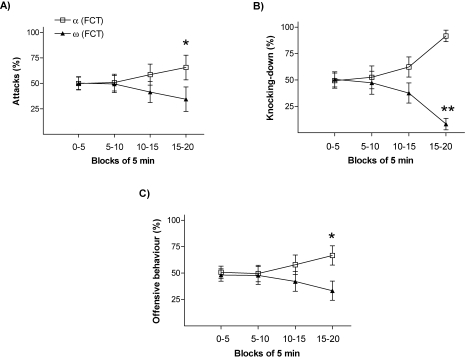
In order to verify the validity of using the “percentage of passes over feeder” during the FCT as an index to categorize animals according to their dominant–submissive status after day 1 interactions, we firstly classified animals in each pair as either “dominant” (α-FCT; the rat with the highest frequency of passes over feeder) or “submissive” (ω-FCT; the rat with the lowest frequency of passes over feeder) and then analyzed their temporal dynamic interactions in a number of agonistic–defensive behaviors displayed during the first social encounter. As shown in Figure 1, the frequency of offensive and defensive behaviors displayed throughout the 20 minutes test between the two rats in the pair evolved over time. During the last 5 minutes block (15–20 minutes), dominant rats (α-FCT) showed significantly higher percentage number of (i) attacks (Figure 1A; p < 0.05), (ii) knocking-downs (Figure 1B; p < 0.01), and (iii) more globally, of the integrated measure including all “offensive behaviors” (Figure 1C; p < 0.05).
Figure 1.
Behavior progression during the 20 minutes of social interaction (pre-FCT) represented in blocks of interval of 5 minutes. The graphs represent rates between opponents of frequency of (A) attacks, (B) knocking-down, and (C) offensive behavior. * p < 0.05, ** p < 0.01.
This validation of the “percentage of passes over feeder” during the FCT as an index to categorize animals' dominant–submissive status on day 1 presents advantages over other dominance/submission measures based on observation of animals' interactions and body postures subjectively judged by human referees. Endpoints based on competition for priority of access to specific resources [similar to the one selected in our study, such as time spent on a feeder (Malatynska et al., 2002; Malatynska and Kostowski, 1984) or number of sucrose pellets consumed (Gentsch et al., 1988)] have been shown to be reliable and convenient for fast and objective automated behavioral analysis.
Impact of stress on the long-term establishment of a social hierarchy between two individuals
To explore whether given equal opportunities to become either dominant or subordinate during a first encounter among two conspecific male rats, stress experienced by one of the animals would affect the long-term establishment of a hierarchy, we set up an experimental procedure in which pairs of rats (matched for both their body weight and anxiety-like behavior in the EPM) were firstly confronted on day 1 and then again 1 week later (day 8). Thirteen pairs of rats were assigned to two groups that were equivalent in their anxiety-like levels (data not shown): (i) in the control condition (non-stress pairs; Pns; n = 7 pairs), rats were directly confronted on day 1 without any prior manipulation (both rats in the pair were not stressed, Rns); (ii) in the stress condition (stress pairs; Ps; n = 6 pairs), one rat (Rs) in the pair was exposed to stress immediately before their first encounter, whereas the other rat (Rns) in the pair was not stressed.
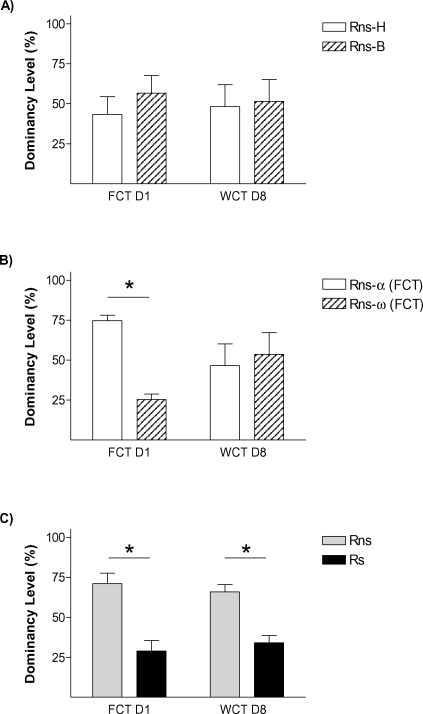
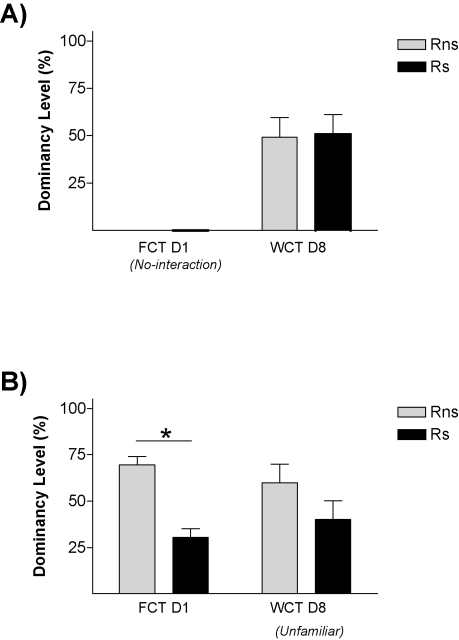
On the first encounter (day 1), those pairs (Ps) in which one animal had been submitted to stress differed in the frequency of passes over the feeder [p < 0.01; t-test vs. 50% p < 0.05], with Rs rats passing only around one third of the times Rns rats pass [Rns = 71.09 ± 6.61; Rs 28.91 ± 6.61] (Figure 3C). In the control condition (Pns; Figure 3A), animals bearing the fur mark-like stressed rats in the Ps group (but not submitted to stress) showed equivalent number of relative passes over the feeder as their counterparts marked as the non-stressed rats in the Ps condition [43.31 ± 11.12% vs. 56.68 ± 11.12%, respectively; all tests n.s.]. Therefore, stress facilitated the establishment of a hierarchy in the dyad, leading the stressed animal to become submissive. We then asked whether stress would also affect the memory for the social hierarchy established. Therefore, data from day 1 about passes over the feeder was re-evaluated to establish whether animals in each pair were either dominant, submissive, or not different to their counterpart (i.e., no hierarchy was established). In the control condition (Pns) (Figure 2B), 1 pair displayed similar number of passes which was interpreted as no hierarchy established, whereas the remaining 6 pairs followed criteria for the establishment of a hierarchy [Rns-α-FCT = 74.64 ± 3.32%; Rns-ω-FCT = 25.33 ± 3.32%; p < 0.01). In the stress condition (Ps; Figure 2C), the six stressed animals in each pair fulfilled criteria to be classified as submissive, and their counterparts as dominant (p < 0.001). On day 8 (WCT), in the control condition (Pns) there was no difference in the percent time drinking spent by rats classified according to their behavior on day 1 as either dominant or submissive. In fact, dominance from day 1 to day 8 was only maintained in 3 out of the 6 pairs in this condition (note that the total numbers of Pns pairs was 7, but 1 of them did not establish a clear dominance on day 1). On the contrary, there was a difference in the percent time spent drinking in the pairs of the stress condition (Ps) (p < 0.01), with stressed rats (Rs = 34.15 ± 4.58%) drinking significantly less time than the not stressed (Rns = 65.84 ± 4.58%). In this pairs (Ps), the dominant–submissive hierarchy was maintained in all the cases from day 1 to day 8, with all stressed animals falling into the criteria of submissive in indices from both days. The difference on the maintenance of the hierarchy from day 1 to day 8 between the stressed and the non-stressed pairs was further confirmed by Chi-square analyses (p < 0.05).
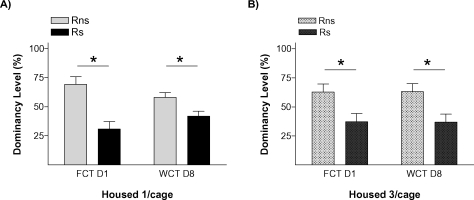
Figure 3.
Social memory is not affected by housing conditions. Graphs show dominancy level percent in two competition tests for (A) isolated rats and (B) triads housed rats. * p < 0.05.
Figure 2.
Stress amplifies memory for social hierarchy. The graphs represent rates between opponents in “dominancy level” (relative percent in the parameter which represents dominancy in each competition test, namely % frequency passes over feeder in FCT day 1, and % water consumption in WCT day 8). Graphs shows % dominancy level for (A) Pns, rats are identified at random with head marks (Rns-H) or body marks (Rns-B), (B) % dominancy level of the same Pns, rats are identified as alpha (highest passes over feeder during FCT) or omega (lowest frequency of passes over feeder), and (C) stress pairs (Ps), rats are identified as rats not stressed (Rns) or rats exposed to stress immediately before the first social encounter (Rs). * p < 0.05.
Role of housing conditions on the impact of stress on long-term social relations
We next questioned whether stress effects on the establishment of long-term social relations might depend on the housing conditions to which animals are allocated upon arrival in our animal facility. We aimed to compare results between animals kept isolated (equal conditions as the previous experiment, which would allow us to replicate it; housed 1/cage: n = 18) and animals housed in groups of 3 per cage (housed 3/cage: n = 20).
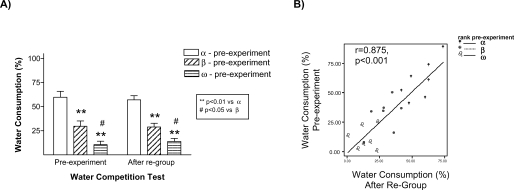
The results of this experiment replicated our finding that stressed animals were the ones that behaved as submissive on days 1 and 8. We also found evidence that this effect of stress on the establishment of long-term memories for a pair hierarchy was not dependent on the homecage conditions: stressed animals (Rs) behaved as subordinate in tests performed on days 1 and 8 both in the isolated (Figure 3A) and in the group-caged (Figure 3B) conditions (all p < 0.05). Therefore, once again, stress induced a long-term memory for the hierarchy established after a first encounter with a novel conspecific. Interestingly, stress did not influence the already well-established hierarchy among the animals in the group-caged condition, as demonstrated in the experiment by the re-establishment of their previous hierarchy when they were re-housed together after the WCT on day 8. On these triads, equivalent hierarchy status was observed in homecage-based WCTs delivered before and after the “social memory” experimental procedures (Figure 4A) with their percent water consumption in each of these competition tests showing a strong positive correlation (r = 0.875, p < 0.001; Figure 4B).
Figure 4.
Group-housed rats maintained their previous hierarchy status after being re-housed together once the social memory experiments were finished (day 8). In the left panel the graph represents the water consumption ratio average between group-cage rats during the WCT performed the week before the experiment started (pre-experiment) and when they were re-housed together after the WCT on day 8 (A). Rats with the highest ratio in time dedicated to water consumption during the WCT pre-experiment were identified as alpha (blank bars), those with the lowest ratio as omega (bars with horizontal lines), and the rats in between both types as beta (bars with slant lines). ** p < 0.01 vs. α-rats, # p < 0.05 vs. β-rats. On the right panel the relationship between the ratio of water consumption during WCT pre-experiment and after re-group is represented (B). Equivalent hierarchy status was observed in homecage-based WCTs delivered before or after the “social memory” experimental procedures.
Testing the specificity of the memory effect induced by stress
Since uncontrollable stress can have important and long-lasting consequences on behavior, it could be argued that the submissive status of stressed animals on day 8 is not related to a memory for the social hierarchy, but to the enduring impact of stress by itself. To evaluate this possibility, we compared behavior in the WCT on day 8 of pairs of animals that were submitted to stress on day 1, but not exposed to the two interaction procedures that are normally delivered on that day. As shown in Figure 5A, stress does not render animals submissive when they participate in a WCT 7 days after stress exposure (i.e., dominancy level for Rns and Rs rats does not differ; n.s.). This result supports the view that the dominant–submissive relationship observed in previous experiments on day 8, in which Rs animals (stressed before their encounter on day 1) display a submissive behavior on day 8, is not the direct consequence of stress rendering individuals in a state of learned helplessness still evident 1 week afterwards, since stressed animals and just submitted to the competition test on day 8 do not become submissive. This result strongly supports the view that stress potentiates a long-term memory for the social relationship.
Figure 5.
Specificity of the memory in stress-induced social hierarchy rank. Graphs show dominancy level percent in (A) Ps confronted only on day 8 (7 days after Rs were stressed), no differences were found in dominancy level between Rns and Rs, indicating that stress does not render animals submissive when they compete on day 8 and (B), stress rats confronted with unfamiliar rats not only during the FCT but as well in the WCT, no differences were found on day 8 between Rns and Rs, overruling the hypothesis that stress potentitates memory for the social status acquired on day 1. * p < 0.05.
However, the question still remains as to whether stress potentiates the memory for “the social rank” evinced on day 1 or whether its effect is more specifically related to a memory for “the particular individual” encountered on day 1 and for their respective associated status. It is well known that social experience can have a major impact on subsequent conflicts, with winners being more likely to win and losers more likely to lose in future encounters with different opponents (Chase et al., 1994; Dugatkin and Druen, 2004; Hsu and Wolf, 1999). This is known as “winner and loser effects,” a mechanism that is widespread in the animal kingdom, and has received great deal of attention in models of aggression and dominance hierarchy formation (Chase et al., 1994; Dugatkin and Earley, 2004; Landau, 1951; Rutte et al., 2006).
We therefore aimed to evaluate whether the effect of stress in our model just responds to winner and loser effects or whether it involves recognition memory of the specific partners involved in the Ps dyads. In particular, we questioned whether behavior of rats in the stress pair condition (Ps) in the WCT on day 8 would differ if instead of being confronted with the familiar rat (their opponent on day 1), they were confronted with an unfamiliar male rat. Importantly, this unfamiliar rat had an equivalent social experience (i.e., social status as defined by its own experience on day 1) as its opponent on day 1; i.e., contests in this experimental condition took place between one dominant and one submissive rat, classified according to their behavior on day 1, and unfamiliar to each other. Thus, pairs of rats in the Ps condition on day 1 were afterwards divided into two equivalent groups that differed in the procedures given on day 8. As shown in Figure 5B, no significant differences were observed in the dominance level displayed in the WCT on day 8 by stressed (Rs) rats and their opponent unfamiliar dominant rat (n.s.). This result clearly differ from those of previous experiments in which stressed (Rs) rats displayed on day 8 a clearly lower dominance level when confronted with the familiar non-stressed rats (Rns). Therefore, in our model, stress is not just potentiating a memory for winner and loser effects; otherwise, the status of animals in the stress condition would have been maintained when faced with unfamiliar rats that had independently undergone previous experience of victory or defeat. Instead, these data strongly suggests that stress reinforces the establishment of a specific recognition memory for the familiar counterpart and their respective social status in such specific dyad. The ability to learn and remember conspecific individuals is a critical requirement for social behavior and for the stability of social groups (Insel and Fernald, 2004). It is an ability shared by different animal species (Beaugrand, 1997).
Involvement of protein synthesis mechanisms on the effects of stress on social memory
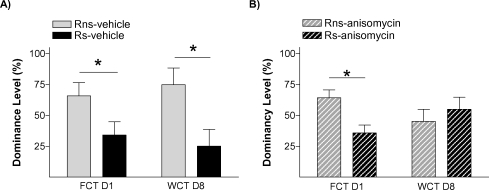
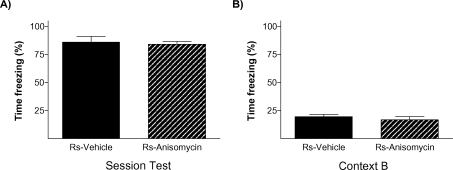
To further verify that stress' effect on the endurance of social status between two specific rats was exerted through memory mechanisms, our next experiment was addressed to study if it is dependent on a neurobiological mechanism widely required for the formation of long-term memories, protein synthesis. Protein synthesis has proved to be a virtually universal requirement for the storage of information into long-term memory, including social recognition memory in mice (Richter et al., 2005). We evaluated the impact of blocking protein synthesis immediately after the first social encounter (day 1) in both animals in the pairs submitted to the stress condition (Ps; i.e., one Rs and one Rns). The main readout of this experiment was the behavior displayed by animals in the WCT on day 8. In this experiment, 13 pairs of rats in the stress condition (Ps) were assigned to two equivalent groups. Immediately after the first encounter on day 1, one group of animals was injected with the protein synthesis inhibitor anisomycin (150 mg/kg i.p.) while the other group received a vehicle injection. This dose of anisomycin has been frequently shown to inhibit protein synthesis by approximately 90% of cerebral protein synthesis and impairing long-term memory formation (Bourtchouladze et al., 1998; Davis and Squire, 1984). As opposed to vehicle-injected pairs of animals that, on day 8, showed the characteristic pattern that stressed rats (Rs) spent a lower percent time drinking than non-stressed rats (Rns) (p < 0.05; Figure 6A), animals injected with anisomycin did not show such pattern (n.s.; Figure 6B). In this group, both stressed (Rs) and non-stressed rats (Rns) displayed similar percent of time drinking (n.s.), indicative of an absence of maintenance of the dominant–submissive status from day 1 (and observed on that day before injection of the inhibitor) to day 8. Hence, administration of the protein synthesis inhibitor anisomycin after the first encounter prevented the stress-induced potentiation of memory that is normally observed on day 8. Chi-square analyses confirmed a difference between vehicle- and anisomycin-injected animals in the maintenance of their dominance hierarchy between days 1 and 8 (p < 0.05). Interestingly, although anisomycin treatment interfered with the formation of the long-term social memory (Figure 6B), it did not affect animals' long-term fear conditioning memory for the context in which they received the stress. Thus, equivalent freezing values were found in vehicle- and anisomycin-injected rats when exposed on day 9 (1 day after the WCT) either to the same context (n.s.; Figure 7A) or, 2 hours later, to a different context (n.s.; Figure 6B).
Figure 6.
Social memory is dependent on protein synthesis. Graph shows dominancy level (%) during two competition encounters (FCT and WCT) of pairs rats treated with (A) vehicle (solid bars) or (B) anisomycin (hatched bars), immediately after FCT. No differences were found in WCT between Rns and Rs in pairs treated with anysomicin, suggesting that stress potentiates a hierarchy-linked recognition memory between specific individuals through mechanisms that involve de novo protein synthesis.
Figure 7.
Emotional memory of stressed rats for the contextual fear conditioning test was not affected by anisomycin treatment. The graphs show the percent time stress rats froze during the test of the CFC on day 9 when they were exposed to (A) the same context they were conditioned (context A) or (B) a new context 2 hours after being tested for fear conditioning in context A. No differences were observed between groups (solid bars, vehicle; hatched bars, anisomycin).
Discussion
Using a novel model for the assessment of long-term hierarchy rank in rats, we show here that stress experienced by one individual at the time of a first social encounter can have a profound impact on the long-term establishment of the individual's rank within that particular social context. Through a series of complementary experiments, we propose that stress potentiates a hierarchy-linked recognition memory between specific individuals through mechanisms that involve de novo protein synthesis.
We found that being stressed during the first interaction with a conspecific male rat of equivalent attributes (such as gender, body weight and size, and trait anxiety) renders the stressed rat eventually submissive, as becomes progressively evident over that initial encounter in a novel neutral confinement and is already clear in the food competition test given immediately afterwards. Importantly, we should emphasize that this is the hierarchical outcome resulting from an uncontrollable stressful situation (fear conditioning) might differ from the impact of experiencing controllable stressful experiences in which the individuals learn to cope with an aversive challenge. In fact, evidence for a context-dependent modulation of behavioral and physiological responses linked to social status has been previously noted (Bartolomucci et al., 2001, 2004).
The main finding of this study is the observation that such stress will not only affect the immediate social hierarchy established within the pair of animals, but it will also determine individuals' rank in their future mutual interactions. Social rank evinced by the food competition test in non-stressed pairs of rats was not maintained 1 week later, but appears to be evident when tests are performed 2 days after their first encounter (Cordero and Sandi, unpublished data obtained with slightly different experimental conditions), which suggests that stress might amplify, rather than promoting a recognition memory. In fact, the development of a certain memory between the two contestants in the control conditions is somehow expected, given that social aggressive interactions by themselves are stressful for both dominant and subordinate animals (Summers and Winberg, 2006) and stress hormones expected to modulate memory consolidation (de Kloet et al., 1999; McGaugh and Roozendaal, 2002; Roozendaal, 1999; Roozendaal et al., 2006; Sandi, 1998). Interestingly, the enduring effect of additional, exogenous stress is specific in potentiating a recognition memory for “the particular individual,” since (i) just exposing one of the males on the pair to stress without an immediate social interaction does not determine the social hierarchy displayed by animals if they are confronted for the first time 1 week after stress delivery; and (ii) a submissive relationship on day 8 is not observed in stressed males that had a first social encounter on day 1 but on day 8 are exposed to an “unfamiliar” dominant male on the WCT. This memory effect appears to be particularly relevant as also indicated by work in lizards showing that memory of opponents in more potent for long-term hierarchies than visual sign stimuli after social hierarchy has been established (Larson et al., 2001; Korzan et al., 2007). Since biphasic effects have been frequently described for the cognitive effects of varying stress intensity (de Kloet et al., 1999; Joels, 2006; Sandi and Pinelo-Nava, 2007) an interesting question that remains for future studies is whether exogenous stress given after a social interaction would also affect long-term expression of hierarchy rank.
We also found that the enduring effect of stress on the establishment of a dominance–submissive hierarchy between two individuals is prevented by administration of the protein synthesis inhibitor anisomycin given after their first social encounter. Anisomycin specifically interfered with their social recognition memory without affecting animals' memory linked to the context where they were stressed (as indicated by freezing values in anisomycin-treated animals equivalent to those displayed by controls when submitted to contextual fear conditioning context generalization tests). The lack of effect of anisomycin treatment on contextual fear conditioning memories can be easily explained by the timing of drug injection. Anisomycin was injected after the food competition test and therefore at least 35 minutes after the conditioning session, a time delay enough to allow the training-induced triggering of protein synthesis mechanisms. In fact, memories for avoidance learning and fear conditioning were shown to be interfered by systemic anisomycin treatments given immediately, but not 10–60 minutes, after training (Bourtchouladze et al., 1998; Davis et al., 1981). Therefore, under our experimental conditions, anisomycin seems to have specifically affected protein synthesis elicited by the social interactions. The requirement of protein synthesis for the consolidation of information into long-term memories has been shown for many animal species and learning tasks (Alberini, 2005; Bailey et al., 1996; Davis and Squire, 1984; Sandi and Rose, 1997), including social recognition memory in mice (Kogan et al., 2000; Richter et al., 2005). Previous studies on social memories have been based on juvenile recognition by an adult male rodent (Kogan et al., 2000; Richter et al., 2005). Here, we extend the implication of this “universal” neurobiological mechanism of memory formation, protein synthesis, to recognition memory linked to acquired hierarchy rank between two adult individuals. However, a note of caution should also be added since mechanisms other than protein synthesis (such as alterations in neurotransmitter levels) might also account for the mnemonic effects induced by anisomycin (Canal et al., 2007).
The ability to recognize other members of one's own species is an important requirement of life in social groups (Ferguson et al., 2002). Extensive work focusing on the mechanisms that maintain dominance hierarchies have shown that they are generally stable and maintained through the recognition of dominant and non-dominant individuals through different sensory channels (Broom, 2002). Recognition memory in animals has frequently been linked to the storage of the “olfactory signature” for a particular conspecific, which in the case of mice memory for a juvenile was shown to last for at least 1 week (Kogan et al., 2000). Future behavioral and pharmacological studies will be addressed to find out if, in our model, the recognition memory facilitated by stress is hold by the stressed and/or the non-stressed paired animal.
An important step in our model is the matching of rats in pairs according to their “intrinsic” attributes. That body weight/size plays key roles in the establishment of social hierarchies in animal species in which dominance–submissive relationships are established through aggressive context, does probably not require an explanation. The relevance of matching animals for their anxiety levels might however be not so obvious. Yet, there is ample evidence indicating a key relationship between anxiety and (i) hierarchy status (Ferrari et al., 1998; Gentsch et al., 1990), (ii) social aggression (Kikusui et al., 2004; Maestripieri et al., 1991; Patin et al., 2005), (iii) social discrimination (Landgraf and Wigger, 2002); and (iv) social investigation (Dunn and File, 1999; File, 1980). Moreover, anxiolytic agents can also have an important impact on social hierarchy rank (Joly and Sanger, 1991, 1992).
Although not always uniform and depending on many factors, studies in rodents frequently show that stress tends to induce social avoidance (Haller and Bakos, 2002) and facilitate submission. However, we should also note that the stress hormones glucocorticoids have been reported to induce rapid increases in aggressive behavior in male rodents (Mikics et al., 2004). Note that, in our model, submissive behavior in the stressed rat develops over time during the 20 minutes of the first social interaction, with the dominance–submissive relationship only becoming evident during the last 5 minutes block of that interaction. Therefore, the submissive status developed by stressed rats does not appear to be simply caused by a “learned helplessness effect” induced by prior shock exposure. In fact, learned helplessness protocols classically require the exposure to much longer and stronger stressors than the procedures used in our study (Maier and Watkins, 2005). This means that for the first 10–15 minutes, stressed rats did not immediately display submissive behavior as a consequence of prior stress (shock) exposure, but that the dominance–submission hierarchy emerged after 10–15 minutes interaction. A possible explanation could be that stress reduces individuals' persistency to fight for dominance, by affecting their engagement in a “war of attrition” (a delayed process, one hopes to wear down its enemy by continuously engaging in battle; Hammerstein and Parker, 1982). Alternatively, stress might eventually diminish individuals' “resource holding power/potential” (Parker, 1974) which can manifest as postural changes that decrease individuals' opportunity to win an escalated contest (Scott and Fredericson, 1951; Maynard Smith, 1982; Archer, 1998). Although the exact mechanism remains to be established, stress seems to affect social dynamics generated during the process of social interaction. Moreover, this effect would be long-lasting and the one prevalent on day 8 competition test. Social dynamics have been proposed to play a key role in the generation of linear hierarchies and social structures (Chase, 1982; Chase et al., 2002; Francis, 1988) and, therefore, our results support an important role of stress on the establishment of hierarchical societies.
Both in animals and humans, there is a relationship between individuals' social status and health, as well as between the magnitude of social status differences in a society and the quality of “social relations” and population health. Developing a better understanding of the social and neurobiological determinants of social status is critical to reduce the deleterious impact of social inequalities in psychological, sociological, and health domains. Some authors have proposed interventions to change social, economic, and cultural determinants of health, all of them representing complex interventions difficult to tackle. Our study showing that memory mechanisms participate in the impact of stress on the establishment of long-term social hierarchies widens the potential interventions to reduce psychosocial and societal problems due to uneven status.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that should be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Professor Stephan Morgenthaler for consultation on dyadic statistical analyses, and Fabian Jordi for his excellent technical assistance with the behavioral evaluations. This work was partially supported by a grant from the Swiss National Science Foundation (3100A0-108102) and the intramural funding from the EPFL (Switzerland).
References
- Alberini C. M. (2005). Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 28, 51–56 10.1016/j.tins.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Albers H., Ferris C. (1986). Role of the flank gland in vasopressin induced scent marking behavior in the hamster. Brain Res. Bull. 17, 387–389 10.1016/0361-9230(86)90242-X [DOI] [PubMed] [Google Scholar]
- Archer J. (1988). The behavioural biology of agression (Cambridge University Press; ) [Google Scholar]
- Baenninger L. (1970). Social dominance orders in the rat: “Spontaneous” food and water competition. J. Comp. Physiol. Psychol. 71, 202–209 10.1037/h0029162 [DOI] [Google Scholar]
- Bailey C. H., Bartsch D., Kandel E. R. (1996). Toward a molecular definition of long-term memory storage. Proc. Natl. Acad. Sci. USA 93, 13445–13452 10.1073/pnas.93.24.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M., Albers H. (1996). Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus). J. Comp. Neurol. 27, 252–263 [DOI] [PubMed] [Google Scholar]
- Bartolomucci A. (2005). Resource loss and stress-related disease: is there a link? Med. Sci. Monit. 11, RA147–154 [PubMed] [Google Scholar]
- Bartolomucci A. (2007). Social stress, immune functions and disease in rodents. Front. Neuroendocrinol. 28, 28–49 10.1016/j.yfrne.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Bartolomucci A., Chirieleison A., Gioiosa L., Ceresini G., Parmigiani S., Palanza P. (2004). Age at group formation alters behavior and physiology in male but not female CD-1 mice. Physiol. Behav. 82, 425–434 10.1016/j.physbeh.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Bartolomucci A., Palanza P., Gaspani L., Limiroli E., Paanerai A. E., Ceresini G., Poli M. D., Parmigiani S. (2001). Social status in mice: behavioural, endocrine and immune changes are context dependent. Physiol. Behav. 73, 401–410 10.1016/S0031-9384(01)00453-X [DOI] [PubMed] [Google Scholar]
- Beaugrand J. (1997). Relative importance of individual differences, agonistic experience, and assessment accuracy during hierarchy formation: a simulation study. Behav. Proc. 41, 177–192 10.1016/S0376-6357(97)00046-6 [DOI] [PubMed] [Google Scholar]
- Beehner J. C., Phillips-Conroy J. E., Whitten P. L. (2005). Female testosterone, dominance rank, and aggression in an Ethiopian population of hybrid baboons. Am. J. Primatol. 67, 101–119 10.1002/ajp.20172 [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R., Abel T., Berman N., Gordon R., Lapidus K., Kandel E. R. (1998). Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn. Mem. 5, 365–374 [PMC free article] [PubMed] [Google Scholar]
- Broom M. (2002). A unified model of dominance hierarchy formation and maintenance. J. Theor. Biol. 219, 63–72 [PubMed] [Google Scholar]
- Chase I. D. (1982). Dynamics of hierarchy formation: The sequential development of dominance relationships. Behavior 80, 218–240 [Google Scholar]
- Chase I. D., Bartolomeo C., Dugatkin L. A. (1994). Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim. Behav. 48, 393–400 10.1006/anbe.1994.1253 [DOI] [Google Scholar]
- Chase I. D., Tovey C., Spangler-Martin D., Manfredonia M. (2002). Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl. Acad. Sci. USA, 99, 5744–5749 10.1073/pnas.082104199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Albon S. D., Guinness F. E. (1984). Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308, 358–360 10.1038/308358a0 [DOI] [Google Scholar]
- Clutton-Brock T. H., Hodge S. J., Spong G., Russell A. F., Jordan N. R., Bennett N. C., Sharpe L. L., Manser M. B. (2006). Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068 10.1038/nature05386 [DOI] [PubMed] [Google Scholar]
- Davis H., Squire L. (1984). Protein synthesis and memory: a review. Psychol. Bull. 96, 518–559 10.1037/0033-2909.96.3.518 [DOI] [PubMed] [Google Scholar]
- Davis H. P., Rosenzweig M. R., Kinkade P. T., Bennett E. L. (1981). Effects of anisomycin on retention of the passive-avoidance habit as a function of age. Exp. Aging Res. 7, 33–44 [DOI] [PubMed] [Google Scholar]
- De Kloet E. R., Oitzl M. S., Joels M. (1999). Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 22, 422–426 10.1016/S0166-2236(99)01438-1 [DOI] [PubMed] [Google Scholar]
- Drew D., Dickey G. (1977). Observational and competitive measures of dominance in rats. Psychol. Rec. 2, 331–338 [Google Scholar]
- Dugatkin L., Druen M. (2004). The social implications of winner and loser effects. Proc. Biol. Sci. 271, S488–S489 10.1098/rsbl.2004.0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugatkin L., Earley R. (2004). Individual recognition, dominance hierarchies, and winner and loser effects. Proc. R. Soc. (Lond.), Ser. B 271, 1537–1540 10.1098/rspb.2004.2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. J., File S. E. (1999). Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Hormon. Behav. 21, 193–202 10.1016/0018-506X(87)90044-4 [DOI] [PubMed] [Google Scholar]
- Ferguson J. N., Young L. J., Insel T. R. (2002). The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 23, 200–224 10.1006/frne.2002.0229 [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., Palanza P., Parmigiani S., Rodgers R. J. (1998). Interindividual variability in Swiss male mice: relationship between social factors, aggression, and anxiety. Physiol. Behav. 63, 821–827 10.1016/S0031-9384(97)00544-1 [DOI] [PubMed] [Google Scholar]
- Ferris C. F., Delville Y., Grzonka Z., Luber-Narod J., Insel T. R. (1993). An iodinated vasopressin (V1) antagonist blocks flank marking and selectively labels neural binding sites in golden hamsters. Physiol. Behav. 54, 737–747 10.1016/0031-9384(93)90085-T [DOI] [PubMed] [Google Scholar]
- Ferris C. F., Pollock J., Albers H. E., Leeman S. E. (1985). Inhibition of flank-marking behavior in golden hamsters by microinjection of a vasopressin antagonist into the hypothalamus. Neurosci. Lett. 55, 239–243 10.1016/0304-3940(85)90027-8 [DOI] [PubMed] [Google Scholar]
- Ferris C. F., Singer E. A., Meenan D. M., Elliott Albers H. (1988). Inhibition of vasopressin-stimulated flank marking behavior by V1-receptor antagonists. Eur. J. Pharmacol. 154, 153–159 10.1016/0014-2999(88)90092-1 [DOI] [PubMed] [Google Scholar]
- File S. E. (1980). The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods, 2, 219–238 10.1016/0165-0270(80)90012-6 [DOI] [PubMed] [Google Scholar]
- Francis R. (1988). On the relationship between aggression and social dominance. Ethology 78, 223–237 [Google Scholar]
- Gentsch C., Lichtsteiner M., Feer H. (1988). Competition for sucrose-pellets in triads of male Wistar rats: the individuals' performances are differing but stable. Behav. Brain Res. 27, 37–44 10.1016/0166-4328(88)90107-6 [DOI] [PubMed] [Google Scholar]
- Gentsch C., Lichtsteiner M., Feer H. (1990). Competition for sucrose-pellets in triads of male Wistar rats: effects of acute and subchronic chlordiazepoxide. Psychopharmacology (Berl) 100, 530–534 10.1007/BF02244007 [DOI] [PubMed] [Google Scholar]
- Gray J. (1987). The psychology of fear and stress (Cambridge University Press; ). [Google Scholar]
- Haller J., Bakos N. (2002). Stress-induced social avoidance: A new model of stress-induced anxiety? Physiol. Behav. 77, 327–332 10.1016/S0031-9384(02)00860-0 [DOI] [PubMed] [Google Scholar]
- Hammerstein P., Parker G. A. (1982). The asymmetric war of attrition. J. Theor. Biol. 96, 647–682 10.1016/0022-5193(82)90235-1 [DOI] [Google Scholar]
- Herrero A. I., Sandi C., Venero C. (2006). Individual differences in anxiety trait are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors. Neurobiol. Learn. Mem. 86, 150–159 10.1016/j.nlm.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Hsu Y., Wolf L. L. (1999). The winner and loser effect: integrating multiple experiences. Anim Behav. 57, 903–910 10.1006/anbe.1998.1049 [DOI] [PubMed] [Google Scholar]
- Insel T. R., Fernald R. D. (2004). How the brain processes social information: searching for the social brain, Annu. Rev. Neurosci. 27, 697–722 10.1146/annurev.neuro.27.070203.144148 [DOI] [PubMed] [Google Scholar]
- Joels M. (2006). Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci. 27, 244–250 10.1016/j.tips.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Joly D., Sanger D. (1991). Social competition in rats: a test sensitive to acutely administered anxiolytics. Behav. Pharmacol. 2, 205–213 10.1097/00008877-199106000-00004 [DOI] [PubMed] [Google Scholar]
- Joly D., Sanger D. (1992). Social competition in dominant rats can be attenuated by anxiogenic drugs. Behav. Pharmacol. 3, 83–88 [DOI] [PubMed] [Google Scholar]
- Kikusui T., Takeuchi Y., Mori Y. (2004). Early weaning induces anxiety and aggression in adult mice. Physiol. Behav. 81, 37–42 10.1016/j.physbeh.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Kim J. J., Diamond D. M. (2002). The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 3, 453–462 10.1038/nrm832 [DOI] [PubMed] [Google Scholar]
- Kogan J. H., Frankland P. W., Silva A. J. (2000). Long-term memory underlying hippocampus-dependent social recognition memory in mice. Hippocampus 10, 47–56 [DOI] [PubMed] [Google Scholar]
- Korzan W. J., Hoglund E., Watt M. J., Forster G. L., Overli O., Lukkes J. L., Summers C. H. (2007). Memory of opponents is more potent than visual sign stimuli after social hierarchy has been established. Behav. Brain Res. 183, 31–42 10.1016/j.bbr.2007.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau H. G. (1951). On dominance relations and the structure of animal societies. I. Effect of inherent characteristics. Bull. Math. Biophys. 13, 1–19 10.1007/BF02478336 [DOI] [Google Scholar]
- Landgraf R., Wigger A. (2002). High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav. Genet. 32, 301–314 10.1023/A:1020258104318 [DOI] [PubMed] [Google Scholar]
- Larson E. T., Summers C. H. (2001). Serotonin reverses dominant social status. Behav. Brain Res. 121, 95–102 10.1016/S0166-4328(00)00393-4 [DOI] [PubMed] [Google Scholar]
- Lucion A., Vogel W. (1994). Effects of stress on defensive aggression and dominance in a water competition test. Integr. Physiol. Behav. Sci. 29, 415–422 10.1007/BF02691361 [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Badiani A., Puglisi-Allegra S. (1991). Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav. Neurosci. 105, 663–668 10.1037/0735-7044.105.5.663 [DOI] [PubMed] [Google Scholar]
- Maier S. F., Watkins L. R. (2005). Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 29, 829–841 10.1016/j.neubiorev.2005.03.021 [DOI] [PubMed] [Google Scholar]
- Malatynska E., Goldenberg R., Shuck L., Haque A., Zamecki P., Crites G., Schindler N., Knapp R. J. (2002). Reduction of submissive behavior in rats: a test for antidepressant drug activity. Pharmacology 64, 8–17 10.1159/000056145 [DOI] [PubMed] [Google Scholar]
- Malatynska E., Kostowski W. (1984). The effect of antidepressant drugs on dominance behavior in rats competing for food. Pol J. Pharmacol. Pharm. 36, 531–540 [PubMed] [Google Scholar]
- Maynard Smith J. (1982). Evolution and the theory of games (Cambridge University Press; ). [Google Scholar]
- McGaugh J. L., Roozendaal B. (2002). Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 12, 205–210 10.1016/S0959-4388(02)00306-9 [DOI] [PubMed] [Google Scholar]
- Mikics E., Kruk M. R., Haller J. (2004). Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology 29, 618–635 10.1016/S0306-4530(03)00090-8 [DOI] [PubMed] [Google Scholar]
- Ötsberg V. (2003). Children in classrooms: peer status, status distribution and mental well-being. Soc. Sci. Med. 56, 17–29 10.1016/S0277-9536(02)00006-0 [DOI] [PubMed] [Google Scholar]
- Parker G. A. (1974). Assessment strategy and the evolution of animal conflicts. Anim Behav. 29, 193–205 [Google Scholar]
- Patin V., Lordi B., Vincent A., Caston J. (2005). Effects of prenatal stress on anxiety and social interactions in adult rats. Dev. Brain Res. 160, 265–274 10.1016/j.devbrainres.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Raleigh M. J., McGuire M. T., Brammer G. L., Pollack D. B., Yuwiler A. (1991). Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 559, 181–190 10.1016/0006-8993(91)90001-C [DOI] [PubMed] [Google Scholar]
- Richter K., Wolf G., Engelmann M. (2005). Social recognition memory requires two stages of protein synthesis in mice. Learn. Mem. 12, 407–413 10.1101/lm.97505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. (1999). Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 25, 213–238 10.1016/S0306-4530(99)00058-X [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Okuda S., de Quervain D. J., McGaugh J. L. (2006). Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience 138, 901–910 10.1016/j.neuroscience.2005.07.049 [DOI] [PubMed] [Google Scholar]
- Rutte C., Taborsky M., Brinkhof M. W. G. (2006). What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21 10.1016/j.tree.2005.10.014 [DOI] [PubMed] [Google Scholar]
- Sandi C. (1998). The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plast. 6, 41–52 10.1155/NP.1998.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C. (2004). Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 5, 917–930 10.1038/nrn1555 [DOI] [PubMed] [Google Scholar]
- Sandi C., Pinelo-Nava M. T. (2007). Stress and memory: behavioral effects and neurobiological mechanisms, neural plasticity 20 pages, 10.1155/2007/78970 [DOI] [PMC free article] [PubMed]
- Sandi C., Rose S. P. R. (1997). Protein synthesis- and fucosylation-dependent mechanisms in corticosterone facilitation of long-term memory in the chick. Behav. Neurosci. 111, 1098–1104 10.1037/0735-7044.111.5.1098 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M. (2005). Sick of poverty. Sci. Am. 293, 92–99 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Share L. J. (1994). Rank-related differences in cardiovascular function among wild baboons: role of sensitivity to glucocorticoids. Am. J. Primatol. 32, 261–275 10.1002/ajp.1350320404 [DOI] [PubMed] [Google Scholar]
- Scott J. P., Fredericson E. (1951). The causes of fighting in mice and rats. Physiol. Zool. 24, 273–309 [Google Scholar]
- Shors T. J. (2006). Significant life events and the shape of memories to come: a hypothesis. Neurobiol. Learn. Mem. 85, 103–115 10.1016/j.nlm.2005.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C. H., Winberg S. (2006). Interactions between the neural regulation of stress and aggression. J. Exp. Biol. 209, 4581–4589 10.1242/jeb.02565 [DOI] [PubMed] [Google Scholar]
- Tamashiro K. L. K., Nguyen M. M. N., Sakai R. R. (2005). Social stress: from rodents to primates. Front. Neuroendocrinol. 26, 27–40 10.1016/j.yfrne.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Vagell M. E., McGinnis M. Y. (1998). The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats. Horm. and Behav. 33, 163–179 10.1006/hbeh.1998.1445 [DOI] [PubMed] [Google Scholar]
- Wilkinson R. (1999). Health, hierarchy, and social anxiety. Ann. NY Acad. Sci. 896, 48–63 10.1111/j.1749-6632.1999.tb08104.x [DOI] [PubMed] [Google Scholar]
- Wilkinson R. G., Pickett K. E. (2006). Income inequality and population health: a review and explanation of the evidence. Soc. Sci. Med. 62, 1768–1784 10.1016/j.socscimed.2005.08.036 [DOI] [PubMed] [Google Scholar]