Abstract
The accumulation of filamentous α-synuclein (α-S) is associated with Parkinson’s disease. It remains controversial as to the mode (antiparallel or parallel) of α-S self-assembly and whether an exact alignment of the central hydrophobic region is essential. In the present study, we performed in vitro assembly using α-S with or without the attachment of artificial leucine zippers (Zips) capable of forming either parallel or antiparallel coiled coils and included a spacer in one derivative. Results showed that Zips accelerate filament assembly in both the parallel and antiparallel fashions, that a precise alignment of the central hydrophobic region is not essential, and that the antiparallel pairs displayed the highest thioflavin T signals. More importantly, cells expressing Zip-fused α-S, but not α-S alone, formed α-S immunopositive and thioflavin S-positive inclusions in 7 days. The results suggest that α-S can assemble in both parallel and antiparallel modes but have a higher tendency to assemble in the latter mode and that cells overexpressing Zip-fused α-S may be used to screen α-S assembly inhibitors due to enhanced ability to form inclusions.—Jiang, P., Ko, L., Jansen, K. R., Golde, T. E., Yen, S.-H. Using leucine zipper to facilitate α-synuclein assembly.
Keywords: aggregation, adeno-associated virus
Alpha-synuclein (α-S) is a highly conserved presynaptic protein containing 140 amino acids, and it contributes to as much as 0.5–1% of the total protein in soluble cytosolic brain fractions (1,2,3). Although its physiological functions are not fully understood, accumulation of α-S aggregates is an important pathological feature of several slow-onset neurodegenerative disorders such as Parkinson’s disease (PD), dementia with Lewy bodies (LBs), and multiple system atrophy diseases, collectively regarded as α-synucleinopathies (4).
Structurally, α-S can be divided into three overlapping regions that include the amino terminus (N) of ∼100 amino acids containing six 11-residue repeats, the central hydrophobic region from amino acids 61–95, and the acidic carboxy terminus (C) (5). In neutral solution, α-S is monomeric and natively unfolded (6), but, on exposure to various chemical and physical factors, it can self-assemble into cross-β amyloid-type filamentous structures (7). Such formation of α-S assemblies requires the central hydrophobic region (8). Because the morphology and physiochemical properties of filaments assembled from recombinant α-S are similar to those accumulated in synucleinopathies, many in vitro studies have been carried out to decipher the molecular mechanisms underlying α-S aggregation (9,10,11).
Information regarding α-S assembly has been obtained through in vitro studies using various techniques, including Fourier transform infrared spectroscopy analysis, spin-label electron spin resonance spectroscopy, electron paramagnetic resonance spectroscopy, polarized infrared technology, and fluorescence lifetime imaging (9, 12,13,14,15,16,17,18). However, it remains controversial as to how α-S proteins self-interact to form dimers, polymers, and filaments. In several studies, α-S was determined to assemble primarily in an antiparallel mode (12,13,14,15). In other studies, α-S assembly appeared to favor a parallel mode (16,17,18).
To address this issue, we explored a new strategy in which α-S was attached with coiled coil at its N or C end to result in enhanced interactions of α-S between the comparable ends of adjacent molecules (i.e., N end to N end, C end to C end), or between the opposite ends (N end to C end). We hypothesize that enhanced interactions of comparable ends would facilitate α-S assembly if the mode of assembly requires a parallel alignment of α-S. In contrast, enhanced interactions between different ends of adjacent α-S molecules would accelerate the assembly if α-S can aggregate in an antiparallel fashion.
A coiled coil is a structural motif found in the basic leucine zipper (Zip) class of transcription factors (19,20,21), as well as in fibrous proteins such as myosin and keratin (22). It has been reported that coiled coils may consist of two or more strongly interacting α-helices supercoiled around one another in a parallel or an antiparallel manner (23). The α-helices of naturally occurring coiled coils are generally parallel. However, a growing number of proteins have been shown to contain antiparallel coiled-coil domains as well (23). Recently, some artificial parallel or antiparallel Zip pairs have been created (23,24,25,26,27,28,29) and used as a tool to study protein interactions (30,31,32,33,34). The advantages of using artificial Zips in this type of study are that 1) they are relatively small (<4 kDa molecular mass); 2) the binding between Zips is specific and strong; 3) some Zips show stronger binding to different types of Zips than to themselves (28, 29, 34); and 4) there is no indication that interactions of Zip-fused non-α-S proteins or peptides lead to the assembly of filamentous aggregates.
In the present study, we used Zips to bring adjacent protein molecules closer to each other and hence increase the local concentration of α-S for self-interactions. We created fusion proteins of wild-type α-S and artificial Zips capable of forming either parallel or antiparallel coiled coils (see schematic in Fig. 1) and determined their effect on α-S aggregation in vitro. Moreover, we overexpressed one of the fusion proteins in cultured cells to test whether the Zip attachment has an effect on α-S self-interactions and aggregation as demonstrated in test tubes. The overexpression was achieved via adeno-associated virus (AAV) transduction, and wild-type α-S without the Zip attachment was included as a control. To our knowledge, this is the first study using Zips to demonstrate the assembly protein aggregates in vitro and in situ.
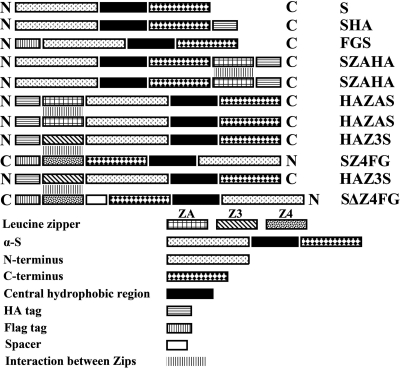
Figure 1.
Schematic for the construction of different recombinants derived from wild-type α-S (S). Different bars represent different fragments of recombinants; N and C denote N or C terminus of protein sequences.
MATERIALS AND METHODS
Construction of α-S recombinants with different artificial Zips and tags
Supplemental Table 1 lists the oligo-DNA used for synthesizing different target recombinants. Supplemental Table 2 presents information concerning the conditions used for polymerase chain reactions (PCRs) and for synthesizing target recombinants. Supplemental Fig. 1 shows an example of how a recombinant gene was generated via PCR. It is necessary to point out that all listed oligo-DNAs contain the restriction enzymatic sites for subcloning and construction of protein expression vectors.
The coding sequences of the different Zips and the spacer, hemagglutinin (HA), and FLAG (FG) are shown in Table 1. Two residues (GG) were added to the junction between the coiled coil and FG or HA, and four residues (GGSG) were added to the junction between Zip and α-S or α-S plus spacer to confer a more flexible protein conformation (30, 34). It has been demonstrated that ZA forms homodimers with high affinity in a parallel manner (28, 29), that Z3 and Z4 form heterodimers in an antiparallel fashion, and that Z4 forms homodimers in the absence of Z3 (33, 34). The addition of a spacer (Δ) enabled us to test whether a precise alignment of the central hydrophobic region between adjacent α-S molecules is essential for antiparallel α-S assembly to take place. We also constructed recombinant genes encoding α-S alone, α-S tagged with HA at its C end, and α-S tagged with FG at its N end as controls for studies of in vitro assembly (see schematic in Fig. 1).
TABLE 1.
Coding sequences of Zips and tags
| Zip or tag | Coding sequence |
|---|---|
| ZA | TVAQLEEKVKTLRAQNYELKSRVQRLREQVAQL |
| Z3 | EQLEKKLQALEKKLAQLEWKNQALEKKLAQ |
| Z4 | ALKKELQANKKELAQLKWE LQALKKELAQ |
| Spacer(Δ) | AGGAGAGGAAGAG |
| HA | YPYDVPDYAYPYDVPDYA |
| FG | DYKDDDDKDYKDDDDK |
Preparation of plasmids for protein expression of different recombinants
We used the Impact system (New England Biolabs, Ipswich, MA, USA) for protein expression. PCR products of SZAHA, SZ4FG, SΔZ4FG, SHA, and α-S groups were cut by restriction enzymes NdeI and Sap I (New England Biolabs), then inserted into linearized pTYB1 vector with the same cohesive ends; HAZAS, HAZ3S, and FGS groups were cut by restriction enzymes Sap I and XhoI (New England Biolabs), then inserted into linearized pTYB11 vector with the same cohesive ends. The plasmids were transformed, amplified, purified, and then verified by DNA sequencing (Sequencing Core Lab, Mayo Clinic, Rochester, MN, USA). They are referred to as pTYB1-SZAHA-intein, pTYB1-SZ4FG-intein, pTYB1-SZΔ4FG-intein, pTYB1-SHA-intein, pTYB1-Syn-intein, pTYB11-intein-HAZAS, pTYB11-intein-HAZ3S, and pTYB11-intein-FGS.
Expression and purification of recombinant proteins
Protein expression and purification were conducted as described by the manufacturer of the Impact system. Proteins were purified by binding of bacterial lysates to a chitin column, followed by the addition of dithiothreitol to cleave intein and the release of target protein. Target proteins were filtered with Amicon Ultra-15 centrifugal filter devices (50,000 NMWL, Millipore, Bedford, MA, USA) to reduce the amount of large-size contaminants [e.g., fusion protein containing intact intein tag (MW>55 kDa)]. The filtrates were loaded onto another Amicon Ultra-15 centrifugal filter device (10,000 NMWL; Millipore, Bedford, MA, USA). On centrifugation, a buffer containing 10 mM phosphate, 2.7 mM KCl, and 137 mM NaCl, pH 7.5, was added to the device and centrifuged. This step was repeated 3 more times to remove contaminants from protein fragments less than 10 kDa in mass. Purified proteins were quantified by the BCA method (Pierce Biotechnology, Rockford, IL); characterized by SDS-PAGE, silver staining, and Western blot analysis; and stored at −80°C. The purity of samples was evaluated by densitometric analysis of silver-stained gels with MCID software (Imaging Research, St. Catharines, ON, Canada).
In vitro aggregation
Purified proteins were diluted in a buffer containing 10 mM phosphate, 2.7 mM KCl, and 137 mM NaCl, pH 7.5, to a final concentration of 10 μM. They were incubated at 70°C for 30 min to dissociate coiled coil formation that could occur during protein storage, and then incubated at 37°C with constant shaking on a vortex. At different time points of incubation (0, 8, 13, 18, 24, 36 and 48 h), small aliquots were collected and analyzed by methods described in the following sections. The assembly study included 10 groups of samples; 8 contained only a single form of recombinant protein (i.e., SZAHA, HAZAS, SZ4FG, SΔZ4FG, HAZ3S, SHA, FGS, or S), and the others contained mixtures of equal amounts of either SZ4FG and HAZ3S, or SΔZ4FG and HAZ3S.
Circular dichroism (CD) and fluorescence spectroscopies
Samples collected from the assembly study were diluted in 10 mM phosphate buffer, pH 7.5, to a final concentration and volume of 5 μM and 140 μl, respectively. Far-UV CD measurements were carried out on a Jasco J-810 spectropolarimeter (Jasco, Easton, MD, USA) over the range of 260–190 nm, with a cell path of 0.1 cm (quartz cuvette; Hellma, Jena, Germany), bandwidth of 1 nm, speed of 50 nm/min, response time of 8 s, and data pitch of 0.1 nm. For all spectra, an average of 3 scans were obtained, smoothed using the “means-movement” algorithm with a convolution width of 25 in the Jasco spectra analysis program, and normalized to mean residue ellipticity, as reported previously (35).
Thioflavin T fluorescence binding assays of protein assembly were performed as described previously (12). Each sample of 10 μl was mixed with 50 μl of 5 μM thioflavin T, and fluorescence signals were measured immediately with a Cary Eclipse fluorescence spectrophotometer (Varian, Walnut Creek, CA, USA).
Western blot analysis
Aliquots (10 μl/sample) collected at different time points of the assembly study were mixed with sample buffer and resolved by SDS-PAGE using 4–20% Criterion Tris HCl Gel (Bio-Rad, Hercules, CA, USA). Precision plus protein standards (Bio-Rad) were included as references. Proteins separated by SDS-PAGE were transferred onto nitrocellulose membranes. The membranes were then incubated with 5% nonfat milk in Tris-buffered saline (TBS) for 30 min, and then processed for immunolabeling with antibodies to α-S (Pan, cat. AB5464; Chemicon, Temecula, CA, USA), HA (Covance Research Products, Berkeley, CA, USA), or FG (Exbio, Praha, Czech Republic). Immunoreactivity was visualized with an enhanced chemiluminescence system (ECL plus, Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK).
Electron and immunoelectron microscopies
Small amounts of samples derived from the assembly study were adsorbed onto carbon-coated copper grids, fixed with 2% formaldehyde in phosphate buffered-saline (PBS), negatively stained with 5% uranyl acetate, and examined with a Philips EM 2008 electron microscope (Phillips, Hillsboro, OR, USA). To determine the width of individual filaments, images were captured at ×40,000, and more than 50 filaments per group were analyzed with MCID software (Imaging Research) by two individuals. To find out whether recombinant proteins SZ4FG and HAZ3S or SΔZ4FG and HAZ3S are able to coassemble, each mixture was adsorbed onto carbon-coated copper grids; fixed in 2% formaldehyde in PBS; blocked with PBS plus 5% normal goat serum, 0.1% BSA, and 0.1% gelatin; and incubated for 1 h with a mixture of anti-HA (mouse, 1:10) and anti-Flag (rabbit, 1:10, F7425, Sigma, St. Louis, MO, USA) antibodies. The grids were then incubated with a mixture of secondary antibodies containing anti-rabbit IgG (1:25) and anti-mouse IgG (1:25), respectively, conjugated with 5 and 10 nm colloidal gold. The immunogold-labeled samples were negatively stained with 2% uranyl acetate and examined by electron microscopy.
Construction of Zip-attached α-S and virus package for AAV transduction
To demonstrate that the artificial Zip attachment affects α-S assembly in intact cells, as demonstrated in test tubes, we used the AAV system to transduce α-S with or without Zip attached at its C end. Here, we chose α-S attached with ZA (SZA) to test. For a control, we used cells transduced with α-S without Zip. The forward primer used for construction of pAAV-SZA was 5′GTCGACAGATCTGCCACCATGGATGTATTCATGAAAGG3′(BglII site underscored), the reverse primer was 5′GGTGGTACTAGTTTAAAGCTGTGCCACCTGTTCCC 3′ (SpeI site underscored), the template for PCR was the constructor pTYB1-SZAHA-intein, the annealing temperature was 46°C, and the product length was 564 bp. The PCR products were cut by restriction enzymes BglII and SpeI and ligated into pAAV empty vector double cut by these enzymes. The vector was derived from AAV of serotype 1. After transformation, isolation of plasmid, and sequencing, endofree-grade plasmid pAAV-SZA and pAAV-S were purified for packaging of AAV. The methods for AAV packaging were described before (36).
AAV infection and immunofluorescence staining of infected cells
Human embryonic kidney (HEK) 293 cells were plated on coverslips (2×104/well) in 24-well plates (Corning, Corning, NY, USA); 24 h later, they were infected with AAV-SZA or AAV-S at a ratio of 105 virus genomes per cell. For a negative control, we used cells without infection. When cell density reached 90% confluency, cells were treated with 50 mM sodium butyrate for 40 h to enhance the expression of target genes SZA and S (37). For detection of both soluble and insoluble α-S, the coverslips were fixed in 100% methanol for 5 min at −20°C, permeabilized with 0.5% Triton X-100 for 5 min, and then processed for α-S immunofluorescence staining with the primary antibody Syn 1 (BD Transduction Lab, San Diego, CA, USA) and goat anti-mouse secondary antibody labeled with Alexa594. For detecting insoluble α-S only, the coverslips were treated with 0.2% Triton X-100 for 5 min before fixation in methanol. This allowed extraction of soluble proteins. For thioflavin S staining, the coverslips were immersed in 0.0005% thioflavin S/PBS/formalin and washed 3 times with 70% ethanol and Tris-buffered saline (pH 7.6). After immuno- and thiofavin S staining, the coverslips were stained with DAPI to locate the nuclei. Samples were evaluated by confocal microscopy (Zeiss LSM 510; Carl Zeiss MicroImaging, Thornwood, NY, USA).
Statistical analysis
For comparison of multiple groups, statistical significance was determined using ANOVA.
RESULTS
Analysis of recombinant α-S and its derivatives by SDS-PAGE and Western blot
Each recombinant preparation separated by SDS-PAGE was demonstrated by silver staining to contain mainly a single band, which was shown by Western blot analysis to display immunoreactivity with antibodies to α-S (Supplemental Fig. 2), but not with those absorbed with wild-type α-S (data not shown). The purity of α-S or its derivatives of most samples was greater than 95%.
Effect of Zip on α-S assembly in vitro
CD spectroscopy
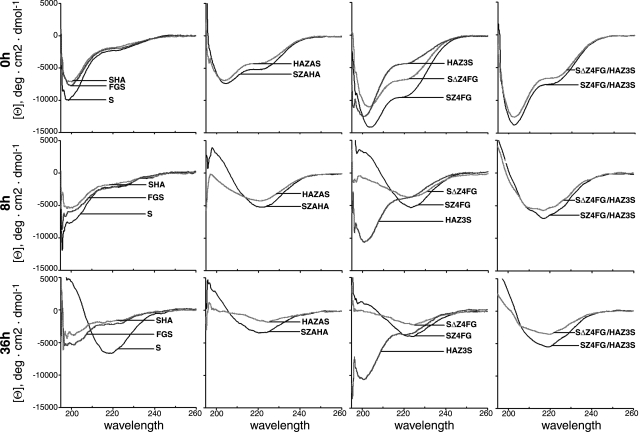
Without incubation (at time 0), the CD spectrum of α-S or derivatives SHA, FGS (control), and HAZ3S exhibited a minimum around 200 nm of molar ellipticity and a small negative shoulder around 220 nm (Fig. 2, 0 h), characteristic of a protein that is primarily random coil. Corresponding samples (at time 0) from other α-S derivatives displayed a minimum around 204 nm and negative shoulder around 220 nm, suggesting that the secondary structure was predominantly random coil with possibly a small percentage of alpha helix content. Samples of SZ4FG/HAZ3S or SΔZ4FG/HAZ3S mixtures (for antiparallel interactions) collected at the 8 h time point (Fig. 2, 8 h) showed a typical β-sheet CD spectrum with a minimum at 218 nm, which is similar to that observed during assembly of untagged wild-type α-S (Fig. 2, 36 h; ref. 38). In comparison, SZAHA, HAZAS, SZ4FG, or SΔZ4FG (all for parallel self-interactions) collected at the same time point showed the β-sheet CD spectrum with a slight shift of the minimum to higher wavelengths (Fig. 2, 8 h) similar to that observed during assembly of α-S with the A53T mutation (38). The molar ellipticity values for the β-sheet conformation decreased gradually with increased incubation time. Such decreases likely reflect a loss of proteins due to sedimentation of large aggregates, since precipitates were observed in samples after incubation, and similar phenomena have been reported by others during amyloid fibril assembly (39). Similar changes of CD spectra were not observed in samples collected at the 8 h time point from S, SHA, FGS, or HAZ3S preparations. Within this group of proteins, β-sheet structure was detected in the S sample only after 36 h (Fig. 2, 36 h). The results demonstrate that the attachment of Zip (except HAZ3S) can effectively increase the propensity of α-S to self-interact.
Figure 2.
Circular dichroism spectra of α-S and derivatives at different time points of assembly. Samples were collected at 0 h (top), 8 h (middle) and 36 h (bottom) of incubation at 37°C.
Thioflavin binding assay
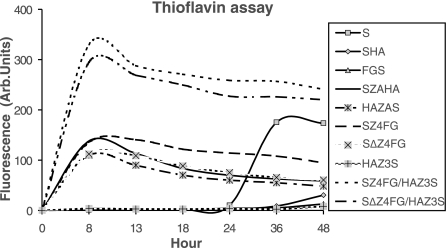
Thioflavin T binding assay demonstrated that the fluorescence signals of SZAHA, HAZAS, SZ4FG, SΔZ4FG, SZ4FG/HAZ3S, or SΔZ4FG/HAZ3S reach the maximum at the 8 h time point (Fig. 3) and decrease slowly afterward due to the aggregation/precipitation of assemblies. In contrast, an increase of fluorescence signal was detected in wild-type α-S collected only after 36 h or longer incubation (Fig. 3). No signal was detected in HA, FGS, and HAZ3S samples with 36 h of vortexing. The results are consistent with those obtained from CD measurement, indicating the facilitation of α-S assembly with Zip attachments (except HAZ3S). Among all groups, the maximum fluorescence signals displayed by antiparallel groups are higher than those displayed by the wild-type α-S and parallel groups. More importantly, there were very little differences between the two mixtures in antiparallel groups in respect to the thioflavin T fluorescence signals.
Figure 3.
Thioflavin T binding assay of α-S and derivatives at different time points of assembly. With the exception of HAZ3S, all samples containing Zip-fused α-S assemble faster than those α-S without any tags, or tagged with HA or FG. Among the Zip fused, those containing the mixtures for antiparallel assembly (i.e., SZ4FG/HAZ3S or SΔZ4FG/HAZ3S) displayed far more intense fluorescence signals than others.
Western blot analysis
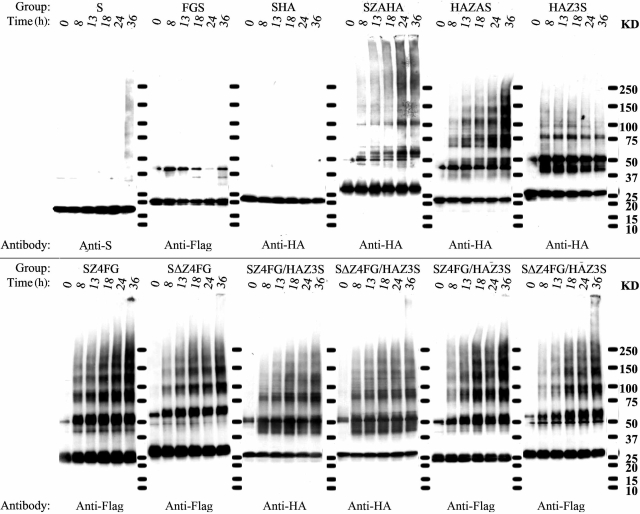
Formation of SDS-stable oligomeric α-S and derivatives was determined by Western blot with antibodies that recognize the N terminus of α-S, HA, or FG tag (Fig. 4). With unmodified α-S, an increase of oligomer assembly was evident after 36 h but not shorter periods of incubation. In comparison, in most Zip-modified preparations, an increase of HA- or FG-immunoreactive, high-molecular-weight species was observed at the 8-h time point.
Figure 4.
Immunoblotting analysis of assemblies derived from α-S and derivatives. Samples were collected at 0, 3, 13, 18, 24, and 36 h of incubation and probed with antibodies to α-S, FG, or HA. Molecular mass markers are labeled between gels; values are listed at right.
When duplicate samples of SZ4FG/HAZ3S or SΔZ4FG/HAZ3S mixtures were probed for HA and FG, both tags were detected in similar sized oligomers, particularly those consistent with trimer or larger oligomers. Some oligomers were more readily distinguishable as bands than others from adjacent smears. A gradual increase of oligomer assembly was observed in preparations containing SZ4FG or SΔZ4FG but not in HAZ3S, SHA, or FGS. Although the smears in most Zip-modified preparations are likely to represent oligomers produced during the process of α-S assembly, those with HAZ3S do not, because the extent of smearing in HAZ3S did not increase with time. The results of Western blot analysis are consistent with those from CD spectrometry and the thioflavin T binding assay, demonstrating the positive effect of enhanced interaction by coiled coils on α-S assembly and, more importantly, the assembly of α-S in both antiparallel and parallel manners.
Ultrastructural characterization and immunogold labeling
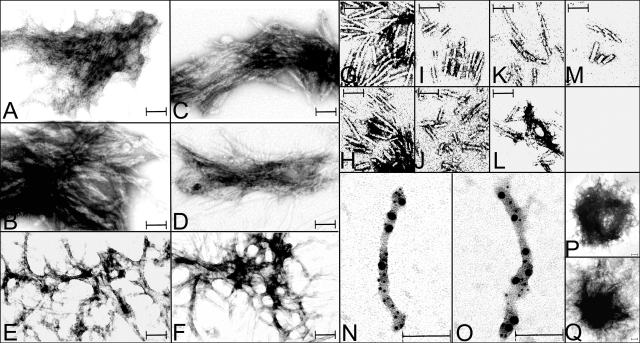
Electron microscopic examination of samples collected at 8 h or at later time points revealed the presence of filamentous aggregates and the absence of dispersed individual filaments in those samples containing 10 μM of SZAHA, HAZAS, SZ4FG, SΔZ4FG, SZ4FG/HAZ3S, or SΔZ4FG/HAZ3S (Fig. 5A–F). To obtain dispersed filaments (Fig. 5G–L), we reduced the rate of assembly by decreasing the concentration of protein to 3 μM, lowering the speed of vortexing the samples, and collecting samples after 16 h of incubation. In preparations containing 10 μM unmodified α-S, dispersed filaments were detected in samples collected at the 36 h time point (Fig. 5M). No filaments were detected in SHA, FGS, and HAZ3S incubated for 36 h.
Figure 5.
Electron micrographs of filaments assembled from α-S and derivatives. Aggregated (A–F) or dispersed filaments (G–M) were assembled from SZAHA (A, G), HAZAS (B, H), SZ4FG (C, I), SΔZ4FG (D, J), SZ4FG/HAZ3S (E, K), SΔZ4FG/HAZ3S (F, L), or Syn (M). Filaments assembled from SZ4FG/HAZ3S (N) and SΔZ4FG/HAZ3S (O) were dual immunogold labeled (5- and 10-nm gold particles) with FG and HA. LB-like aggregates were detected in Zip-fused α-S (e.g., SZAHA) (P) after 36 h of incubation, and in unmodified α-S (Q) only after much longer incubation (>1 wk). Scale bars = 100 nm.
Far more filaments were assembled at a low concentration of Zip-modified samples than at higher concentration of their unmodified counterparts (compare Fig. 5G–L, M). Our assessment of more than 50 filaments in each sample demonstrated that the diameters of these filaments are 11.62 ± 0.19 nm (average±sem) for unmodified S, 11.86 ± 0.18 nm for SZAHA, 11.40 ± 0.16 nm for HAZAS, 11.64 ± 0.19 nm for SZ4FG, 11.69 ± 0.16 nm for SΔZ4FG, 11.83 ± 0.21 nm for SZ4FG/HAZ3S, and 11.48 ± 0.19 nm for SΔZ4FG/HAZ3S. There are no significant differences in diameter between filaments from Zip-modified proteins and those from their unmodified counterparts. However, the presence of different Zips appeared to have some effect on the manner in which filaments interact. In preparations containing a single form of Zip (for parallel interactions) collected at the 8-h time point, we observed aggregates containing filaments packed tightly against each other (Fig. 5A–D). In comparison, in those containing two forms of Zips (for antiparallel interactions), we found aggregates containing loosely packed filaments that appear as meshwork (Fig. 5 E, F). The size and the shape of the aggregates changed with time. By 36 h of incubation, the aggregates assumed a shape resembling LBs. Such LB-like aggregates were detected in the unmodified α-S only after a much longer incubation (>1 wk) (Fig. 5P, Q).
Our immunogold labeling of SZ4FG/HAZ3S or SΔZ4FG/HAZ3S assemblies showed the codecoration of filamentous structures with both 5 nm (for FG tag) and 10 nm (for HA tag) gold particles (Fig. 5N, O). Such dual-labeled filaments were not detected in samples containing a single form of modified or unmodified α-S (data not shown).
Effect of Zip on α-S assembly in cultured cells
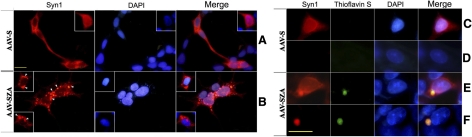
By α-S immunofluorescence staining, we demonstrated that AAV-SZA- or AAV-S-infected cells express the intended gene products in cell bodies and short processes. When cells were fixed with methanol then permeabilized with 0.5% Triton X-100, α-S immunoreactivities were located in multiple small clusters and large inclusions in cells overexpressing SZA (Fig. 6B, E) but distributed diffusely in cells overexpressing α-S without Zip (Fig. 6A, C). The small aggregates were thioflavin S negative, and the large inclusions were positive. When cells were treated with 0.2% Triton X-100 first and then fixed in methanol, only large inclusions positive with Syn1 antibody and thioflavin S were observed in the AAV-SZA-infected cells (Fig. 6F). No fluorescence signals were detected in the cells infected with AAV-S (Fig. 6D). About 40% of the AAV-SZA-infected cells contained aggregated α-S. Cells without the infection displayed only very weak or no staining regardless of the fixation protocol used (data not shown).
Figure 6.
Formation of small aggregates and large inclusions in HEK293 cells infected with ZipA tagged α-S (AAV-SZA). Small aggregates (B) and larger inclusions (B, E, F) recognized by antibodies to α-S were demonstrated after 7 days of infection. The large inclusions were thioflavin S positive and preserved regardless of fixation/permeabiliation protocols: fixed with methanol then permeabilized with 0.5% Triton X-100 (E) or permeabilized first then fixed with methanol (F). In contrast, the small aggregates were thioflavin S negative and well preserved in cells fixed first then permeabilized (B) but not in those permeabilized first then fixed with methanol (data not shown). Cells infected with untagged α-S (AAV-S) displayed α-synuclein immunoreactivity in a diffuse pattern if they were fixed first then permeabilized (A, C) but showed no fluorescence signals if they were permeabilized before fixation (D). Nuclei were marked with DAPI stain. Scale bars = 25 μM.
DISCUSSION
In the present study, we investigated the mode of wild-type α-S self-interactions using derivatives containing three artificial Zips (ZA, Z3, and Z4). All Zips are capable of forming homodimers of coiled coils in parallel fashion, and Z3 and Z4 have a high tendency to form heterodimers in antiparallel fashion with each other. On the basis of the Zip type, the site of Zip attachment and HA or FG labeling, and the presence or absence of a spacer, the recombinant proteins generated from α-S with or without Zip or tags were regarded as SZAHA, HAZAS, HAZ3S, SZ4FG, SΔZ4FG, SHA, FGS, and S. In total, we designed 10 groups for assembly: SZAHA and HAZAS for parallel aggregation; SZ4FG/HAZ3S and SΔZ4FG/HAZ3S for antiparallel aggregation; SZ4FG, SΔZ4FG, and HAZ3S for controls of antiparallel aggregation (can also be considered as parallel aggregation); SHA and FGS for controls of Zip attachment; and S for control of all attachments.
On one hand, CD analysis and thioflavin T binding assay of our samples collected at different time points of in vitro assembly revealed a marked increase in the propensity of α-S to form β-structures or filaments by attachment of parallel or antiparallel Zips; moreover, thioflavin T binding assay showed that the emission of fluorescence signals in antiparallel aggregation groups (SZ4FG/HAZ3S and SΔZ4FG/HAZ3S) are more intense than those in parallel ones, and that the assembly kinetics of SZ4FG/HAZ3S are comparable to that of SΔZ4FG/HAZ3S. These results suggested that α-S can assemble in both antiparallel and parallel manners, that the process of antiparallel assembly does not require a precise alignment of the central hydrophobic region, and that α-S may have a higher tendency to assemble in an antiparallel mode. On the other hand, they also showed a lack of positive impact of Z3, HA, or FG attachment on α-S interaction or thioflavin T binding during assemblies. We do not know why Z3 differed from other Zips in lacking a positive effect on α-S assembly. It is possible that the concentration of Z3 in our samples is too low to form coiled coils, because Z3 self-interaction was demonstrated previously at a concentration above 200 μM (34), far exceeding the concentration used in the present study. Alternatively, the charge or the location of Z3 may reduce the ability of α-S to assemble in a parallel fashion. Besides, although it is reasonable to assume that both SZ4FG or SΔZ4FG and HAZ3S can coassemble, assemblies derived from SZ4FG or SΔZ4FG may induce the self-assembly of HAZ3S. This issue was examined subsequently by Western blot analysis and immunoelectron microscopy.
Consistent with the results of CD spectrometry and thioflavin T binding assay, our Western blot analysis also demonstrated the positive effect of Zips on α-S assembly and the assembly of α-S in both antiparallel and parallel manners. More importantly, duplicate gels probed for HA and FG in SZ4FG/HAZ3S or SΔZ4FG/HAZ3S groups showed similar increasing patterns of both tags with different sized oligomers, which suggests that both SZ4FG or SΔZ4FG and HAZ3S can coassemble. Besides, we noted that dimeric α-S and derivatives in different amounts were detected at the 0 h time point. Because they were not readily detected in the stock of purified proteins, the formation of these α-S species appears to occur during the process of preparing samples for assembly. As described in Materials and Methods, all samples were subjected to 30 min heating to 70°C for the purpose of reducing coiled coils formed during storage.
By electron microscopy we demonstrated that α-S attached with Zips (except HAZ3S) can form filaments much faster than those without Zip attachment. We also noted that, for α-S, the changes of morphology due to Zip attachment are not in the diameter of its single filament, but in the shape of filamentous aggregates. The significance of such a difference (loosely or tightly packed filamentous aggregates) might be due to the different fashion (antiparallel or parallel) of α-S self-interaction. With respect to filament packing, note that LBs and Lewy neurites contain loosely packed filaments in random orientation (40). To resolve the issue of whether filaments assembled in SZ4FG/HAZ3S or SΔZ4FG/HAZ3S were due to coassembly of α-S with different Zips, we dual stained the filaments with antibodies to HA or Flag and successfully detected both tags by immunoelectron microscopy. Combined with Western blot pattern aforementioned, the result demonstrates conclusively the coassembly of α-S in an antiparallel manner.
We overexpressed one Zip-tagged α-S (SZA) in cultured cells via AAV infection to study the effect of Zip attachment on α-S self-interaction and showed the accumulation of small aggregates and large inclusions in the AAV-SZA-infected but not in the AAV-S-infected cells. Our findings that large- and small-sized aggregates differ with regard to their susceptibility to detergent extraction suggest that small-sized aggregates are less stable than large inclusions. Although it remains unclear whether there is a chronological order in the formation of small clusters vs. large inclusions, it is possible that large inclusions may result from the congregation of small clusters. It is worth noting that cells bearing inclusions or aggregates have intact nuclei, suggesting that such accumulation of α-S may not be cytotoxic. Further studies of cells at multiple time points after infection with AAV-SZA are necessary to draw a conclusion.
Nevertheless, our results indicate that α-S assembly can be facilitated by coiled coil interactions in situ, as well as in vitro. In view of the finding of abundant α-S inclusions and aggregates in cultured cells only a few days (≤7 days) after overexpressing Zip-modified proteins, we propose that such cultures may be used as a model system for initial screening of potential agents that impede protein aggregation. For drug screening, this model is likely to be more cost effective and less time consuming than using animal models.
Exposure to metals or neurotoxins is a risk factor for PD (41), and genomic multiplication of wild-type α-S gene has been linked to familial PD (42). It has been demonstrated that α-S self-interactions can be promoted by various experimental treatments, including exposure to metals or neurotoxins (43, 44), increases in the temperature of incubation (45), the addition of fatty acids and related anionic detergents (46, 47), and the addition of polycations or polyamines (48, 49). Although the initial effects of different factors on α-S self-interactions may be different, it is conceivable that they all eventually lead to increases in the local concentration of α-S and/or the stability of dimers/oligomers as in the case with Zip attachment.
CONCLUSIONS
Our results, summarized in Table 2, indicate that 1) α-S proteins can assemble via both parallel and antiparallel modes but have a higher tendency to assemble via the latter mode; 2) a precise alignment of the central hydrophobic region is not essential for α-S assembly; 3) coiled coils can strongly facilitate wild-type α-S assemblies to aggregate in vitro and in situ; and 4) cells overexpressing Zip attached α-S may be used for drug screening.
TABLE 2.
Summary
| Group | Change detected, earliest time point (h)
|
Aggregates in cells | |||
|---|---|---|---|---|---|
| β-Sheet: CD | ThT assay (value) | Western blot analysis | EM | ||
| Control for all | |||||
| S | 36 | 36 (175) | 36 | 36 | No |
| SHA | ≫36 | ≫36 | ≫36 | ≫36 | ND |
| FGS | ≫36 | ≫36 | ≫36 | ≫36 | ND |
| Parallel | |||||
| SZAHA | 8 | 8 (137) | 8 | 8 | Yes |
| HAZAS | 8 | 8 (110) | 8 | 8 | ND |
| Antiparallel | |||||
| SZ4FG/HAZ3S | 8 | 8 (330) | 8 | 8 | ND |
| SΔZ4FG/HAZ3S | 8 | 8 (294) | 8 | 8 | ND |
| Control for antiparallel | |||||
| SZ4FG | 8 | 8 (135) | 8 | 8 | ND |
| SΔZ4FG | 8 | 8 (112) | 8 | 8 | ND |
| HAZ3S | ≫36 | ≫36 | ≫36 | ≫36 | ND |
EM, electron microscopy; ND, not done; ThT, thioflavin T.
Acknowledgments
We thank Drs. Terrone Rosenberry and Vijayaraghavan Rangachari for assistance in CD analysis and valuable comments. We are grateful to Dr. Wen-Lang Lin for assistance in electron microscopy, to Dr. Tania Gendron for her critical reading, and to Ms. Carolina Ceballos-Diaz and Ms. Ena Causevic for technical assistance in AAV packaging and Western blot analysis. This study was supported by the U.S. National Institutes of Health (P50-NS40256) and the Mayo Foundation.
References
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva H A, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli J T, Scheller R H. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Muller T, Riess O. Involvement of alpha-synuclein in Parkinson’s disease and other neurodegenerative disorders. J Neural Transm. 2000;107:31–40. doi: 10.1007/s007020050002. [DOI] [PubMed] [Google Scholar]
- Galvin J E, Lee V M, Trojanowski J Q. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero D A, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb P H, Zhen W, Poon A W, Conway K A, Lansbury P T., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- Lundvig D, Lindersson E, Jensen P H. Pathogenic effects of alpha-synuclein aggregation. Brain Res Mol Brain Res. 2005;134:3–17. doi: 10.1016/j.molbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Giasson B I, Murray I V, Trojanowski J Q, Lee V M. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- Conway K A, Harper J D, Lansbury P T. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- El-Agnaf O M, Jakes R, Curran M D, Wallace A. Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of alpha-synuclein protein implicated in Parkinson’s disease. FEBS Lett. 1998;440:67–70. doi: 10.1016/s0014-5793(98)01419-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hsu L J, Sisk A, Xia Y, Takeda A, Sundsmo M, Masliah E. Human recombinant NACP/alpha-synuclein is aggregated and fibrillated in vitro: relevance for Lewy body disease. Brain Res. 1998;799:301–306. doi: 10.1016/s0006-8993(98)00514-9. [DOI] [PubMed] [Google Scholar]
- Conway K A, Harper J D, Lansbury P T., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood S J, Steavenson S, Jiang Y, Wu G M, Anafi D, Kaufman S A, Martin F, Sitney K, Denis P, Louis J C, Wypych J, Biere A L, Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan M, Jensen P H, Marsh D. Association of alpha-synuclein and mutants with lipid membranes: spin-label ESR and polarized IR. Biochemistry. 2006;45:3386–3395. doi: 10.1021/bi052344d. [DOI] [PubMed] [Google Scholar]
- Klucken J, Outeiro T F, Nguyen P, McLean P J, Hyman B T. Detection of novel intracellular alpha-synuclein oligomeric species by fluorescence lifetime imaging. FASEB J. 2006;20:2050–2057. doi: 10.1096/fj.05-5422com. [DOI] [PubMed] [Google Scholar]
- Chen M, Margittai M, Chen J, Langen R. Investigation of alpha-synuclein fibril structure by site-directed spin labeling. J Biol Chem. 2007;282:24970–24979. doi: 10.1074/jbc.M700368200. [DOI] [PubMed] [Google Scholar]
- Der-Sarkissian A, Jao C C, Chen J, Langen R. Structural organization of alpha-synuclein fibrils studied by site-directed spin labeling. J Biol Chem. 2003;278:37530–37535. doi: 10.1074/jbc.M305266200. [DOI] [PubMed] [Google Scholar]
- Del Mar C, Greenbaum E A, Mayne L, Englander S W, Woods V L., Jr Structure and properties of alpha-synuclein and other amyloids determined at the amino acid level. Proc Natl Acad Sci U S A. 2005;102:15477–15482. doi: 10.1073/pnas.0507405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W H, Johnson P F, McKnight S L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- O'Shea E K, Rutkowski R, Kim P S. Evidence that the leucine zipper is a coiled coil. Science. 1989;243:538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- O'Shea E K, Klemm J D, Kim P S, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- Cohen C, Parry D A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7:1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Oakley M G, Kim P S. A buried polar interaction can direct the relative orientation of helices in a coiled coil. Biochemistry. 1998;37:12603–12610. doi: 10.1021/bi981269m. [DOI] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- Kohn W D, Mant C T, Hodges R S. Alpha-helical protein assembly motifs. J Biol Chem. 1997;272:2583–2586. doi: 10.1074/jbc.272.5.2583. [DOI] [PubMed] [Google Scholar]
- Bryson J W, Betz S F, Lu H S, Suich D J, Zhou H X, O'Neil K T, DeGrado W F. Protein design: a hierarchic approach. Science. 1995;270:935–941. doi: 10.1126/science.270.5238.935. [DOI] [PubMed] [Google Scholar]
- Oakley M G, Kim P S. Protein dissection of the antiparallel coiled coil from Escherichia coli seryl tRNA synthetase. Biochemistry. 1997;36:2544–2549. doi: 10.1021/bi962391t. [DOI] [PubMed] [Google Scholar]
- Arndt K M, Pelletier J N, Muller K M, Alber T, Michnick S W, Pluckthun A. A heterodimeric coiled-coil peptide pair selected in vivo from a designed library-versus-library ensemble. J Mol Biol. 2000;295:627–639. doi: 10.1006/jmbi.1999.3352. [DOI] [PubMed] [Google Scholar]
- Pelletier J N, Arndt K M, Pluckthun A, Michnick S W. An in vivo library-versus-library selection of optimized protein-protein interactions. Nat Biotechnol. 1999;17:683–690. doi: 10.1038/10897. [DOI] [PubMed] [Google Scholar]
- Kim B M, Oakley M G. A general method for selection and screening of coiled coils on the basis of relative helix orientation. J Am Chem Soc. 2002;124:8237–8244. doi: 10.1021/ja020275+. [DOI] [PubMed] [Google Scholar]
- Sharma A, Antoku S, Fujiwara K, Mayer B J. Functional interaction trap: a strategy for validating the functional consequences of tyrosine phosphorylation of specific substrates in vivo. Mol Cell Proteomics. 2003;2:1217–1224. doi: 10.1074/mcp.M300078-MCP200. [DOI] [PubMed] [Google Scholar]
- Devit M, Cullen P J, Branson M, Sprague G F, Jr, Fields S. Forcing interactions as a genetic screen to identify proteins that exert a defined activity. Genome Res. 2005;15:560–565. doi: 10.1101/gr.3259905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh I, Hamilton A D, Regan L. Antiparallel leucine zipper-directed protein reassembly: application to the green fluorescent protein. J Am Chem Soc. 2000;122:5658. [Google Scholar]
- Magliery T J, Wilson C G, Pan W, Mishler D, Ghosh I, Hamilton A D, Regan L. Detecting protein-protein interactions with a green fluorescent protein fragment reassembly trap: scope and mechanism. J Am Chem Soc. 2005;127:146–157. doi: 10.1021/ja046699g. [DOI] [PubMed] [Google Scholar]
- Rangachari V, Reed D K, Moore B D, Rosenberry T L. Secondary structure and interfacial aggregation of amyloid-beta(1–40) on sodium dodecyl sulfate micelles. Biochemistry. 2006;45:8639–8648. doi: 10.1021/bi060323t. [DOI] [PubMed] [Google Scholar]
- Levites Y, Jansen K, Smithson L A, Dakin R, Holloway V M, Das P, Golde T E. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci. 2006;26:11923–11928. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W Y, Bailey E C, McCune S L, Dong J Y, Townes T M. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc Natl Acad Sci U S A. 1997;94:5798–5803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell L C, Berriman J, Jakes R, Goedert M, Crowther R A. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci U S A. 2000;97:4897–4902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjernberg L O, Tjernberg A, Bark N, Shi Y, Ruzsicska B P, Bu Z, Thyberg J, Callaway D J. Assembling amyloid fibrils from designed structures containing a significant amyloid beta-peptide fragment. Biochem J. 2002;366:343–351. doi: 10.1042/BJ20020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima K, Ueda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, Kawai M. Immunoelectron-microscopic demonstration of NACP/alpha-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson’s disease and in dementia with Lewy bodies. Brain Res. 1998;808:93–100. doi: 10.1016/s0006-8993(98)00734-3. [DOI] [PubMed] [Google Scholar]
- Gorell J M, Johnson C C, Rybicki B A, Peterson E L, Kortsha G X, Brown G G, Richardson R J. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20:239–247. [PubMed] [Google Scholar]
- Singleton A B, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson M R, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Uversky V N, Li J, Fink A L. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- Uversky V N, Li J, Bower K, Fink A L. Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: implications for Parkinson’s disease. Neurotoxicology. 2002;23:527–536. doi: 10.1016/s0161-813x(02)00067-0. [DOI] [PubMed] [Google Scholar]
- Uversky V N, Lee H J, Li J, Fink A L, Lee S J. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem. 2001;276:43495–43498. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- Necula M, Chirita C N, Kuret J. Rapid anionic micelle-mediated alpha-synuclein fibrillization in vitro. J Biol Chem. 2003;278:46674–46680. doi: 10.1074/jbc.M308231200. [DOI] [PubMed] [Google Scholar]
- Cole N B, Murphy D D, Grider T, Rueter S, Brasaemle D, Nussbaum R L. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- Goers J, Uversky V N, Fink A L. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci. 2003;12:702–707. doi: 10.1110/ps.0230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony T, Hoyer W, Cherny D, Heim G, Jovin T M, Subramaniam V. Cellular polyamines promote the aggregation of alpha-synuclein. J Biol Chem. 2003;278:3235–3240. doi: 10.1074/jbc.M208249200. [DOI] [PubMed] [Google Scholar]