Abstract
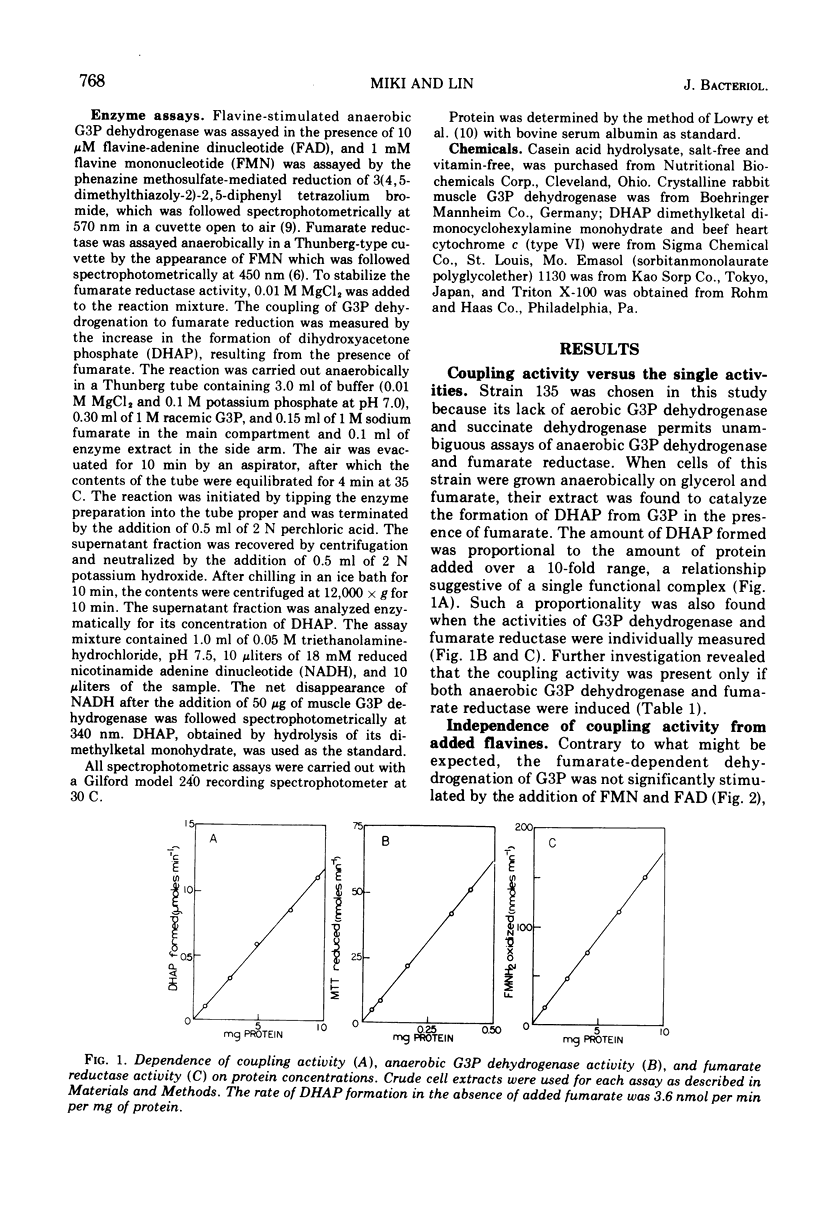
Anaerobic growth of Escherichia coli on glycerol as carbon source and fumarate as hydrogen acceptor results in the induction of both anaerobic sn-glycerol-3-phosphate (G3P) dehydrogenase and fumarate reductase. In previous studies, the G3P dehydrogenase was measured by the G3P-dependent reduction of a tetrazolium dye mediated by phenazine methosulfate in the presence of flavine mononucleotide and flavine adenine dinucleotide, and fumarate reductase was measured by the fumarate-dependent oxidation of reduced flavine mononucleotide. Results from the present study indicate that these two enzymes actually constitute a functional complex which is sedimentable at 200,000 × g in 2 h and which can catalyze the dehydrogenation of G3P at the expense of fumarate without any added cofactor. This coupling activity is not constituted by simply mixing an extract containing anaerobic G3P dehydrogenase with another extract containing fumarate reductase. Additional evidence for an organized complex is provided by the high degree of sensitivity of the coupled reaction to low concentrations of the detergents Triton X-100 and Emasol 1130 in comparison with the individual activities of the two component enzymes measured with the aid of artificial cofactors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Faust P. J., Vandemark P. J. Phosphorylation coupled to NADH oxidation with fumarate in Streptococcus faecalis 10Cl. Arch Biochem Biophys. 1970 Apr;137(2):392–398. doi: 10.1016/0003-9861(70)90454-6. [DOI] [PubMed] [Google Scholar]
- GEST H. Oxidation and evolution of molecular hydrogen by microorganisms. Bacteriol Rev. 1954 Mar;18(1):43–73. doi: 10.1128/br.18.1.43-73.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- HIRSCHMANN H. The nature of substrate asymmetry in stereoselective reactions. J Biol Chem. 1960 Oct;235:2762–2767. [PubMed] [Google Scholar]
- Hatchikian E. C., Le Gall J. Evidence for the presence of a b-type cytochrome in the sulfate-reducing bacterium Desulfovibrio gigas, and its role in the reduction of fumarate by molecular hydrogen. Biochim Biophys Acta. 1972 Jun 23;267(3):479–484. doi: 10.1016/0005-2728(72)90175-2. [DOI] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Purification and properties of the flavine-stimulated anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli. J Bacteriol. 1972 Oct;112(1):539–547. doi: 10.1128/jb.112.1.539-547.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PECK H. D., Jr, SMITH O. H., GEST H. Comparative biochemistry of the biological reduction of fumaric acid. Biochim Biophys Acta. 1957 Jul;25(1):142–147. doi: 10.1016/0006-3002(57)90431-6. [DOI] [PubMed] [Google Scholar]
- Quastel J. H., Stephenson M. Further Observations on the Anaerobic Growth of Bacteria. Biochem J. 1925;19(4):660–666. doi: 10.1042/bj0190660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Stephenson M., Whetham M. D. Some Reactions of Resting Bacteria in Relation to Anaerobic Growth. Biochem J. 1925;19(2):304–317. doi: 10.1042/bj0190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


