Abstract
In cardiac myocytes there is evidence that activation of some receptors can regulate protein kinase A (PKA)-dependent responses by stimulating cAMP production that is limited to discrete intracellular domains. We previously developed a computational model of compartmentalized cAMP signaling to investigate the feasibility of this idea. The model was able to reproduce experimental results demonstrating that both β1-adrenergic and M2 muscarinic receptor-mediated cAMP changes occur in microdomains associated with PKA signaling. However, the model also suggested that the cAMP concentration throughout most of the cell could be significantly higher than that found in PKA-signaling domains. In the present study we tested this counterintuitive hypothesis using a freely diffusible fluorescence resonance energy transfer-based biosensor constructed from the type 2 exchange protein activated by cAMP (Epac2-camps). It was determined that in adult ventricular myocytes the basal cAMP concentration detected by the probe is ∼1.2 μM, which is high enough to maximally activate PKA. Furthermore, the probe detected responses produced by both β1 and M2 receptor activation. Modeling suggests that responses detected by Epac2-camps mainly reflect what is happening in a bulk cytosolic compartment with little contribution from microdomains where PKA signaling occurs. These results support the conclusion that even though β1 and M2 receptor activation can produce global changes in cAMP, compartmentation plays an important role by maintaining microdomains where cAMP levels are significantly below that found throughout most of the cell. This allows receptor stimulation to regulate cAMP activity over concentration ranges appropriate for modulating both higher (e.g., PKA) and lower affinity (e.g., Epac) effectors.
Keywords: β-adrenergic receptor signaling, muscarinic receptor signaling, live cell imaging, fluorescence resonance energy transfer, biosensors
many different neurotransmitters and hormones control a wide range of cellular processes by regulating the production of a common second messenger, cAMP (32). This signaling pathway plays a particularly important role in sympathetic regulation of cardiac function, where β1-adrenergic receptor (β1AR) activation generates significant changes in the electrical, mechanical, and metabolic properties of the heart by stimulating the production of cAMP and activating protein kinase A (PKA) (4). Although other G protein-coupled receptors are also able to affect cAMP production in the heart, they do not all produce the same responses. This has led to the hypothesis that cAMP production is compartmentalized and that different receptors can regulate cAMP production in specific microdomains (31).
The recent development of several different biosensors capable of monitoring cAMP activity in intact living cells has provided a means of obtaining more direct proof that compartmentation occurs (20, 33, 37). Some of these sensors are targeted to specific subcellular locations. This includes the fluorescence resonance energy transfer (FRET)-based biosensor constructed using the type II regulatory and catalytic subunits of PKA (38). Like endogenous type II PKA, this probe is targeted to the peripheral plasma membrane and along the transverse tubules through its interaction with A kinase anchoring proteins (34, 38). Because of its targeted expression, the PKA-based probe is appropriate for detecting cAMP responses occurring in PKA signaling domains (12). Some other biosensors are expressed more uniformly. This includes the FRET-based biosensor constructed with the nucleotide binding domain of the type 2 exchange protein activated by cAMP (Epac2-camps). The absence of anchoring sequences found in the full-length protein results in homogeneous expression of this probe throughout the cytoplasm of cells (19, 35). Consequently, this probe is expected to respond to changes in cAMP occurring anywhere in the cell.
When expressed in adult ventricular myocytes, the PKA- and Epac2-based probes both respond to β1AR stimulation (35). However, only the Epac2-camps responds to prostaglandin receptor activation, which indicates that it is able to detect changes in cAMP occurring in subcellular locations outside of those where type II PKA is found (35). This raises the question of whether or not β1AR stimulation elicits a uniform increase in cAMP throughout the cell. It has actually been suggested the βAR activation selectively stimulates cAMP production in discrete microdomains where the PKA-based probe is expressed (38). If this is true, then the same amount of cAMP averaged over the greater cytoplasmic volume monitored by the Epac2-based probe should appear as a lower concentration. However, to date this biosensor has only been used to measure relative changes in cAMP activity.
Previously we used a computational modeling approach to demonstrate that compartmentation of cAMP signaling is a feasible explanation for β1-adrenergic as well as M2 muscarinic receptor (M2R) regulation of cAMP responses in adult ventricular myocytes (12). However, one of the predictions of that model is β1AR stimulation produces changes in cAMP throughout the entire cell. Furthermore, it predicts that the basal concentration of cAMP in the bulk cytoplasmic compartment is actually much greater than that found in microdomains where type II PKA signaling occurs. Another prediction is that M2R activation can elicit stimulatory as well as inhibitory effects on cAMP levels in PKA signaling domains, without producing corresponding changes in cAMP concentrations in the bulk cytosolic compartment. The initial goal of the present study was to test those predictions by using the Epac2-based biosensor to estimate the actual concentration of total cAMP in intact adult cardiac myocytes. In addition to supporting the hypothesis that compartmentation of cAMP is important for explaining different types of receptor mediated responses, our results also demonstrate that compartmentation is important under basal conditions, by maintaining microdomains where cAMP levels are kept well below bulk cytoplasmic levels to preserve PKA-dependent signaling.
METHODS
Myocyte isolation and biosensor expression.
Cardiac ventricular myocytes were isolated from adult Hartley guinea pigs by methods previously described (2, 16). The protocol used was approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. Microbial contamination of cell suspensions was minimized by gravity sedimentation and subsequent plating (105 rod shaped cells/plate) in sterile, serum-free, Dulbecco's modified Eagle medium (GIBCO-Invitrogen) containing 1% penicillin-streptomycin. The Epac2-camps biosensor used in this study consists of the cAMP binding domain of Epac2 with yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) attached to the amino and carboxy terminal ends, respectively (18). An adenovirus expressing this biosensor (Ad-YFP-Epac2-CFP) was created by use of the Clonetech Adeno-X-expression system (BD Biosciences), as described previously (35). Myocytes were exposed to virus-containing media (MOI 10 to 50) for up to 48 h. Epac2-camps expression was monitored by measuring CFP fluorescence.
Live cell FRET measurements.
Infected cells were transferred to a chamber (model 26G, Warner Instruments) mounted on the stage of an inverted microscope (model IX70, Olympus) and perfused with a solution containing (in mM) 140 NaCl, 5.4 KCl, 2.5 MgCl2, 1.5 CaCl2, 11 glucose, and 5.5 HEPES (pH 7.4). Images were obtained with a ×40 water-immersion objective (1.3 numerical aperture; Olympus) and CCD camera (Orca ER, Hamamatsu). CFP excitation was achieved by using a 175-W xenon arc lamp (Lambda DG-4, Sutter Instruments) with a D436/20 band-pass filter and a 455DCLP dichroic mirror. CFP and YFP (FRET) emissions were measured with D480/30 and D535/30 band-pass filters, respectively. Ratiometric FRET measurements were made by simultaneously recording CFP and YFP fluorescence via a Dual View Micro Imager (Optical Insights) equipped with a 505DCXR beam splitter. All filters were obtained from Chroma Technology. Cells were excited for 50–200 ms once every 20 s. Fluorescence images were acquired by 2 × 2 binning and analyzed with Simple PCI imaging software (Compix).
Spectral bleed-through was measured in cells expressing CFP or YFP constructs alone. The band-pass filter used for excitation of CFP caused no detectible excitation of YFP. However, direct excitation of CFP did result in CFP emission detected in the YFP channel. CFP fluorescence detected in the YFP channel was 25% of that measured in the CFP channel. FRET responses (F) were defined as the change in bleed-through corrected CFP/YFP whole cell fluorescence intensity ratio (ΔR) over the baseline ratio (R0).
In vitro FRET measurements.
Epac2-camps expressed in Sf9 cells was used to calculate the concentration of cAMP that produces half-maximal activation of the probe (EC50) as described previously (18). Purified protein was suspended in a solution containing 110 mM K-aspartate, 20 mM KCl, 5 mM MgATP, and 5 mM HEPES (pH 7.2). The purpose of this solution was to mimic the ionic environment inside a cardiac myocyte. The EC50 and Hill coefficient (nH) for cAMP activation of the probe was determined to 1.1 μM and 1, respectively (see Supplemental Fig. S1). The absolute maximal FRET response measured in vivo (Fabs) was defined as the percent increase in magnitude of the FRET response observed in the presence of a saturating concentration of cAMP over that observed in the absence of cAMP. Under these conditions Fabs was determined to be 43%.
Calculation of cAMP concentration.
The concentration of cAMP detected by Epac2-camps in intact cells was estimated by a generalization of the approach described previously (12). The FRET response measured in intact cells (F) was compared with the relationship between cAMP and the FRET response measured in vitro. The magnitude of the maximum FRET response observed in intact cells (Fmax) was significantly smaller than the absolute maximum FRET response measured in vitro (Fabs) (see results). Assuming that Fmax and Fabs both represent saturation of cAMP binding, this suggests that in intact cells basal levels of cAMP are high enough to cause significant activation of the probe. To calculate the cAMP concentration detected by Epac2-camps, we used the following relationship (see supplement for derivation):
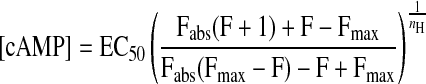 |
(1) |
Under unstimulated conditions, when F = 0, the basal concentration of cAMP can be estimated by measuring Fmax in intact cells and using the values for EC50, nH, and Fabs determined in vitro.
Drugs.
Isoproterenol (Iso) and acetylcholine were prepared fresh as aqueous stock solutions on the day of experiments. Ascorbic acid (50 μM) was added to all Iso-containing solutions. The phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) was prepared as stock solution in DMSO. All chemicals used were purchased from either Sigma-Aldrich or Calbiochem.
Statistical analysis.
All data are expressed as means ± SE obtained from n number of cells. Statistical comparisons were conducted by Student's t-test, with statistical significance defined by P values <0.05.
Computational modeling.
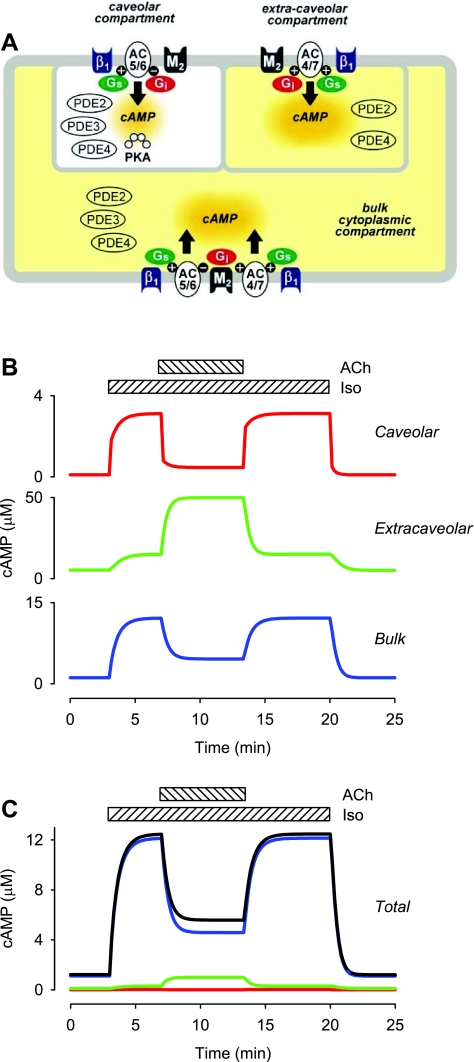
Our previously published computational model of β1AR and M2R regulation of cAMP production in a cardiac ventricular myocyte was used as the basis for this study (12). In this model, existing kinetic data on the signaling pathways involved in regulating cAMP production and degradation are incorporated into a theoretical cell consisting of three different compartments. The first is the subsarcolemmal space associated with the caveolar membrane domains of the cell. The key signaling elements included in that compartment have been found in cholesterol-rich membrane fractions that are associated with caveolin 3, the muscle specific form of caveolin that is involved in creating signaling complexes necessary for producing functional responses (13, 21, 30). This compartment is assumed to include ∼1% of the total cytosolic volume and 10% of the plasma membrane surface area (4). The second compartment reflects the subsarcolemmal space associated with cholesterol-rich lipid rafts that do not include caveolin. It is assumed to comprise ∼2% of the cytosolic volume and 20% of the plasma membrane surface area. The size and dimensions of these submembrane compartments are predictions that arise from the ability of the model to explain previously published experimental data obtained using the PKA-based biosensor (12) and experimental data presented in the current manuscript, which were obtained using the Epac2-based biosensor. The size of these domains is consistent with previously published estimates of the size of submembrane compartments for cAMP made by Rich et al. (23). The final domain is the bulk cytoplasmic compartment, which makes up the balance of the cytosolic volume and is associated with the remainder of the plasma membrane. In the original model, the distribution of signaling elements was based on studies using biochemical methods to detect the presence of those proteins in different cellular fractions. Most membrane proteins found outside of caveolae were assumed to exist solely in the extracaveolar domain. In the present study, the model has been modified so that those proteins are equally distributed between the extracaveolar and bulk cytoplasmic domains. Numerical values of all parameters used in the present version of the model can be found in Supplemental Table S1.
RESULTS
β-Adrenergic responses.
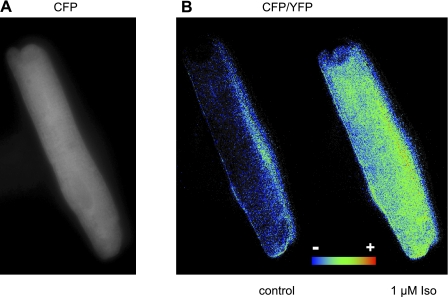
Guinea pig ventricular myocytes were chosen for this study because they express only the β1AR subtype (11, 22). Exposure of these cells to adenovirus containing the DNA sequence for the Epac2-based biosensor exhibited significant expression 24–48 h after infection (Fig. 1A). Exposure of these cells to the βAR agonist Iso produced a change in the probe's FRET response (F) consistent with an increase in cAMP production (Fig. 1). Agonist stimulation resulted in an apparent homogeneous change in the CFP-to-YFP ratio images. This supports the idea that as in other cell types (18) this probe is able to diffuse freely throughout the cytoplasm of cardiac myocytes.
Fig. 1.
Guinea pig ventricular myocyte 48 h after infection with adenovirus expressing the type 2 exchange protein activated by cAMP (Epac2- camps) biosensor. A: expression pattern illustrated by cyan fluorescent protein (CFP) fluorescence. B: pseudocolor image of the CFP-to yellow fluorescent protein (YFP) (CFP/YFP) fluorescence ratio before and after exposure to a maximally stimulating concentration (1 μM) of the β-adrenergic receptor agonist isoproterenol (Iso).
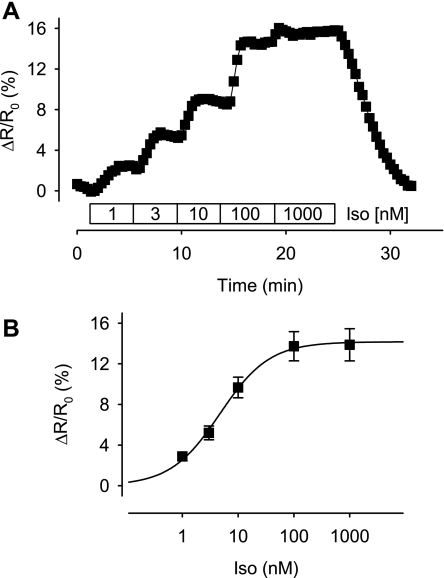
The concentration dependence of the Iso response due to activation of β1ARs is illustrated in Fig. 2. In these experiments the response to various concentrations of Iso were compared with the magnitude of the response produced by a maximally stimulating concentration (1 μM) of Iso in the same cell. The maximum FRET response produced by 1 μM Iso in this set of experiments was 13.9 ± 1.6% (n = 6) over baseline, and the Iso EC50 was 4.7 nM.
Fig. 2.
Concentration dependence of the type 2 exchange protein activated by cAMP (Epac2-camps) response to β-adrenergic receptor stimulation with Iso. A: time course of the relative change in CFP/YFP emission intensity ratio (ΔR/R0) produced by Epac2-camps during exposure of a ventricular myocyte to increasing concentrations of Iso. B: concentration response relationship for the Epac2-camps response to Iso. Data points were fit by a Hill equation with the following parameters: EC50, 4.7 nM; maximum response, 14%; Hill coefficient, 1.
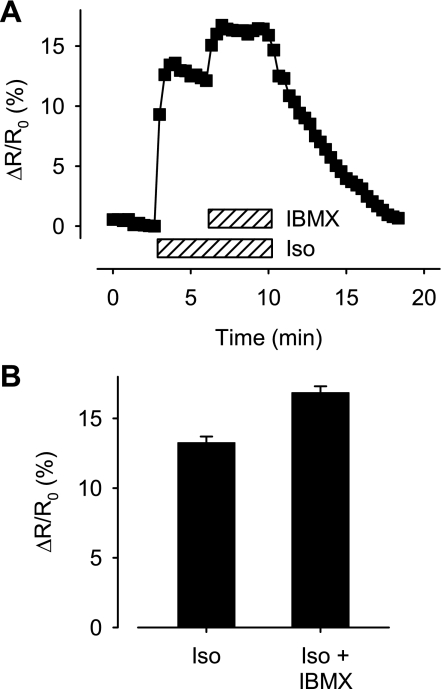
To estimate the actual concentration of cAMP sensed by the Epac2-based probe, we used a generalization of the approach we previously introduced for the PKA-based probe (12). This first required determining the maximum FRET response that can be observed in intact cells (Fmax). We did this by exposing myocytes to a maximally effective concentration of the phosphodiesterase (PDE) inhibitor IBMX in the presence of 1 μM Iso. Inhibiting cAMP hydrolysis while maximally stimulating production is expected to increase cAMP to a level sufficient to maximally activate Epac2-camps expressed in these cells. In this set of experiments, exposure to 1 μM Iso increased the FRET response by 13.3 ± 0.45% over baseline (n = 5). Subsequent addition of 100 μM IBMX further increased this to 16.8 ± 0.46% (Fig. 3). This represents a 27 ± 1.5% increase (P < 0.05) and demonstrates that the concentration of cAMP produced by maximum β1AR stimulation does not saturate binding of Epac2-camps.
Fig. 3.
Maximum response of Epac2-camps in intact cells. A: time course of changes in the relative CFP/YFP emission intensity ratio (ΔR/R0) produced by Epac2 sensor during exposure to a maximally stimulating concentration (1 μM) of the β-adrenergic receptor agonist Iso followed by subsequent addition of a maximally effective concentration (100 μM) of the nonselective phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX). B: average responses to 1 μM Iso and Iso plus 100 μM IBMX (P < 0.05).
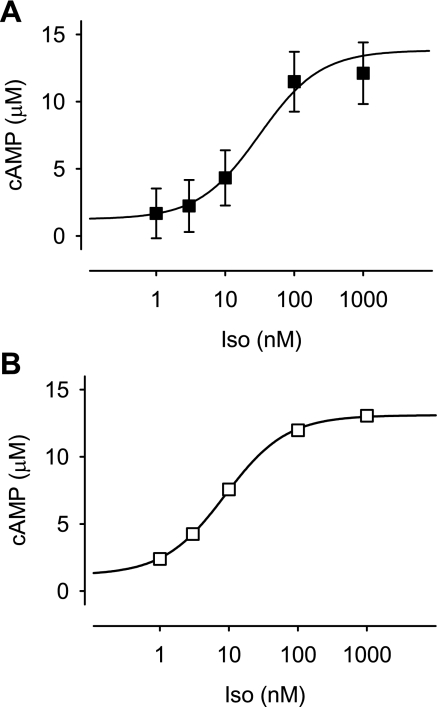
Despite the fact that inhibition of PDE activity caused a further increase in cAMP production, the maximum response observed in the presence of Iso plus IBMX is still significantly less than the absolute maximum response measured in vitro (Fabs). In the presence of a maximally activating concentration of cAMP, the Epac2-based probe is capable of producing a FRET response that is 42.9 ± 1.6% (n = 4) over that observed in the absence of cAMP (see Supplemental Fig. S1). This suggests that basal cAMP levels in these cells are high enough to partially activate this biosensor. Using Eq. 1, we were able to estimate the concentration of cAMP detected by Epac2-camps under basal conditions and following exposure to Iso (Fig. 4A). These data indicate that the concentration of cAMP under basal conditions is 1.2 μM and Iso increases this to a maximum of 13.5 μM.
Fig. 4.
Concentration of cAMP detected by Epac2-camps in adult ventricular myocytes. A: estimated concentration of cAMP produced by different concentrations of the β-adrenergic receptor agonist Iso. Relative CFP/YFP emission intensity ratio measurements from Fig. 2B were converted to cAMP concentration by Eq. 1. Parameters of fit to experimental data points: EC50, 31 nM Iso; minimum cAMP concentration, 1.2 μM; maximum cAMP concentration, 13.5 μM; Hill coefficient, 1. B: effect of Iso on the total cAMP concentration predicted by computational model of cAMP signaling in an adult ventricular myocyte (see methods). Parameters of fit to predicted data points: EC50, 8.8 nM Iso; minimum cAMP concentration, 1.2 μM; maximum cAMP concentration, 13.1 μM; and Hill coefficient 1. Note: experimental and predicted values are consistent with one another.
Muscarinic responses.
To further evaluate what happens to total cAMP levels during receptor activation of signaling mechanisms believed to involve compartmentation, we examined the more complex responses produced by acetylcholine (ACh) activation of the M2R. ACh is able to antagonize responses to agonists that stimulate cAMP production. This is referred to as accentuated antagonism (9). It is now generally accepted that such effects are due to a decrease in cAMP production caused by direct inhibition of adenylyl cyclase (AC) types 5 and/or 6 (AC5/6) through a Gi-dependent signaling mechanism (9). However, this has not always been the case. Some early studies measuring cAMP levels in homogenized tissue or whole cell lysates found that muscarinic inhibitory responses do not always correlate with changes in cAMP (8). An explanation offered for this apparent paradox has been that muscarinic inhibition of cAMP activity is localized to specific subcellular compartments.
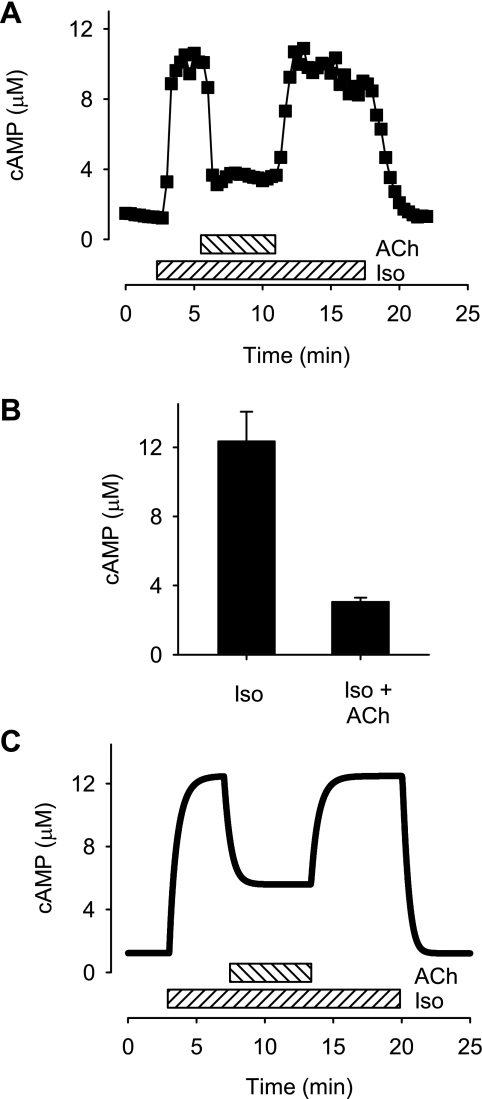
Previously we demonstrated that exposure to ACh produces a decrease in the cAMP activity that can be detected by the PKA-based biosensor in the presence of βAR stimulation (34). This proves that M2R activation is capable of regulating cAMP activity in PKA-signaling domains. To determine whether M2R stimulation has an effect on total cAMP activity, we examined the response detected by the Epac2-based sensor. In this set of experiments, exposure to a maximally stimulating concentration of Iso produced a FRET response of 13.2 ± 0.51% (n = 7). Subsequent addition of 10 μM ACh reduced the FRET response to 7.2 ± 0.56% (P < 0.05). These values correspond to cAMP concentrations of 12.4 μM in the presence of Iso and 3.1 μM following addition of ACh. The effect of ACh represents a 75% decrease in the magnitude of the β-adrenergic response (Fig. 5). These results suggest that M2R activation inhibits cAMP production throughout the entire cell and not just in microdomains where PKA signaling occurs.
Fig. 5.
Muscarinic inhibition of β-adrenergic response. A: time course of changes in total cAMP concentration in a ventricular myocyte exposed to a maximally stimulating concentration (0.2 μM) of Iso and subsequent addition of 10 μM acetylcholine (ACh). The concentration of cAMP detected by Epac2-camps was estimated using Eq. 1. B: averaged response to 0.2 μM Iso and Iso plus 10 μM ACh (P < 0.05). C: effects of Iso and ACh on the total cAMP concentration predicted by computational model of cAMP signaling in an adult ventricular myocyte (see methods). Note: baseline cAMP concentration, cAMP concentration in the presence of a maximally stimulating concentration of Iso, and cAMP concentration in the presence of Iso plus ACh are consistent with experimental observations.
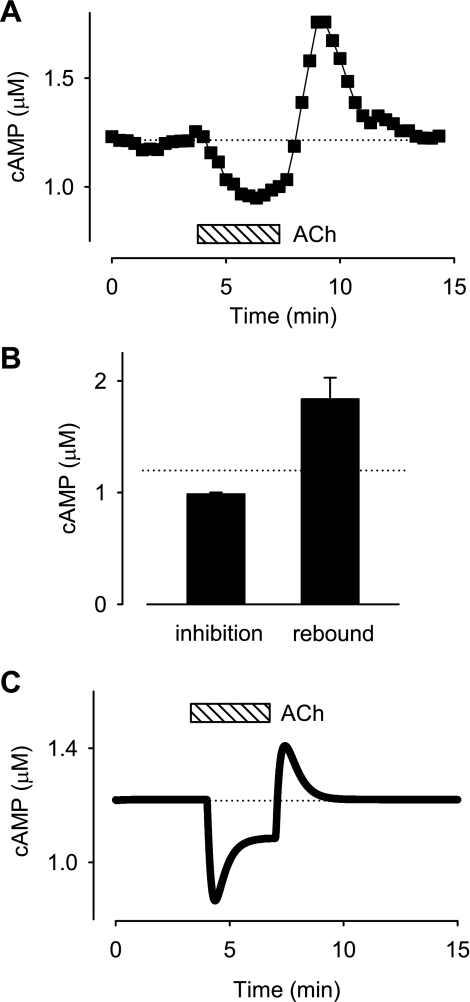
In addition to inhibiting cAMP responses, it is well documented that M2R activation can also produce stimulatory effects (9). The inhibitory effect turns on and off rapidly, whereas the stimulatory effect turns on and off more slowly. In the presence of M2R activation, the inhibitory effect is dominant, but upon termination of M2R activation the inhibitory effect turns off quickly, revealing a stimulatory or rebound response. In ventricular myocytes, the stimulatory effect can be explained by the observation that these cells express adenylyl cyclase types 4 and/or 7 (AC4/7) in addition to AC5/6, and the fact that M2R activation of Gi can actually stimulate AC4/7 whereas it inhibits AC5/6 (3). However, AC5/6 is predominantly found in caveolar membrane fractions, whereas AC4/7 is extracaveolar (7, 21, 27). This suggests that M2R activation inhibits and stimulates cAMP production in distinctly different microdomains.
Computational modeling predicts that the complex temporal response to M2R activation can be explained by the time-dependent flux of cAMP down its concentration gradient from extracaveolar to caveolar compartments (12). Consistent with this prediction, we have demonstrated that the PKA-based biosensor is able to detect a rebound stimulatory effect following washout of ACh that correlates well with the changes in cAMP predicted to occur in the caveolar compartment of our model (34). However, the model also predicts that ACh should not produce any significant rebound in the total cAMP concentration.
Rebound stimulatory effects are not readily observed in the presence of maximum β-adrenergic stimulation. Therefore, we examined the effect of ACh alone on basal cAMP activity. Exposure to 10 μM ACh decreased the Epac2-based biosensor response by 1.7 ± 0.13% (n = 5). The fact that M2R activation was able to produce a decrease in the FRET response in the absence of βAR stimulation clearly demonstrates that basal cAMP levels are high enough to produce significant binding to and activation of the probe. Furthermore, washout of ACh produced a rebound increase in the FRET response to a peak value of 3.4 ± 0.73% before returning to baseline. This corresponds to a steady-state cAMP concentration of 0.99 μM in the presence of ACh and a peak concentration of 1.8 μM following washout of the agonist. The size of these responses appears small compared with those observed in the presence of Iso (see Fig. 5). However, the dynamic range is large enough to significantly affect PKA activity, which has a Kd for cAMP that is ≤300 nM (1, 17). These results suggests that although compartmentation of cAMP may be necessary to produce a transient stimulatory response, the actual transient is reflected by changes in total cAMP, not just cAMP in PKA signaling domains.
Modeling Epac2-camps responses.
If Epac2-camps is uniformly distributed throughout the cytoplasm of the cell, the responses it detects should reflect the total cAMP level in all compartments combined. The original version of the model correctly predicted that basal levels of total cAMP are ∼1 μM. However, it also predicted that maximal β1AR stimulation would only increase total cAMP approximately twofold, M2R activation would only decrease this response ∼30%, and M2R activation in the absence of β1AR stimulation would have little or no effect on total cAMP (12). These later predictions are not consistent with the results obtained by using Epac2-camps.
In the original version of the model, it was assumed that most membrane proteins not associated with caveolae are located in the extracaveolar domain. The bulk cytoplasmic compartment was primarily inert. It was assumed that there were no receptors that might stimulate or inhibit AC activity. Because of this, changes in cAMP levels in the bulk cytoplasmic compartment were determined solely by the flux of cAMP from caveolar and extracaveolar domains. This hypothesis might explain why the model did not predict larger changes in total cAMP in response to agonist stimulation.
We tested this hypothesis by modifying the model so that most of the membrane proteins not associated with caveolae are distributed equally between the extracaveolar and bulk cytoplasmic domains (see Fig. 7A and Supplemental Table S1). This alternative configuration was chosen as a first approximation because it made the fewest assumptions. With this simple modification, the model is able to reproduce the concentration dependence of the response elicited by Iso and explain the greater change in total cAMP concentration produced by maximal β1AR activation (Fig. 4B). This modification of the model also results in the ability of M2R activation to produce significant inhibition of total cAMP in the presence β1AR activation (Fig. 5C), as well as the ability of M2R activation to produce both inhibitory as well as stimulatory responses in the absence of β1AR stimulation (Fig. 6C).
Fig. 7.
Computational modeling of cAMP concentrations in different microdomains in adult ventricular myocytes. A: diagram of different subcellular compartments included in computational model (relative size is not drawn to scale): β1, β1-adrenergic receptor; M2, M2 muscarinic receptor; AC5/6, adenylyl cyclase 5 and/or 6; AC4/7, adenylyl cyclase 4 and/or 7; Gi, inhibitory G protein; Gs, stimulatory G protein. See text for full details. B: effect of 0.2 μM Iso and 10 μM ACh on time course of changes in cAMP concentration in the different subcellular compartments. C: relative contribution of each subcellular compartment to total cAMP concentration.
Fig. 6.
Muscarinic inhibition and stimulation of basal cAMP. A: time course of changes in cAMP concentration during exposure to 10 μM ACh and following ACh washout. The concentration of cAMP detected by Epac2-camps was estimated by Eq. 1. B: average inhibitory response observed in the presence of 10 μM ACh and peak rebound stimulatory response observed after washout of ACh. C: effects of ACh on basal cAMP concentration predicted by computational model (see methods). Note: steady-state inhibition of basal cAMP concentration by exposure to ACh and rebound increase in cAMP concentration following washout of ACh are consistent with experimental observations.
Reversal of Iso responses, either by addition of ACh or upon washout of Iso, is explained by a decrease in adenylyl cyclase activity and subsequent metabolism of cAMP by PDEs. The time course of the decrease in cAMP response measured experimentally is limited by the time required to change the solution in the perfusion chamber. We were not able to instantaneously change the solution bathing the cells. When a supramaximally stimulating concentration of ACh was washed in, it is unlikely that there was a complete exchange of solution in the perfusion chamber before M2Rs were maximally activated. However, when washing out a supramaximally stimulating concentration of Iso, near complete exchange of bathing solution was necessary before β1AR stimulation of AC activity could begin to reverse. The model on the other hand assumes instantaneous changes in agonist concentration. This may explain the slight difference between experimental and model results.
Relative contribution of domains to Epac2-camps response.
If the Epac2-based probe is distributed uniformly throughout the cytoplasm of the cell, as we assume, this means that it must be sensing what is happening in the caveolar and extracaveolar domains, as well as the bulk cytoplasmic compartment. Figure 7B illustrates the effect that β1AR and M2R activation is predicted to have on the cAMP concentrations in all three of these compartments. Because the model assumes that the caveolar and extracaveolar compartments represent only 1 and 2% of the total cytosolic volume of the cell, respectively, they would be expected to contribute little to the total cAMP concentration detected by Epac2-camps. Figure 7C illustrates the relative contribution of cAMP in each compartment to the total cAMP concentration of the cell, as represented by the model. These results suggest that responses detected by Epac2-camps primarily reflect what is happening in the bulk cytosolic compartment, which makes up 97% of the cytoplasmic volume.
DISCUSSION
The present study demonstrates that the Epac2-camps biosensor can be used to monitor β-adrenergic as well as muscarinic receptor-mediated changes in cAMP activity in adult cardiac myocytes. We also presented a generalized method for estimating the actual concentration of cAMP sensed by FRET-based probes that respond to direct cAMP binding. This extends the use of these biosensors beyond measuring relative changes in cAMP activity. Use of this approach requires knowledge of the absolute sensitivity of the probe to changes in cAMP concentration in vitro. It is also based on the assumption that in intact cells the cAMP affinity and absolute maximum response of the probe are the same as they are in vitro. Finally, it requires the ability to maximally activate the probe in intact cells. The accuracy of values obtained by this approach is expected to be greatest when cAMP concentrations are near the affinity constant for binding to the probe.
Compartmentation of basal cAMP.
Before the availability of biosensors to record activity in intact cells, cAMP was typically measured by radioimmunoassay of homogenized tissue samples or whole cell lysates. By using that kind of data, it has been estimated that the basal level of cAMP in cardiac myocytes is 1 μM (15). Our results obtained by using Epac2-camps support the conclusion that the basal concentration of total cAMP in intact ventricular myocytes is 1.2 μM, which is close to that value.
Verifying that the average concentration of cAMP under basal conditions is in the micromolar range is important because it is significantly higher than the apparent affinity of endogenous PKA (≤300 nM) for cAMP (1, 17). This suggests that total cytosolic cAMP is too high to regulate PKA-dependent functional responses, unless cAMP levels in local microdomains where PKA is found are maintained at a significantly lower level. Consistent with this idea, we previously estimated the basal concentration of cAMP detected by the PKA-based probe to be ∼100 nM (12). This is low enough to allow a subsequent increase in cAMP to regulate PKA-dependent responses, without having to assume that affinity of PKA for cAMP is much lower than what has been determined experimentally (28).
High total cAMP levels have also been found in sinoatrial node (SAN) cells (33a). In fact, cAMP levels in SAN cells were found to be higher than those found in ventricular myocytes. Furthermore, the high level of cAMP observed under basal conditions in SAN cells is associated with significant activation of PKA, which plays an important role in regulating pacemaker activity. More recently, Younes et al. (36a) reported that the high basal level of cAMP in SAN cells is associated with Ca2+-stimulated AC activity localized in lipid raft microdomains of the plasma membrane. This would correlate with the higher concentration of AC activity included in the lipid raft domains of the model of ventricular myocytes used in the present study. However, the model does not include any Ca2+-stimulated AC isoforms, which is consistent with what is currently known about ventricular myocytes (13a). Furthermore, in the model, high total levels of cAMP are explained by the presence of the small fraction of total AC activity included in the plasma membrane associated with the bulk cytoplasmic compartment.
Compartmentation of cAMP and β1-adrenergic responses.
Compartmentation of cAMP signaling is perhaps more commonly thought of in the context of receptor activation stimulating the localized production of cAMP. Recent evidence obtained by use of the PKA-based probe has suggested that βAR stimulation of cAMP in neonatal ventricular myocytes is limited exclusively to discrete microdomains (38). This raises the question of whether or not β1AR stimulation produces a similar type of localized response in adult myocytes. Unlike responses detected by the PKA-based probe in neonatal myocytes, we observed no obvious pattern to the change in cAMP detected by the Epac2-based probe in our cells (see Fig. 1). Although this might seem to argue against the idea that β1AR stimulation produces compartmentalized responses, the size of at least some microdomains relevant to cAMP signaling are likely to be too small to be resolved by optical microscopy. In support of this conclusion we found that Iso stimulated FRET responses produced by Epac2-camps with an EC50 of 4.7 nM. This is nearly 10-fold less sensitive than the Iso responses detected by the PKA-based probe (34). This difference might be explained in part by the difference in cAMP sensitivity of the two probes. Epac2-camps is activated by cAMP with an EC50 of 1.1 μM, whereas the PKA-based probe is activated by cAMP with an EC50 of 300 nM (17). However, when results obtained with the two biosensors are converted to cAMP concentrations via Eq. 1, there are clear differences in the sensitivity and magnitude of the responses. Results obtained with Epac2-camps indicate that maximum β1AR stimulation increases the concentration of cAMP to more than 10 μM, whereas previous results obtained with the PKA-based probe indicate that cAMP in the caveolar domain increases to only ∼2 μM (12). We previously demonstrated that the results obtained with the PKA-based probe are consistent with what our computational model predicts is happening in the caveolar or type II PKA signaling domain. The present findings suggest that the results obtained with Epac2-camps are consistent with what the model predicts is happening in the bulk cytoplasmic compartment.
The present study also supports the idea that β1AR stimulation is capable of increasing cAMP levels throughout the cell, but there are distinct differences in the actual concentration of cAMP produced in different domains (see Fig. 7). Intuitively this makes sense because both PKA and Epac are important singling proteins in cardiac myocytes (5, 10, 26). Furthermore, they each have distinctly different affinities for cAMP binding and activation. PKA is activated by cAMP with and EC50 of 90–300 nM (1, 17), whereas Epacs are activated with an EC50 of 1.2–2.8 μM (10). Therefore, it is not surprising to find evidence for compartments in these cells where βAR stimulation produces changes in cAMP concentration that are appropriate for regulating each of these effectors.
Potential limitations.
Although the results of the present study support the conclusion that Epac2-camps can be used to estimate the actual concentration of cAMP in live cells, there are limitations to the accuracy of the values it predicts. The probe's response to cAMP binding is only linear over a limited concentration range. The estimate of basal cAMP levels is likely to be the most accurate, since 1.2 μM cAMP is near the affinity constant for binding. The estimate that maximal β1AR stimulation increases total cAMP concentration to ∼13.5 μM may be somewhat less accurate. However, it is clear that maximal β1AR stimulation does not produce saturating concentrations of cAMP, since subsequent inhibition of PDE activity caused a significant increase in the FRET response of the probe (see Fig. 3A). These results support the conclusion that maximal β1AR stimulation produces more than a 10-fold increase in total cAMP concentration. This is in line with previous estimates using biochemical methods that β-adrenergic stimulation is capable of increasing cAMP levels by up to 20-fold over baseline.
Although to some an increase in cAMP to >10 μM might seem unexpectedly high, it is consistent with results obtained by using a similar biosensor to estimate cAMP concentrations in cultured mammalian cells (14). Other studies have used cyclic nucleotide-gated (CNG) ion channels as reporters of cAMP activity in the subsarcolemmal space of various cell types, including cardiac myocytes (23–25). Responses detected by isoforms of these channels that have an EC50 for activation by cAMP of ∼10 μM suggest that levels can reach high micromolar concentrations (23). Furthermore, because CNG channels appear to be found primarily outside of caveolar membrane fractions (6), this would be consistent with our experimental and modeling results suggesting that cAMP activity is higher specifically in domains associated with extracaveolar and/or bulk cytoplasmic compartments.
Another factor that must be taken into consideration when using these biosensors to estimate cAMP concentrations in intact cells is the possibility that other molecules found in the cytoplasm may interfere with cAMP binding. However, it has previously been determined that the cAMP affinity of Epac2-camps is not significantly different when measured with purified protein or when measured in cell lysates (18). Furthermore, it has been demonstrated that the affinity of purified Epac2-camps for cAMP is significantly higher than it is for other nucleotides (e.g., AMP, >10 mM; cGMP, 11 μM) that might potentially compete for binding to and activation of the probe. It was also reported that the probe could be activated by free ATP with an EC50 of 2.5 mM (18). This might be a perceived problem since the ATP concentration in cardiac myocytes is 5 mM. Although this appears to be a common effect of ATP on FRET responses involving CFP and YFP, it also appears to be specific for free ATP, because it is significantly reduced in the presence of Mg2+, which binds ATP (36). Under normal conditions, intracellular ATP is believed to be saturated by Mg2+ (29). Therefore, to mimic the conditions expected inside a cardiac myocyte, purified Epac2-camps was suspended in a solution containing 5 mM MgATP when measuring its affinity for cAMP. Under these conditions, the EC50 was 1.1 μM, which is similar to that measured in the absence of ATP (18).
There are also potential limitations of the model used in the present study. Our laboratory previously published data (3) supporting the conclusion that in cardiac ventricular myocytes the rebound stimulatory response observed upon washout of ACh can be explained by Gi βγ subunit stimulation of AC4/7 activity. That evidence alone, however, did not explain the difference in the temporal aspects of the inhibitory and stimulatory effects associated with M2R activation. Because it has been demonstrated that AC5/6 and AC4/7 are expressed in different membrane domains, we hypothesized that stimulatory responses could be explained by M2R receptor regulation of the various AC isoforms in different microdomains. One of the reasons for developing the original model was to test the feasibility of that hypothesis. Our previously published work (12) supported it as a possible explanation. One could potentially envision more complex signaling schemes being involved, but our simple first approximation was consistent with results previously published using the PKA-based sensor, and with only minor modifications it was able to produce responses consistent with the new experimental results obtained by use of Epac2-camps in the present study.
Conclusions.
Compartmentation is often thought of as receptor stimulation selectively regulating cAMP production in microdomains responsible for producing functional responses. Results of the present study support the idea that β1-adrenergic and M2 muscarinic receptor activation actually produce more global changes in total cAMP. However, these changes in total cAMP do not accurately reflect what is happening in microdomains where PKA signaling occurs. By estimating the actual concentration of cAMP detected by the Epac2-camps biosensor, we found that total cAMP levels may actually be high enough to maximally activate PKA, even under basal conditions. This suggests that compartmentation plays an important role by maintaining microdomains where cAMP levels are kept significantly below that found throughout most of the cell. This allows receptor stimulation to regulate cAMP concentrations in subcellular compartments, such as the caveolar domain, over a range that can modulate the activity of high-affinity effectors such as PKA. These conclusions are consistent with results obtained by using a quantitative model of compartmentalized cAMP signaling in cardiac ventricular myocytes.
GRANTS
This work was supported by grants from the National Institutes of Health and the American Heart Association.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature 349: 694–697, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Belevych AE, Juranek I, Harvey RD. Protein kinase C regulates functional coupling of β1-adrenergic receptors to Gi/o-mediated responses in cardiac myocytes. FASEB J 18: 367–369, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Belevych AE, Sims C, Harvey RD. ACh-induced rebound stimulation of L-type Ca2+ current in guinea-pig ventricular myocytes, mediated by Gbetagamma-dependent activation of adenylyl cyclase. J Physiol 536: 677–692, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bers DM Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer, 2001.
- 5.Bos JL EpaC proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Brady JD, Rich TC, Le X, Stafford K, Fowler CJ, Lynch L, Karpen JW, Brown RL, Martens JR. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol Pharmacol 65: 503–511, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DM Compartmentalization of adenylate cyclase and cAMP signalling. Biochem Soc Trans 33: 1319–1322, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Hartzell HC Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Molec Biol 52: 165–247, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol 139: 1074–1084, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor EpaC. J Physiol 577: 5–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hool LC, Harvey RD. Role of β1- and β2-adrenergic receptors in regulation of Cl- and Ca2+ channels in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 273: H1669–H1676, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Iancu RV, Jones SW, Harvey RD. Compartmentation of cAMP signaling in cardiac myocytes: a computational study. Biophys J 92: 3317–3331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insel PA, Head BP, Ostrom RS, Patel HH, Swaney JS, Tang CM, Roth DM. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann NY Acad Sci 1047: 166–172, 2005. [DOI] [PubMed] [Google Scholar]
- 13a.Ishikawa Y, Homcy CJ. The adenylyl cyclases as integrators of transmembrane signal transduction. Circ Res 80: 297–304, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Jiang LI, Collins J, Davis R, Lin KM, DeCamp D, Roach T, Hsueh R, Rebres RA, Ross EM, Taussig R, Fraser I, Sternweis PC. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282: 10576–10584, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kameyama M, Hofmann F, Trautwein W. On the mechanism of β-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflügers Arch 405: 285–293, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123: 1–11, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res 95: 67–75, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res 99: 1084–1091, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Nikolaev VO, Lohse MJ. Monitoring of cAMP synthesis and degradation in living cells. Physiology (Bethesda) 21: 86–92, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol 143: 235–245, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranu HK, Mak JC, Barnes PJ, Harding SE. Gi-dependent suppression of β1-adrenoceptor effects in ventricular myocytes from NE-treated guinea pigs. Am J Physiol Heart Circ Physiol 278: H1807–H1814, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116: 147–161, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, Vandecasteele G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res 98: 1081–1088, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rochais F, Vandecasteele G, Lefebvre F, Lugnier C, Lum H, Mazet JL, Cooper DM, Fischmeister R. Negative feedback exerted by cAMP-dependent protein kinase and cAMP phosphodiesterase on subsarcolemmal cAMP signals in intact cardiac myocytes: an in vivo study using adenovirus-mediated expression of CNG channels. J Biol Chem 279: 52095–52105, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Ruehr ML, Russell MA, Bond M. A-kinase anchoring protein targeting of protein kinase A in the heart. J Mol Cell Cardiol 37: 653–665, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem 275: 41447–41457, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem 278: 47997–48003, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Silverman HS, Di Lisa F, Hui RC, Miyata H, Sollott SJ, Hansford RG, Lakatta EG, Stern MD. Regulation of intracellular free Mg2+ and contraction in single adult mammalian cardiac myocytes. Am J Physiol Cell Physiol 266: C222–C233, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 19: 7289–7304, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41: 751–773, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland EW Studies on the mechanism of hormone action. Science 177: 401–408, 1972. [DOI] [PubMed] [Google Scholar]
- 33.Vandecasteele G, Rochais F, Abi-Gerges A, Fischmeister R. Functional localization of cAMP signalling in cardiac myocytes. Biochem Soc Trans 34: 484–488, 2006. [DOI] [PubMed] [Google Scholar]
- 33a.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Warrier S, Belevych AE, Ruse M, Eckert RL, Zaccolo M, Pozzan T, Harvey RD. β-Adrenergic- and muscarinic receptor-induced changes in cAMP activity in adult cardiac myocytes detected using a FRET-based biosensor. Am J Physiol Cell Physiol 289: C455–C461, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Warrier S, Ramamurthy G, Eckert RL, Nikolaev VO, Lohse MJ, Harvey RD. cAMP microdomains and L-type Ca2+ channel regulation in guinea-pig ventricular myocytes. J Physiol 580: 765–776, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willemse M, Janssen E, de LF, Wieringa B, Fransen J. ATP and FRET—a cautionary note. Nat Biotechnol 25: 170–172, 2007. [DOI] [PubMed] [Google Scholar]
- 36a.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, Vinogradova TM, Lukyanenko YO, Li Y, Ruknudin AM, Boheler KR, van Eyk J, Lakatta EG. Ca2+-stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem 283: 14461–14468, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaccolo M Use of chimeric fluorescent proteins and fluorescence resonance energy transfer to monitor cellular responses. Circ Res 94: 866–873, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295: 1711–1715, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.