Abstract
OBJECTIVE— Melanocortin-4 receptor (MC4R) deficiency is the most frequent genetic cause of obesity. However, there is uncertainty regarding the degree of penetrance of this condition, and the putative impact of the environment on the development of obesity in MC4R mutation carriers is unknown.
RESEARCH DESIGN AND METHODS— We determined the MC4R sequence in 2,257 obese individuals and 2,677 nonobese control subjects of European origin and established the likely functional impact of all variants detected. We then included relatives of probands carriers and studied 25 pedigrees, including 97 carriers and 94 noncarriers from three generations.
RESULTS— Of the MC4R nonsynonymous mutations found in obese subjects, 68% resulted in a loss of function in vitro. They were found in 1.72% of obese versus 0.15% of nonobesed subjects (P = 6.9 × 10−10). Among the families, abnormal eating behavior was more frequent in both MC4R-deficient children and adults than in noncarriers. Although BMI was inversely associated with educational status in noncarrier adults, no such relationship was seen in MC4R mutation carriers. We observed a generational effect, with a penetrance of 40% in MC4R-deficient adults aged >52 years, 60% in 18- to 52-year-old adults, and 79% in children. The longitudinal study of adult carriers showed an increasing age-dependent penetrance (37% at 20 years versus 60% at >40 years).
CONCLUSIONS— We have established a robust estimate of age-related penetrance for MC4R deficiency and demonstrated a generational effect on penetrance, which may relate to the development of an “obesogenic” environment. It remains to be seen whether appropriate manipulation of environmental factors may contribute to preventing the development of obesity even in those strongly genetically predisposed to it.
The leptin-melanocortin axis controls human energy homeostasis, and the melanocortin-4 receptor (MC4R) is a key player in its central regulation (1). Loss-of-function mutations in MC4R cause severe familial forms of obesity (2,3), and infrequent gain-of-function polymorphisms have been associated with protection against obesity (4,5). At least 72 nonsynonymous mutations have been discovered so far, but some have no obvious functional consequences (6,7), highlighting the importance of functional characterization of MC4R mutations in the determination of potential pathogenicity. MC4R is a membrane-bound G-protein–coupled receptor that activates adenylate cyclase. Loss-of-function mutations result in a partial or complete loss of function as measured by cAMP production. The majority of missense mutations in MC4R result in intracellular retention of the mutated protein, whereas some primarily affect ligand binding or ligand/receptor activation (8,9). In some cases, the alteration of the basal activity of the receptor (8,10) has been reported.
The prevalence of loss-of-function MC4R mutations ranges from 0.5 to 5.8% in childhood-onset obesity (11–14). The contribution of MC4R mutations to late-onset obesity is still debated (13,15–18). Obesity due to MC4R mutations has been extensively studied, and although heterozygous loss-of-function mutations can clearly cause familial obesity, they can be found in individuals who are not obese (19). There is a need for reliable estimates of penetrance. Furthermore, no study has thoroughly assessed the effect of loss-of-function MC4R mutations in elderly subjects. Previous studies using part of our French cohort evidenced the first mutation in MC4R and demonstrated that most of them lead to an intracellular retention of the receptor (2,13,18).
Although hyperphagia is a key feature of MC4R deficiency, with increased food intake at an ad libitum test meal reported in severely obese MC4R-deficient children (10), an apparent amelioration of obesity and food intake disturbances has been suggested in adulthood in some studies (6,11). Obesity is a complex trait, and MC4R mutations offer a unique opportunity to analyze the effects of both aging and shared environment on the evolution of body mass in this paradigm. In this extensive study of 2,257 unrelated obese subjects, 2,677 control subjects of European descent, and 25 multigenerational pedigrees with several MC4R mutations carriers, we provide a comprehensive picture of the prevalence of this condition and of factors that determine the expression of the obesity phenotype and support previous observations reported in a German familial study (20).
RESEARCH DESIGN AND METHODS
The study protocol was approved by all local ethics committees, and informed consent was obtained from each subject before participation in the study, in accordance with the Declaration of Helsinki principles. For children younger than 18 years, oral consent was obtained, and parents provided written informed consent.
We used the 90th and 97th BMI percentiles as thresholds for childhood overweight and obesity, respectively, according to the recommendations of the European Childhood Obesity Group study (21) in a French reference population (22). The classes of adiposity in adult subjects were defined as lean (BMI <27 kg/m2), overweight (BMI ≥27 and <30 kg/m2), class I-II obese (BMI ≥30 and <40 kg/m2), and class III obese (BMI ≥40 kg/m2). Populations are described in Table 1.
TABLE 1.
Phenotypic description of the studied cohorts and results of the mutation screening
| n (% male) | Mean age (SD) | Mean BMI (SD) | Mutations | Synonymous | Nonsynonymous | Nonsynonymous loss of function | |
|---|---|---|---|---|---|---|---|
| Control subjects | 2,677 (41) | 48 (11) | 22.6 (2.7) | 0.75 (20) | 0.22 (6) | 0.52 (14) | 0.15 (4) |
| Children | 526 (47) | 10 (4) | — | 2.66 (14) | 0.38 (1) | 2.47 (13) | 1.81 (11) |
| Lille | 433 (49) | 11 (3) | — | 2.30 (10) | 0.00 (0) | 2.31 (10) | 2.31 (10) |
| Toulouse | 93 (36) | 5 (2) | — | 4.30 (4) | 1.08 (1) | 3.23 (3) | 1.08 (1) |
| French adults | 863 (30) | 44 (14) | 41.8 (8.8) | 3.24 (28) | 0.23 (2) | 3.01 (26) | 2.32 (20) |
| Class I-II | 160 (36) | 48 (15) | 36.8 (2.9) | 2.50 (4) | 0.00 (0) | 2.50 (4) | 1.25 (2) |
| Class III | 703 (24) | 44 (12) | 47.0 (7.2) | 3.41 (24) | 0.28 (2) | 3.13 (22) | 2.56 (18) |
| Swiss adults | 868 (23) | 43 (11) | 43.2 (7.3) | 1.61 (14) | 0.23 (2) | 1.38 (12) | 0.92 (8) |
| Class I-II | 297 (23) | 42 (11) | 36.3 (3.2) | 0.67 (2) | 0.34 (1) | 0.34 (1) | 0.34 (1) |
| Class III | 571 (23) | 43 (10) | 46.8 (6.2) | 2.10 (12) | 0.18 (1) | 1.93 (11) | 1.23 (7) |
| All adults | 1,731 (28) | 46 (13) | 42.3 (8.4) | 2.43 (42) | 0.23 (4) | 2.19 (38) | 1.61 (28) |
| Class I-II | 457 (33) | 47 (14) | 34.9 (3.1) | 1.31 (6) | 0.21 (1) | 1.09 (5) | 0.65 (3) |
| Class III | 1,274 (24) | 45 (12) | 47.4 (6.9) | 2.82 (36) | 0.26 (3) | 2.59 (33) | 1.96 (25) |
| All obese | 2,257 | — | — | 2.48 (56) | 0.22 (5) | 2.26 (51) | 1.72 (39) |
Data are percent of carriers in the cohort (n of carriers) unless otherwise indicated. BMI of the Swiss individuals was documented before surgery.
A total of 433 unrelated obese children were recruited through a multimedia campaign run by the Centre National de la Recherche Scientifique (CNRS)-Unité Mixte de Recherche (UMR) 8090, and 93 obese children were patients of Toulouse Children's Hospital. French obese adults were recruited by the CNRS UMR8090 and the Department of Nutrition of Paris Hotel Dieu Hospital. The cohort includes 160 class I-II and 703 class III unrelated obese individuals. Some of them have been used in previous studies (18), and the others were recruited under the same criteria between 1999 and 2003. We also sequenced 868 Swiss obese subjects, recruited after gastric surgery in Zurich (15). Their BMI was documented before surgery.
From the D.E.S.I.R. general prospective study, 2,302 control subjects were selected (23). Selection criteria were BMI <27 kg/m2 and fasting glucose <5.6 mmol/l at baseline and during the 9-year study follow-up; 375 unrelated white French adults with BMI <27 kg/m2 and normal glucose tolerance after an oral glucose tolerance test recruited at the CNRS UMR8090 were also used as control subjects. Case and control subjects were not matched for age and sex. Their mean age at inclusion was 48 years (22.6 kg/m2; 41% men).
Sequencing.
We amplified the coding sequence of the MC4R gene from a patient's genomic DNA in three overlapping fragments. PCR conditions and primers sequences are available on request. Sequencing was performed using the automated ABI Prism 3730xl DNA sequencer in combination with the Big Dye Terminator Cycle Sequencing Ready Reaction kit 3.1 (Applied Biosystems). Sequences were assembled and analyzed with Sequencher software.
Functional characterization of the new mutations.
All mutant receptors were generated from the wild type as previously described (24). Wild-type and mutant MC4Rs were transiently transfected into HEK293 cells using Lipofectamine 2000 (Stratagene), according to the manufacturer's instructions. One hundred nanograms of wild-type or mutant receptors was cotransfected with 100 ng Bos-β-galactosidase construct as a control of transfection efficiency.
The response of cells transfected with wild-type and mutant MC4Rs to the addition of α-melanocyte–stimulating hormone (MSH) was measured as previously described (9). β-Galactosidase assay was performed with remaining lysate from cAMP enzyme immunosorbent assay (EIA) as previously described. The amount of cAMP produced per well was calculated on the standard curve derived from EIA using GraphPad Prism 4.0 and corrected by β-galactosidase absorbance to correct for transfection efficiency. The data were fitted to the sigmoidal dose-response curve using GraphPad Prism 4.0. Each experiment was conducted in duplicate at least four times. Mutations detected in this study were classed as loss of function if they showed evidence of impaired cAMP generation either in reported studies or in our investigations.
Familial study.
We sequenced MC4R in the voluntary relatives of all individuals carrying loss-of-function mutations. Pedigrees were available only for patients recruited by the CNRS UMR8090 unit. This first recruitment already included parents of obese individuals. Proband carriers and children who were too young to be legally included the previous times were then contacted until 2006 for the extension of the study to other family members.
Generation determination.
To compare the effects of loss-of-function MC4R mutations between generations, we classified the relatives of obese MC4R-deficient probands according to the following thresholds: The end of childhood was determined as age 18 years, and a generational criterion was defined by the average age when people became grandparents in our cohort. Thus, we classified adult parents as aged 18–52 years and grandparents as aged >52 years.
Body composition and growth.
Probands and relatives have been similarly phenotyped and genotyped. Weight and height were measured during medical consultation, and BMI was calculated as weight in kilograms divided by the square of height in meters. The weights and heights of the children at different ages from birth to the recruitment were obtained from each child's health notebook when available. We then estimated the age of the rebound as previously described (25). The adiposity rebound corresponds to the second rise in BMI curve that usually occurs between ages 5 and 7 years. Age of the obesity onset was defined as the age when BMI first exceeded the 97th percentile for sex and age.
Eating behavior.
In obese adults, eating behavior was assessed by the three-factor eating questionnaire (TFEQ) (26), which evaluates the cognitive restraint of eating, disinhibition, and hunger. The range of scores for hunger was 1–14; for disinhibition, 1–18; and for restraint, 1–21. We transformed the criteria of Stunkard into binary traits using the mean of each criterion for the thresholds (7, 9, and 10.5, respectively).
Because the TFEQ has not been validated in obese children, we used an in-house questionnaire administrated by a trained physician (27,28). Three questions related to eating behavior during or between meals identified binge eating, eating large amounts of food during meals, and snacking as binary phenotypes (yes or no).
Statistical analysis.
Comparison of means was done with unpaired Student's t test and contingency table χ2 analysis using SPSS 14.0 for Windows. When the sample size was <30, the statistical significance of the difference between groups was assessed by the Mann-Whitney U test and the Kolmogorov-Smirnov Z test for nonparametric results. The comparison of prevalence has been tested with the Fisher's exact test because of theoretical sample size <5. Quantitative traits were also analyzed by a linear regression adjusted for age, sex, and BMI. All reported P values are two sided. P values of <0.05 were considered to indicate statistical significance. Generalized estimating equations with an exchangeable correlation matrix were used with STATA software to take familial relationships into account when testing the effects of the mutations.
We used allelic frequencies in case and control subjects from the initial screening to estimate penetrances, i.e., the probability (PGi), of a given a genotype (Gi) being affected, where G1 is the wild type, G2 is the heterozygote, and G3 is the mutant homozygote. From Bayes’ theorem, PGi can be written as P(A Gi) = P(Gi A) × P(A)/[P(Gi A) × P(A)+P(Gi U) × P(U)], where A stands for affected (obese), U for unaffected, and P(A) is the prevalence of being obese, here identified as 15% in the European population.
RESULTS
Initial screening of loss-of-function MC4R mutations
Mutation detection.
The baseline characteristics of the 1,731 obese adults, 526 obese children, and 2,677 nonobese control subjects, all of European descent, are reported in Table 1. We considered as a mutation any rare variant (frequency <1%) found in the coding sequence. In this regard, T112M was thus considered as a mutation (12,29), whereas the more frequent V103I and I251L were excluded (4,5,30). Among the 2,257 obese subjects, we identified 36 different mutations (4 synonymous, 31 missense [6 new], and 1 frameshift) in 56 carriers (2.48%) (Tables 1 and 2; Fig. 1). Two adults were homozygous carriers for I301T, and one patient was a compound heterozygous carrier for Y80X and I301T. In the control group, we identified 20 carriers of 15 different mutations (0.75%; 5 synonymous and 10 [5 new] missense). The prevalence rates are given in Table 1. The enrichment of nonsynonymous mutations in the obese group compared with the control subjects is statistically significant (Fisher's exact test, 2.26 vs. 0.52%, P = 9.8 × 10−8), but there was no difference in the prevalence of synonymous mutations between the two groups (0.22 vs. 0.22%, P = 1).
TABLE 2.
Summary of the mutations, name, carriers, and functional characterization
| Mutation | Obese carriers | Nonobese carriers | Functional characterization | Ref. |
|---|---|---|---|---|
| Known | ||||
| T11A | 1 (45.61) | Like wild type | (36) | |
| R18C | 1 (52.59) | Partial | (33) | |
| R18H | 1 (40.65) | Partial | (33) | |
| S30F | 4 (21.92; 20.10; 20.80; 20.40) | Like wild type | (36) | |
| Y35X + D37V | 2 (32.42; 53.67) | No activity | (29) | |
| InsG48 | 1 (41.52) | No activity | (18) | |
| F51L | 2 (42.52; 50.68) | Like wild type | (15) | |
| I69M | 1 (45.51) | No activity | (17) | |
| Y80X | 2 (32.51; 49.68 compound htz I301T) | No activity | (17) | |
| V95I | 1 (42.78) | No activity | (33) | |
| I102T | 2 (35.25; 41.09) | Partial | (17) | |
| T112M | 7 (37.17; 20.34; 33.21; 47.58; 31.35; 39.97; 53.93) | 1 (24.70) | Partial | (10) |
| S127L | 2 (36.35; 27.22) | Partial | (13) | |
| T150I | 2 (47.87; 49.94) | Partial | (10) | |
| A154D | 2 (42.62; 39.51) | Like wild type | (19) | |
| R165W | 2 (50.86; 66.95) | 1 (24.00) | Partial | (10) |
| I170V | 3 (33.67; 49.53; 21.90) | Partial | (10) | |
| M200V | 2 (47.62; 45.00) | Like wild type | (6) | |
| F202L | 1 (22.90) | Like wild type | (34) | |
| G231S | 1 (41.62) | Partial | (17) | |
| A244E | 1 (26.28) | Like wild type | (36) | |
| L250Q | 1 (58.82) | Partial | (13) | |
| G252S | 1 (49.78) | Partial | (36) | |
| V253I | 1 (34.47) | Like wild type | (10) | |
| C271R | 1 (28.03) | Like wild type | (11) | |
| N274S | 1 (40.34) | Like wild type | (36) | |
| S295P | 1 (42.19) | 2 (25.50; 24.80) | Like wild type | (17) |
| P299H | 3 (23.30; 26.45; 36.8) | No activity | (13) | |
| I301T | 3 (42.91 hmz; 40.47 hmz; 31.78 htz) | Partial | (13) | |
| New | ||||
| H76R | 1 (30.84) | Like wild type | — | |
| S94N | 1 (67.86) | Partial | — | |
| D126Y | 1 (42.65) | No activity | — | |
| D146N | 1 (19.20) | Like wild type | — | |
| Del170V | 1 (22.22) | Not tested | — | |
| F201L | 1 (22.30) | Partial | — | |
| G231V | 1 (24.70) | Like wild type | — | |
| P260Q | 2 (23.30; 25.88) | Partial | — | |
| I289L | 1 (20.30) | No activity | — | |
| R305S | 1 (34.31) | Partial | — | |
| Y332C | 1 (20.13) | Like wild type | — | |
| Y332H | 1 (23.00) | Like wild type | — |
Data are n (BMI of the carriers, expressed as weight in kilograms divided by the square of height in meters). hmz, homozygous; htz, heterzygous.
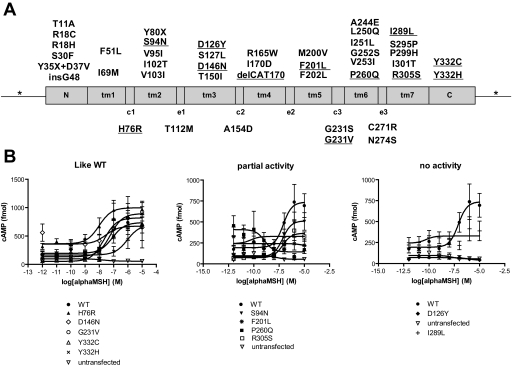
FIG. 1.
Mutations identified in MC4R in the screening of 5,620 subjects. A: Localization of the identified mutation according to MC4R conformation. The transmembrane domains are indicated as tm1–7, cytoplasmic loops c1–3, and extracellular loops e1–3. Mutations identified are listed according to the domain in which they are located. Underlined mutations are new. B: Functional characterization of the new nonsynonymous mutations: cAMP EIA. The graphs indicate the response of mutant and wild-type MC4R to the addition of a logarithmic increase of α-MSH. Each point represents the mean ± SE of at least four independent experiments performed in duplicate.
Functional characterization.
We characterized all newly identified missense mutations by testing the ability of mutated receptors to generate cAMP in response to increasing concentrations of α-MSH. Results are reported in Fig. 1 and Table 2 (Emax and half-maximal effective concentration are given in supplementary Table 1, available in an online appendix at http://dx.doi.org/10.2337/db08-0153): S94N, F201L, P260Q, and R305S showed a partial cAMP response to α-MSH. D126Y and I289L showed a complete inability to signal to cAMP. We observed no effect on the cAMP response to increasing concentrations of α-MSH for H76R, D146N, G231V, Y332C, and Y332H. The other 30 mutations have been characterized previously in in vitro assays (references in Table 2). Of the 34 mutations observed in obese subjects, only 23 (68%) result in a loss of function in vitro. From this stage, we only analyzed carriers of loss-of-function MC4R mutations.
Prevalence of loss-of-function MC4R mutations.
We calculated the prevalence of loss-of-function MC4R mutations in our European population. In obese children, the prevalence was doubled in case of family history of obesity compared with sporadic obesity (2.31 vs. 1.08%, respectively). But the difference was not significant, which showed the relative homogeneity of the cohorts and allowed the calculation of a general prevalence in children: altogether, 1.81% of obese children carried loss-of-function MC4R mutations. In adults, likewise, the prevalence was higher in probands of obese families compared with obese subjects in whom there was no family history of obesity (2.32 vs. 0.92%, P = 0.02). Moreover 1.96% of class III obese subjects harbored loss-of-function MC4R mutations compared with 0.65% in class I-II, but the difference was not significant (P = 0.08). The mean prevalence of loss-of-function MC4R mutations in adult obesity was then 1.61%. Altogether, the prevalence in obese children and adults was 1.72%, whereas 0.15% of nonobese individuals (mean age 44 years; mean BMI 22.8 compared with 22.6 kg/m2 in the whole control population; one man and three women) carried a loss-of-function mutation (11-fold increase; P = 6.9 × 10−10; Table 1).
Penetrance and mode of inheritance.
We evaluated the penetrance of the loss-of-function MC4R mutations in two ways. First, we calculated the proportion of carriers of loss-of-function mutations with obesity in our entire population: 63.5% for heterozygous and 94.6% for homozygous/compound heterozygous carriers, respectively, according to the Bayes’ theorem. We also assessed the penetrance of loss-of-function MC4R mutations in 25 French multigenerational nonconsanguineous pedigrees where the proband was obese and carried a loss-of-function MC4R mutation (supplementary Fig. 1). The familial penetrance was 60% for heterozygous carriers. It was 100% in homozygous and compound heterozygous carriers.
For mutation I301T, we observed a codominant mode of inheritance: two homozygous unrelated individuals presented a BMI of 40.5 and 42.9 kg/m2 compared with a BMI of 31.78 kg/m2 for a I301T heterozygous carrier. Moreover, this heterozygous individual was the father of two compound heterozygous carriers of I301T plus Y80X with BMIs of 49.68 and 54.43 kg/m2, respectively (supplementary Fig. 1). The mother carrying mutation Y80X had a BMI of 33.05 kg/m2. The third generation of this family included two overweight children of 10 and 14 years (BMI 19.0 and 22.3 kg/m2, respectively; BMI ≥90th percentile) carrying either the I301T or the Y80X mutations. The increase in the BMI in homozygous/compound heterozygous compared with heterozygous carriers was significant (P = 0.008).
We observed no impact of the severity of the mutations (Table 2, partial activity vs. no activity) on the severity of the phenotype of the carriers. Mean BMI for carriers of mutations leading to a receptor with a partial activity was 33.62 vs. 31.45 kg/m2 for carriers of one nonfunctional receptor (P = 0.413).
Effects of age on obesity phenotype in MC4R deficiency.
We compared obesity-related phenotypes between carriers and their relatives for children (n = 19 vs. 13) and adults (n = 78 vs. 81). Within the 25 pedigrees, as expected, in each generation, the mean BMI was higher in mutation carriers compared with relatives with a normal MC4R genotype (supplementary Table 2). We observed a gender effect in the whole family sample (+4.3 kg/m2 in men and +8.7 kg/m2 in women; ref. 20) but the difference is not significant (P = 0.19). Children probands were not bigger than their siblings carrying loss-of-function mutations (26.2 vs. 22.6 kg/m2, P = 0.19), but the difference was significant in adults (45.02 vs. 31.93 kg/m2, P = 0.007).
Onset of obesity and evolution of BMI with age.
We obtained heights and weights of children at different ages from the individual health record. Although there was a trend toward earlier development of obesity in MC4R mutation carriers compared with obese subjects with a normal MC4R genotype (3.2 vs. 6.0 years), this was not statistically significant (P = 0.11). The adiposity rebound (a strong predictor of the development of childhood obesity) occurred 3 years earlier in children with MC4R mutations (2.0 vs. 5.2 years; P = 0.006).
In adults, we calculated BMI at 20 years of age from self-reported heights and weights and compared this with measured BMI at the age at examination (mean 44 years). The mean reported BMI of loss-of-function MC4R mutation carriers at 20 years of age (BMI20) was 26.8 kg/m2. Probands were all obese at 20 years; among the carrier-relatives, 22 subjects were normal weight at this age (BMI <90th percentile, 43%), 10 were overweight (BMI ≥90th percentile <97th percentile, 20%), and 19 were obese (BMI ≥97th percentile, 37%). The BMI20 was slightly but significantly lower in relatives with a normal MC4R genotype (24.21 kg/m2, P = 0.04). The BMI of carriers of loss-of-function MC4R mutations increased significantly during this time frame compared with nonmutation carrier relatives (13.75 vs. 6.18 kg/m2, P = 1.66 × 10−5). Seven of the 22 (32%)carriers that were nonobese at 20 years showed late-onset obesity, and 8 of the 10 individuals (80%) who were overweight became obese.
Effect of generation on BMI and obesity penetrance.
Comparing the carriers of loss-of-function mutations to their relatives with a normal MC4R genotype in the 25 pedigrees, we observed a significant BMI increase of 6.78 kg/m2 (26.37 vs. 33.15 kg/m2, P = 5.95 × 10−6).
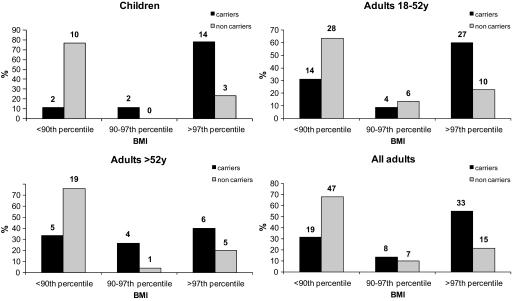
Adult carriers <52 years were 3 kg/m2 heavier at 20 years of age than the previous generation at the same age (data not shown). The disease penetrance was 78% in children, 60% in adults <52 years old, and 40% in adults >52 years old for obesity (supplementary Table 3). The decrease in penetrance was significant between children and adults >52 years old (P = 0.038). In noncarriers, the prevalence of obesity was the same in each generation (23%; supplementary Table 3).
Although carrying a MC4R mutation was consistently associated with obesity in every generation, the risk of obesity in carriers was multiplied by 3.4 for children (P = 10−100, Fisher's exact test), by 2.9 for adults <52 years old (P = 10−84), and by only 1.7 for adults >52 years old (P = 10−11) compared with noncarrier individuals. This decrease in risk was significant between adults >52 and <52 years old (P = 3.0 × 10−5) and between children and adults >52 years old (P = 9.6 × 10−4; Fig. 2).
FIG. 2.
Distribution of individuals according to BMI. We used the official threshold of BMI, percentiles 90 and 97, to discriminate between lean, overweight, and obese subjects (21) within the 97 carriers and 81 noncarrier relatives from intrafamilial study. The absolute sample sizes of each subgroup are given above the bars.
We looked at the genotype distribution of FTO rs1421085 (31) among the generations and found no differences between carriers and noncarriers or among children, adults <52 years old, and adults >52 years old (data not shown).
Eating behavior.
An in-house questionnaire distributed to children assessed three abnormal food intake behaviors. There were twice as many children who ate large amounts of food during meals in the carrier group compared with their noncarrier relatives (61.1 vs. 37.5%; P = 0.25). No obese child was a binge eater, and snacking was equally frequent in the two groups.
In adults, we used the well-established TFEQ questionnaire (26) and observed a significant increase of the disinhibition score in MC4R mutation carriers compared with noncarrier relatives (7.2 vs. 5.1, P = 0.007; supplementary Table 2). Moreover, the proportion of carriers harboring a disinhibition rating above the mean score was 37 vs. 12% in noncarrier relatives (P = 0.01). The same was true for the hunger score: 31.0% of carriers above the mean vs. 10% in noncarrier subjects (P = 0.047). Using these methods, eating behavior was not impaired in adult nonobese carriers compared with their noncarrier relatives, suggesting that the effects of MC4R on eating behavior may exhibit incomplete penetrance.
We observed a negative relationship between educational level and BMI in noncarrier adults (P = 0.045) within the families (n = 81), as previously reported (32), but no such correlation was observed for the 78 adult carriers of loss-of-function MC4R mutations (P = 0.42; data not shown). Apart from BMI and reported eating behavior, we observed no consistent difference for the other analyzed clinical traits between carriers and noncarriers (supplementary Table 2) in this study.
Exclusion of the probands.
To exclude any recruitment bias, we performed the analyses of the same phenotypes without the probands. Effects of the mutations on BMI remained significant (+5.17 kg/m2, P = 1.37 × 10−3); effects on the other clinical features seemed to remain as trends, but we were no longer able to observe the generational effect on penetrance (supplementary Table 4).
DISCUSSION
Mutations in MC4R associated with obesity have been extensively reported, but the true prevalence of this condition has been obscured by several factors. First, the fundamental difference among loss-of-function, neutral, and gain-of-function mutations in their major implication on weight has been ignored by several groups and has led to conflicting and/or inconclusive results regarding the prevalence of MC4R mutations in different ethnic groups (14,15). In this study, we highlighted that only 68% of nonsynonymous mutations in MC4R result in a loss-of-function in vitro in obese subjects. Thus, the prevalence of loss-of-function MC4R mutations in our large sample set of obese subjects of European origin is 1.72%. Although our mode of recruitment of obese patients may have tended to overestimate the proportion of severe phenotypes, the prevalence is consistent with that previously published in other obese cohorts (14,18,33–35). Our findings in the control cohort are consistent with other large, population-based cohorts (6), such as the KORA-S4 study, where the prevalence of MC4R mutations was also 0.15%. It remains also possible that more subtle mutation effects, such as alteration of the response to other agonists or antagonists, have not been evidenced by us or by others (36) and that the prevalence of loss-of-function MC4R is slightly higher.
By studying 2,257 obese individuals and their family members (25 multigenerational pedigrees) and 2,677 nonobese control subjects, we identified 108 subjects who harbored loss-of-function MC4R mutations. This represents the largest group of MC4R mutation carriers studied up to now. Our family cosegregation data are consistent with a codominant mode of inheritance as reported previously (11). We found that MC4R loss-of-function mutations result in obesity in 63.5% of mutation carriers, and this value is similar using two different ways of calculation (case-control study and pedigrees). Thus, the obesity phenotype associated with loss-of-function MC4R mutations exhibits variable penetrance.
On the contrary, some noncarrier relatives exhibit obesity. This may arise from the so-called phenomenon of “assortive mating” (i.e., obese people more often get married with other obese people).
This study establishes the first robust estimates of age-related penetrance for this condition. However, loss-of-function MC4R mutation adult carriers at 20 years of age displayed a low penetrance of obesity (37%). One explanation could be the difference in the environmental pressure between France, Switzerland, and the U.K. during the 1980s and an underreported weight at 20 years in self-administered questionnaires, a recognized phenomenon especially in overweight/obese people (37). In some subjects, there was evidence for the development of obesity caused by MC4R mutations in adult life. Longitudinal familial analysis also provides evidence that the current obesogenic lifestyle may worsen the effect of loss-of-function MC4R mutations on childhood and adult energy, but educational level does not positively impact on obesity risk among carriers. Nonobese carriers used as control subjects can develop obesity later in life, even if our control subjects have been selecting for stable weight <27 kg/m2 during 9 years; their mean age was 44 years at inclusion, so they are unlikely to become obese in the near future.
We also find that the penetrance of obesity in those carrying loss-of-function MC4R mutations seems to have increased when comparing grandparents, parents, and children in multigenerational pedigrees. Of note, the prevalence of obesity was similar in each generation in wild-type carriers of the 25 pedigrees, showing that the environment alone cannot explain such an effect.
We demonstrated that a common FTO variant does not influence severity or penetrance of obesity in our sample (data not shown). However, sex, age, and generation impact penetrance and severity of obesity, which confirms previous observations made by Dempfle et al. (20). MC4R mutation carriers born around the time of the second World War were much less likely to be obese in childhood, compared with their offspring born in the 1960s and 1970s and especially with the 1990s–2000s generation. It is plausible that the food constraints imposed in France during and after the second World War (there was ticketing limited access to food until 1947) have delayed obesity, as suggested by the difference in BMI at 20 years among mutation carriers of different generations. Our results agree both with an oligogenic (20) and a codominant (11) mode of inheritance. We cannot exclude that the increasing distant relationships between the family members could be a cause of the observed impact of age and generation on penetrance, but direct effects of relationship distance and other genetic and environmental factors remain to be tested to confirm this assumption.
Given the age- and generation-related penetrance of obesity in carriers of functionally significant MC4R mutations, we show that environmental factors contribute to the development of obesity even in those strongly genetically predisposed to it. It remains to be seen whether timely manipulation of environmental factors may be able to prevent or delay the development of obesity or reduce its severity in subjects carrying loss-of-function MC4R mutations.
In conclusion, we have established the first robust estimates of age-related penetrance for this condition and demonstrated a generational effect on penetrance that may relate to the development of an obesogenic environment. In addition, educational level does not impact on obesity risk among carriers. MC4R deficiency may sometimes present as obesity developing in adult life. Our study also highlighted the importance of functional analysis in genetic diagnosis of this condition.
Supplementary Material
Acknowledgments
S.O. has received support from the Wellcome Trust and Medical Research Council (MRC). I.S.F. has received support from the Wellcome Trust and MRC. P.F. has received support from the Wellcome Trust and MRC. This work was supported in part by Conseil Regional Nord-Pas de Calais/Fonds Européen de Dèveloppement Règional and Association Française des Diabétiques funding.
We thank the patients and families that participated in this study. We also thank S. Gaget, C. Lecoeur, M. Deweirder, and F. Allegaert for technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 16 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cone RD: Anatomy and regulation of the central melanocortin system. Nat Neurosci 8 :571 –578,2005 [DOI] [PubMed] [Google Scholar]
- 2.Vaisse C, Clement K, Guy-Grand B, Froguel P: A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 20 :113 –114,1998 [DOI] [PubMed] [Google Scholar]
- 3.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S: A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20 :111 –112,1998 [DOI] [PubMed] [Google Scholar]
- 4.Geller F, Reichwald K, Dempfle A, Illig T, Vollmert C, Herpertz S, Siffert W, Platzer M, Hess C, Gudermann T, Biebermann H, Wichmann HE, Schafer H, Hinney A, Hebebrand J: Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am J Hum Genet 74 :572 –581,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, Tounian P, Levy-Marchal C, Buzzetti R, Pinelli L, Balkau B, Horber F, Bougneres P, Froguel P, Meyre D: Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet 16 :1837 –1844,2007 [DOI] [PubMed] [Google Scholar]
- 6.Hinney A, Bettecken T, Tarnow P, Brumm H, Reichwald K, Lichtner P, Scherag A, Nguyen TT, Schlumberger P, Rief W, Vollmert C, Illig T, Wichmann HE, Schafer H, Platzer M, Biebermann H, Meitinger T, Hebebrand J: Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab 91 :1761 –1769,2006 [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie RG: Obesity-associated mutations in the human melanocortin-4 receptor gene. Peptides 27 :395 –403,2006 [DOI] [PubMed] [Google Scholar]
- 8.Lubrano-Berthelier C, Cavazos M, Le Stunff C, Haas K, Shapiro A, Zhang S, Bougneres P, Vaisse C: The human MC4R promoter: characterization and role in obesity. Diabetes 52 :2996 –3000,2003 [DOI] [PubMed] [Google Scholar]
- 9.Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S: Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 12 :561 –574,2003 [DOI] [PubMed] [Google Scholar]
- 10.Nijenhuis WA, Garner KM, van Rozen RJ, Adan RA: Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. J Biol Chem 278 :22939 –22945,2003 [DOI] [PubMed] [Google Scholar]
- 11.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S: Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348 :1085 –1095,2003 [DOI] [PubMed] [Google Scholar]
- 12.Gu W, Tu Z, Kleyn PW, Kissebah A, Duprat L, Lee J, Chin W, Maruti S, Deng N, Fisher SL, Franco LS, Burn P, Yagaloff KA, Nathan J, Heymsfield S, Albu J, Pi-Sunyer FX, Allison DB: Identification and functional analysis of novel human melanocortin-4 receptor variants. Diabetes 48 :635 –639,1999 [DOI] [PubMed] [Google Scholar]
- 13.Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, Ferron C, Froguel P, Vaisse C: Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet 12 :145 –153,2003 [DOI] [PubMed] [Google Scholar]
- 14.Miraglia Del Giudice E, Cirillo G, Nigro V, Santoro N, D'Urso L, Raimondo P, Cozzolino D, Scafato D, Perrone L: Low frequency of melanocortin-4 receptor (MC4R) mutations in a Mediterranean population with early-onset obesity. Int J Obes Relat Metab Disord 26 :647 –651,2002 [DOI] [PubMed] [Google Scholar]
- 15.Branson R, Potoczna N, Kral JG, Lentes KU, Hoehe MR, Horber FF: Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med 348 :1096 –1103,2003 [DOI] [PubMed] [Google Scholar]
- 16.Hebebrand J, Geller F, Dempfle A, Heinzel-Gutenbrunner M, Raab M, Gerber G, Wermter AK, Horro FF, Blundell J, Schafer H, Remschmidt H, Herpertz S, Hinney A: Binge-eating episodes are not characteristic of carriers of melanocortin-4 receptor gene mutations. Mol Psychiatry 9 :796 –800,2004 [DOI] [PubMed] [Google Scholar]
- 17.Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang S, Bertrais S, Hercberg S, Basdevant A, Clement K, Vaisse C: Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab 91 :1811 –1818,2006 [DOI] [PubMed] [Google Scholar]
- 18.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P: Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106 :253 –262,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao YX, Segaloff DL: Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J Clin Endocrinol Metab 90 :5632 –5638,2005 [DOI] [PubMed] [Google Scholar]
- 20.Dempfle A, Hinney A, Heinzel-Gutenbrunner M, Raab M, Geller F, Gudermann T, Schafer H, Hebebrand J: Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J Med Genet 41 :795 –800,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poskitt EM: Defining childhood obesity: the relative body mass index (BMI). European Childhood Obesity Group. Acta Paediatr 84 :961 –963,1995 [DOI] [PubMed] [Google Scholar]
- 22.Rolland-Cachera MF, Cole TJ, Sempe M, Tichet J, Rossignol C, Charraud A: Body mass index variations: centiles from birth to 87 years. Eur J Clin Nutr 45 :13 –21,1991 [PubMed] [Google Scholar]
- 23.Balkau B: [An epidemiologic survey from a network of French Health Examination Centres, (D.E.S.I.R.): epidemiologic data on the insulin resistance syndrome]. Rev Epidemiol Sante Publique 44 :373 –375,1996 [PubMed] [Google Scholar]
- 24.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S: Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106 :271 –279,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyre D, Lecoeur C, Delplanque J, Francke S, Vatin V, Durand E, Weill J, Dina C, Froguel P: A genome-wide scan for childhood obesity-associated traits in French families shows significant linkage on chromosome 6q22.31–q23.2. Diabetes 53 :803 –811,2004 [DOI] [PubMed] [Google Scholar]
- 26.Stunkard AJ, Messick S: The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29 :71 –83,1985 [DOI] [PubMed] [Google Scholar]
- 27.Durand E, Boutin P, Meyre D, Charles MA, Clement K, Dina C, Froguel P: Polymorphisms in the amino acid transporter solute carrier family 6 (neurotransmitter transporter) member 14 gene contribute to polygenic obesity in French Caucasians. Diabetes 53 :2483 –2486,2004 [DOI] [PubMed] [Google Scholar]
- 28.Ghoussaini M, Vatin V, Lecoeur C, Abkevich V, Younus A, Samson C, Wachter C, Heude B, Tauber M, Tounian P, Hercberg S, Weill J, Levy-Marchal C, Le Stunff C, Bougneres P, Froguel P, Meyre D: Genetic study of the melanin-concentrating hormone receptor 2 (MCHR2) in childhood and adulthood severe obesity. J Clin Endocrinol Metab 92 :4403 –4409,2007 [DOI] [PubMed] [Google Scholar]
- 29.Hinney A, Schmidt A, Nottebom K, Heibult O, Becker I, Ziegler A, Gerber G, Sina M, Gorg T, Mayer H, Siegfried W, Fichter M, Remschmidt H, Hebebrand J: Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab 84 :1483 –1486,1999 [DOI] [PubMed] [Google Scholar]
- 30.Young EH, Wareham NJ, Farooqi S, Hinney A, Hebebrand J, Scherag A, O'Rahilly S, Barroso I, Sandhu MS: The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29 563 individuals. Int J Obes 31 :1437 –1441,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougneres P, Kovacs P, Marre M, Balkau B, Cauchi S, Chevre JC, Froguel P: Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 39 :724 –726,2007 [DOI] [PubMed] [Google Scholar]
- 32.Burke V, Beilin LJ, Dunbar D: Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. Int J Obes Relat Metab Disord 25 :147 –157,2001 [DOI] [PubMed] [Google Scholar]
- 33.Hinney A, Hohmann S, Geller F, Vogel C, Hess C, Wermter AK, Brokamp B, Goldschmidt H, Siegfried W, Remschmidt H, Schafer H, Gudermann T, Hebebrand J: Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J Clin Endocrinol Metab 88 :4258 –4267,2003 [DOI] [PubMed] [Google Scholar]
- 34.Jacobson P, Ukkola O, Rankinen T, Snyder EE, Leon AS, Rao DC, Skinner JS, Wilmore JH, Lonn L, Cowan GS Jr, Sjostrom L, Bouchard C: Melanocortin 4 receptor sequence variations are seldom a cause of human obesity: the Swedish Obese Subjects, the HERITAGE Family Study, and a Memphis cohort. J Clin Endocrinol Metab 87 :4442 –4446,2002 [DOI] [PubMed] [Google Scholar]
- 35.Larsen LH, Echwald SM, Sorensen TI, Andersen T, Wulff BS, Pedersen O: Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab 90 :219 –224,2005 [DOI] [PubMed] [Google Scholar]
- 36.Xiang Z, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, Millard WJ, Haskell-Luevano C: Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry 45 :7277 –7288,2006 [DOI] [PubMed] [Google Scholar]
- 37.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, Keil M, Reynolds JC, Yanovski JA: A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics 117 :1203 –1209,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.