Abstract
Bordetellae are Gram-negative bacilli causing respiratory tract infections of mammals and birds. Clinically important are B. pertussis, B. parapertussis and B. bronchiseptica. B. pertussis vaccines have been successful in preventing pertussis in infants and children. Veterinary vaccines against B. bronchiseptica are available, but their efficacy and mode of action are not established. There is no vaccine against B. parapertussis. Based on the concept that immunity to non-capsulated Gram-negative bacteria may be conferred by serum IgG anti-LPS we studied chemical, serological and immunological properties of the O-specific polysaccharides (O-SP) of B. bronchiseptica and B. parapertussis obtained by different degradation procedures. One type of the B. parapertussis and two types of B. bronchiseptica O-SP were recognized based on the structure of their non-reducing end saccharide; no cross-reaction between the two B. bronchiseptica types was observed. Competitive inhibition assays showed the immunodominance of the non-reducing end of these O-SP. Conjugates of B. bronchiseptica and B. parapertussis O-SP were prepared by two methods: using the Kdo residue exposed by mild acid hydrolysis of the LPS or the core glucosamine residue exposed by deamination of the LPS, for binding to an aminooxylated protein. Both coupling methods were carried out at a neutral pH, room temperature, and in a short time. All conjugates, injected as saline solutions at a fraction of an estimated human dose, induced antibodies in mice to the homologous O-SP. These methodologies can be applied to prepare O-SP-based vaccines against other Gram-negative bacteria.
Keywords: bordetella, LPS, vaccine, conjugate
1. Introduction
Vaccination has been proven effective for preventing infection of humans and animals by Bordetella spp. Killed whole cell and subunit vaccines have been used to immunize infants and children against Bordetella pertussis, the cause of pertussis, a highly contagious, severe respiratory infection especially of young children. No vaccine is available against B. parapertussis which causes a milder and less frequent form of pertussis in humans and a respiratory infection in sheep [1]. B. pertussis is confined to humans. Infection with B. parapertussis does not confer immunity to pertussis [2]. B. bronchiseptica causes serious respiratory infections in a variety of hosts: kennel cough in dogs, atrophic rhinitis in piglets, bronchopneumonia in rabbits and guinea pigs [3]. Rarely, B. bronchiseptica infects humans, mostly young children, animal handlers and immuno-compromised individuals [4]. Cellular and subcellular veterinary vaccines are available for this pathogen but they are of limited efficacy [5,6,7]. Among all bordetellae only B. pertussis expresses pertussis toxin [8,9].
Serum IgG anti-LPS has been shown to confer immunity to Gram-negative bacteria [10,11,12]. Monoclonal antibodies to B. pertussis LPS were shown to have complement-dependent bactericidal activity [13]. The LPS of all three bordetellae is of low molecular weight, < 10 kDa, rendering their isolated saccharides non-immunogenic. B. pertussis LPS is comprised of a Lipid A domain and a branched dodecasaccharide, composed of unusual sugars with free amino and carboxylic groups [14]. By SDS-PAGE, B. pertussis LPS shows two bands, A and B. Band B contains lipid A and a branched nonasaccharide core, Band A contains further substituted Band B by a trisaccharide unit. An almost identical core structure to B. pertussis was reported for B. bronchiseptica LPS [15]. The core region of B. parapertussis has a shorter heptasaccharide structure and does not contain the Band A trisaccharide [14]. In contrast to B. pertussis, which produces no O-specific polysaccharide (O-SP), B. bronchiseptica and B. parapertussis synthesize short O-SP containing about 12– 18 sugars. Initially, it was reported that the O-SP of both these organisms is identical and composed of a linear polymer of 1,4-linked 2,3-diacetamido-2,3-dideoxy-α-galacturonic acid (GalNAc3NAcA) [16]. Later, serological differences between B. bronchiseptica strains were ascribed to the structural variations of the non-reducing end-groups of the O-SP [17,18]. Similar observations were made for the serotypes Ogawa and Inaba of Vibrio cholerae O1 that differ only by a methyl group at the non-reducing end of Ogawa [19] and for Salmonella O40 and O43 serotypes [18].
The objectives of this study were to define and correlate structural and immunological data of B. bronchiseptica and B. parapertussis O-SP to enable proving experimental vaccines of wide coverage. Different conjugation methods were devised and the serum antibody responses elicited by these investigational vaccines in young outbred mice were assayed.
2. Materials and methods
2.1. Bacteria and cultivation
B. bronchiseptica ATCC 10580, Rb50 (ATCC BAA-588), and B. parapertussis ATCC 15989 were obtained from ATCC (Manassas, VA). B. bronchiseptica 15374, 3145 and B. parapertussis 12822 were obtained from Dr. M. Perry (NRC, Ottawa, Canada). Bacteria were grown on Bordet-Gengou (BG) agar plates and transferred to Stainer-Scholte (S-S) media [20]. After 16 to 24 hours of cultivation at 37 °C with shaking in buffled flasks, bacteria were harvested by centrifugation, killed by boiling for 1 h and stored at −20 °C for LPS extraction. Haemophilus ducreyi LPS used as a control was a gift from Teresa Lagergård (Göteborg, Sweden).
2.2. Oligosaccharides
LPS was isolated by hot phenol-water extraction and purified by enzyme treatment and ultracentrifugation as described [21]. Two methods were used for LPS degradation: (1) LPS (100 mg) was heated in 10 ml 1 % acetic acid for 60 min at 100 °C, ultracentrifuged at 35000 rpm for 5h at 4 °C and the carbohydrate-containing supernatant was passed through a 1 ×100 cm column of BioGel P-4 in pyridine/acetic acid/water buffer (4/8/988 ml) monitored with a Knauer differential refractometer. Twenty eight mg of O-SP were recovered from the void volume; (2) LPS (100 mg) was deaminated in 18 ml of a solution containing 30% acetic acid/5% sodium nitrite/water (1/1/1) for 6 h, at room temperature, on a magnetic stirrer, followed by ultracentrifugation [22]. The supernatant was freeze-dried and purified on the BioGel P-4 column as above. Twenty three mg of saccharide fraction, designated as O-SPdeam, were recovered from the void volume.
For isolation of oligosaccharides used in competitive inhibition assays, LPS was dissolved in anhydrous HF (100 mg LPS, 8 ml HF, 25°C, 24 h), then evaporated at room temperature, in a hood, on a plastic Petri dish. The residue was dissolved in pyridine/acetic acid/water buffer (4/8/988 ml), passed through a 2.5 × 80 cm column of Sephadex G-50 and further purified by HPLC on 250 × 9.5 mm Phenomenex Aqua column in 0.1 % TFA (for the first 10 min), then with a gradient of 0–50 % acetonitrile in 0.1 % TFA. The effluent was monitored by UV 220 nm absorption. Isolated oligosaccharides contained average 15 repeats of diacetamidouronic acid (average molecular mass of 4158 Da, as assayed by MALDI-TOF) and were chemically characterized previously [17].
2.3. Conjugation
2.3.1. O-SP conjugates
Bovine serum albumin (BSA, Sigma, St. Louis, MO) was derivatized to aminooxylated derivatives in a two step procedure: (1) BSA was treated with succinimidyl 3-(bromoacetamido)propionate (SBAP, Pierce, Pittsburgh, PA) to introduce thiol-reactive bromoacetamido moieties (BSA-Br); (2) BSA-Br was coupled with O-(3-thiopropyl)hydroxylamine, a heterobifunctional linker, to form the aminooxylated protein through stable thioether linkages (BSA-ONH2) as described [23]. For conjugation, BSA-ONH2 (5 mg) was reacted with 10 mg of O-SP of B. bronchiseptica 10580 (Bb10580), B bronchiseptica Rb50 (BbRb50) or B. parapertussis 10595 (Bpp15989) in 1.5 ml Buffer A (PBS, 0.1% glycerol, 5 mM EDTA), at pH 5.7, for 15 hours with stirring, at room temperature. The reaction mixture was passed through a 1 × 100 cm Sephadex G-100 column in 0.2 M NaCl and the void volume fraction characterized by protein concentration, immunodiffusion, SDS-PAGE and MALDI-TOF spectroscopy. The conjugates were designated BSA-ONH2/Bb10580 (#1), BSAONH2/ BbRb50 (#2) and BSA-ONH2/Bpp15989 (#3).
2.3.2. O-SPdeam conjugates
BSA-ONH2 (5 mg) were reacted with 10 mg of O-SPdeam of the listed strains using the described conditions. The products were designates BSA-ONH2/Bb10580deam (#4), BSA-ONH2/BbRb50deam (#5) and BSA-ONH2/Bpp15989deam (#6).
2.4. Immunization
5–6 weeks-old female NIH Swiss Webster mice were injected s.c. 3 times at 2 weeks intervals with 2.5 µg saccharide as a conjugate in 0.1 ml PBS. Groups of 10 mice were exsanguinated 7 days after the second or third injections [24]. Controls received PBS. Hyperimmune sera against B. bronchiseptica strains 10580 and Rb50, and against B. parapertussis strain 15989 were prepared with heat-killed whole bacteria as described [25].
2.5. Analytic
Protein concentration was measured by the method of Lowry [26]. SDS-PAGE used 14% gels according to the manufacturer's instructions (Bio-Rad, Hercules, CA). Double immunodiffusion was performed in 1 % agarose gel in PBS. Endotoxic activity was measured by the limulus amebocyte lysate assay as described by the manufacturer (Cambrex, Walkersville, MD).
2.6. Spectroscopy
MALDI-TOF mass spectra of the derivatized carrier proteins and of the conjugates were obtained with an OmniFlex MALDI-TOF instrument (Bruker Daltonics, Billerica, MA) operated in the linear mode. Samples for analysis were desalted and 1 µl, mixed with 20 µl of sinnapinic acid matrix made in 30% CH3CN and 0.1% trifluroacetic acid. Next, 1 µl of mixture was dried on the sample stage and placed in the mass spectrometer. NMR spectra were recorded at 30°C in D2O on a Varian UNITY INOVA 600 instrument using acetone as reference for both proton (2.225 ppm) and carbon (31.5 ppm) spectra. Varian standard programs COSY, NOESY (mixing time of 400ms), TOCSY (spinlock time 120 ms), HSQC, and gHMBC (long-range transfer delay 70 ms) were used [17].
2.7. Antibodies
Serum IgG antibodies were measured by ELISA [27]. Nunc Maxisorb plates were coated with B. bronchiseptica 10580, Rb50 or B. parapertussis 15989 LPS at 5 µg/ml in PBS containing 1% trichloroacetic acid and assayed as described [28], with the modification of 1 % HSA in PBS instead of BSA was used in a blocking step. The optimal concentration of the coating antigen was determined by checkerboard titration. PA MRX Dynatech reader was used. Antibody levels were calculated relative to the hyperimmune standard serum diluted 1:20,000 for B. bronchiseptica 10580; 1:15,000 for B. bronchiseptica Rb50 and 1:10,000 for B. parapertussis 15989, each assigned a value of 1000 ELISA Units (EU). Results were computed with an ELISA data processing program provided by the Biostatistics and Information Management Branch, CDC [29].
Competitive inhibition ELISA was done by incubating hyperimmune mice sera, diluted to the concentration that gave an A405 absorption of about 1.0, with 10 or 50 µg of inhibitor per well, for 1 h at 37 °C and overnight at 4°C. The assay was then continued as above. Sera at the same dilution with and without inhibitor were compared. Percent inhibition was defined as (A405 adsorbed serum/ A405 non-adsorbed serum) × 100%.
3. Results
3.1. Chemical characterization of LPS
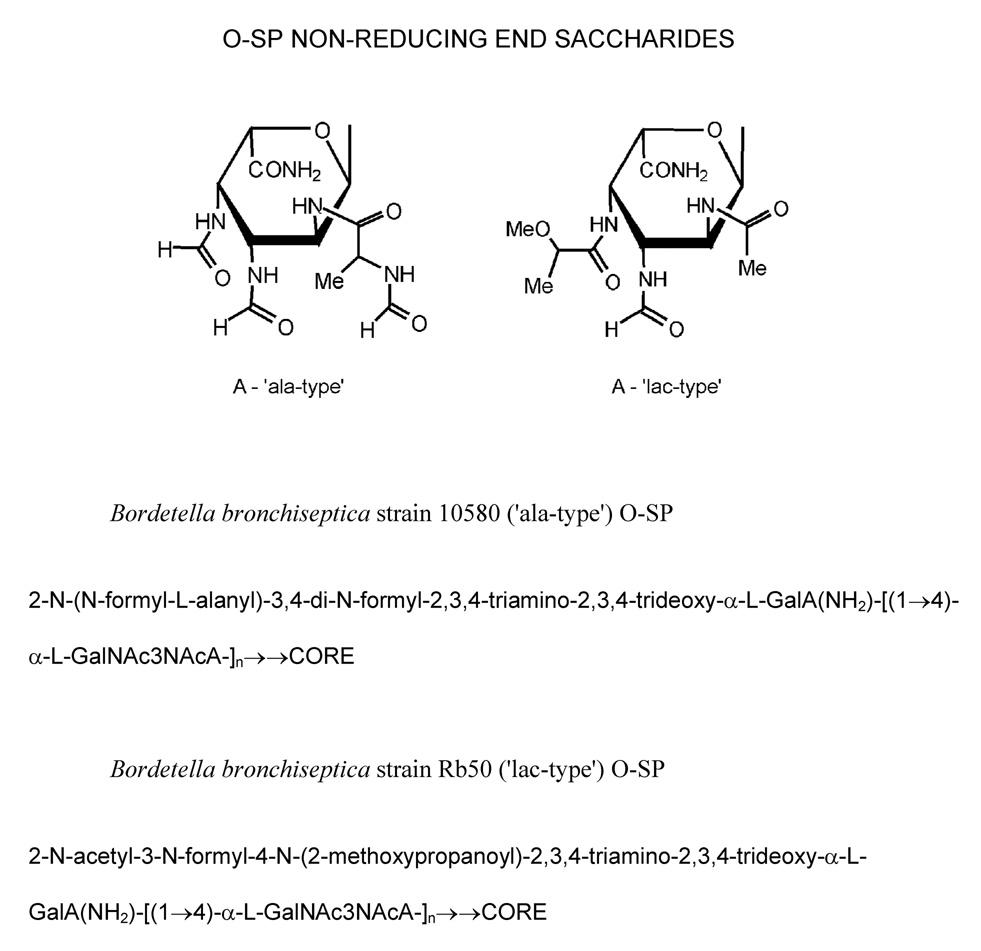
B. bronchiseptica 10580, 15374, 5137 and B. parapertussis 15989 and 12822 strains had an alanine substituent at the terminal non-reducing GalNAc3NAcA residue of the O-SP and thus was designated as an ‘ala-type’. B. bronchiseptica Rb50 had an O-methyl lactic acid substituent at the terminal non-reducing end of the O-SP, thus representing a ‘lac-type’ (Figure 1). The second GalNAc3NAcA residue from non-reducing-end (penultimate) was present as a free acid in B. bronchiseptica 10580, Rb50 and 5137 and was amidated in B. bronchiseptica 15374 and in B. parapertussis 15989 and 12822 strains.
Fig. 1.
Non-reducing end-group types of B. bronchiseptica O-SP.
3.2. Immunological characterization of LPS
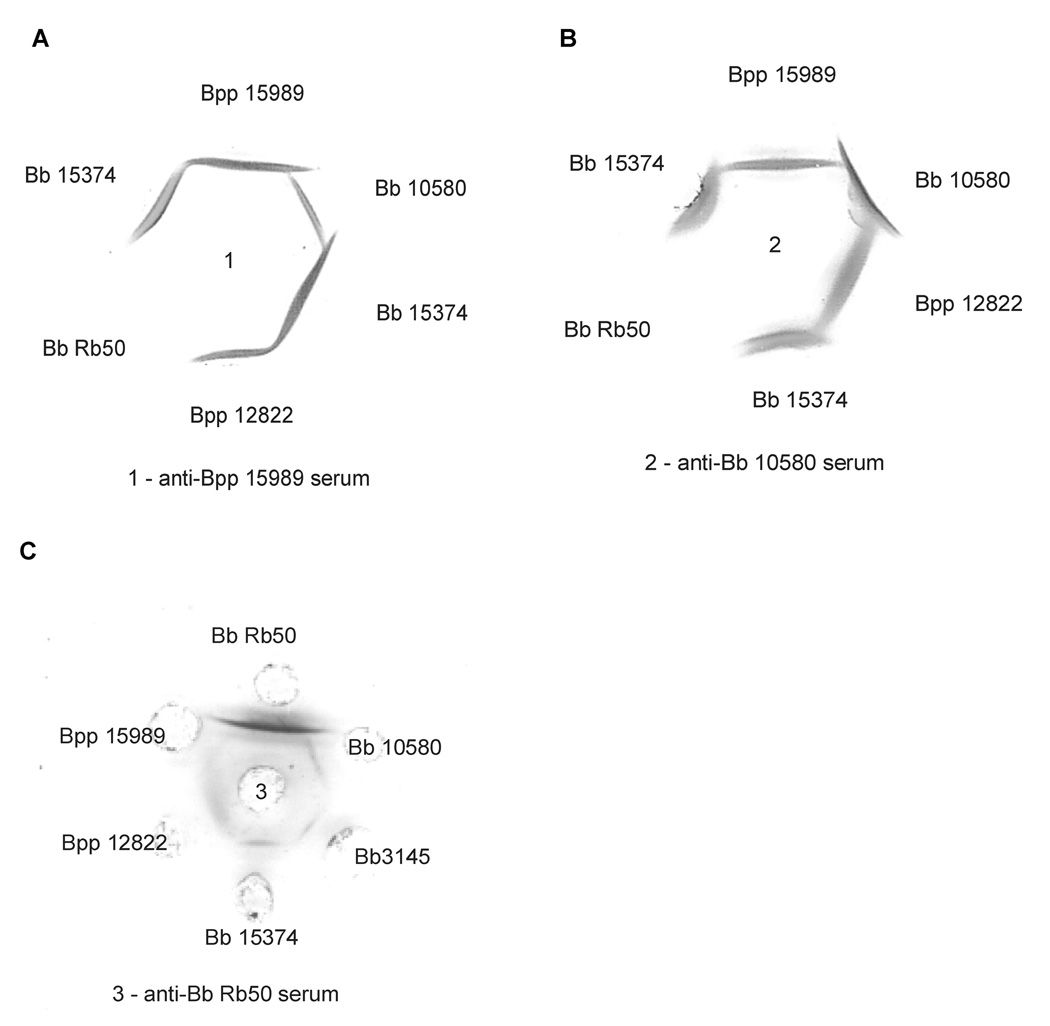
Immunodiffusion studies showed the following: (1) no cross-reaction between ‘ala-type’ and ‘lac-type’ LPS; (2) the ‘ala-type’ LPS from B. bronchiseptica 10580 and B. parapertussis 15989 were cross-reactive but with a spur of B. bronchiseptica 10580 LPS over that of B. parapertussis 15989 when tested against B. bronchiseptica 10580 antiserum. Using B. parapertussis 15989 serum, a spur of B. parapertussis 15989 LPS over that of B. bronchiseptica 10580 was seen (Figure 2). These two strains differ in their penultimate residue, being a free acid in B. bronchiseptica 10580 LPS and amidated in B. parapertussis15989. The LPS of B. bronchiseptica 15374, which like B. parapertussis 15989 LPS was an ‘ala-type’ and had amidated penultimate sugar, showed a line of identity with B. parapertussis 15989 LPS tested against anti-B. parapertussis 15989. Competitive inhibition ELISA corroborated the immunodiffusion data. Oligosaccharides of ‘ala-type’ did not inhibit binding of antibodies to ‘lac-type’ LPS (Table 1) and conversely, oligosaccharides of ‘lac-type’ did not inhibit binding of antibodies to ‘alatype’ LPS. Serum raised against B. parapertussis 15989 of ‘ala-type’ LPS was inhibited only 50% by B. bronchiseptica 10580 oligosaccharides also of ‘ala-type’. Conversely, serum raised against B. bronchiseptica 10580 LPS was inhibited only 50% by B. parapertussis 15989 ‘oligosaccharides. This partial inhibition may be ascribed to the amidation of penultimate sugar in B. parapertusis 15989 LPS and lack of it in B. bronchiseptica 10580 LPS.
Fig. 2.
Immunodifusion assays of anti-B parapertussis (Bpp) 15989 (A) and anti-B. bronchiseptica (Bb) 10580 (B) with Bordetella LPS. The LPS from Bpp15989 forms a spur over Bb10580 LPS, both ‘ala-type’, when tested against anti-Bpp15989 serum; Bb10580 LPS forms a spur over Bpp15989 using anti-Bb10580 serum. No reaction was seen between with BbRb50 LPS (‘lac’ type) and the two antisera.
Table 1.
Competitive inhibition ELISA. Plates were coated with B. bronchiseptica 10580, Rb50 or B. parapertussis 15989 LPS at 5 ug/ml and reacted with anti-B. bronchiseptica 10580, anti-Rb50 or anti-B. parapertussis 15989 hyperimmune mice sera. Inhibitors were used at 50 ug/well.
| Inhibitor | O-SP end-group | Amidation of 2nd O-SP sugar | % of inhibition of hyperimmune whole cell sera |
||
|---|---|---|---|---|---|
| anti-Bb10580 | anti-BbRb50 | anti-Bpp15989 | |||
| Bb 10580 O-SP | Ala | − | 92 | 2 | 50 |
| Bb 110H O-SP | Ala | − | 96 | 3 | 45 |
| Bb Rb50 O-SP | Lac | − | 0 | 93 | 1 |
| Bb 512 O-SP | Lac | − | 1 | 95 | 3 |
| Bpp 15989 O-SP | Ala | + | 42 | 5 | 97 |
| Bpp 15311 O-SP | Ala | + | 50 | 3 | 98 |
| H. ducreyi O-SP | na1 | na | 0 | 0 | 0 |
na - not applicable
3.3. Characterization of conjugates
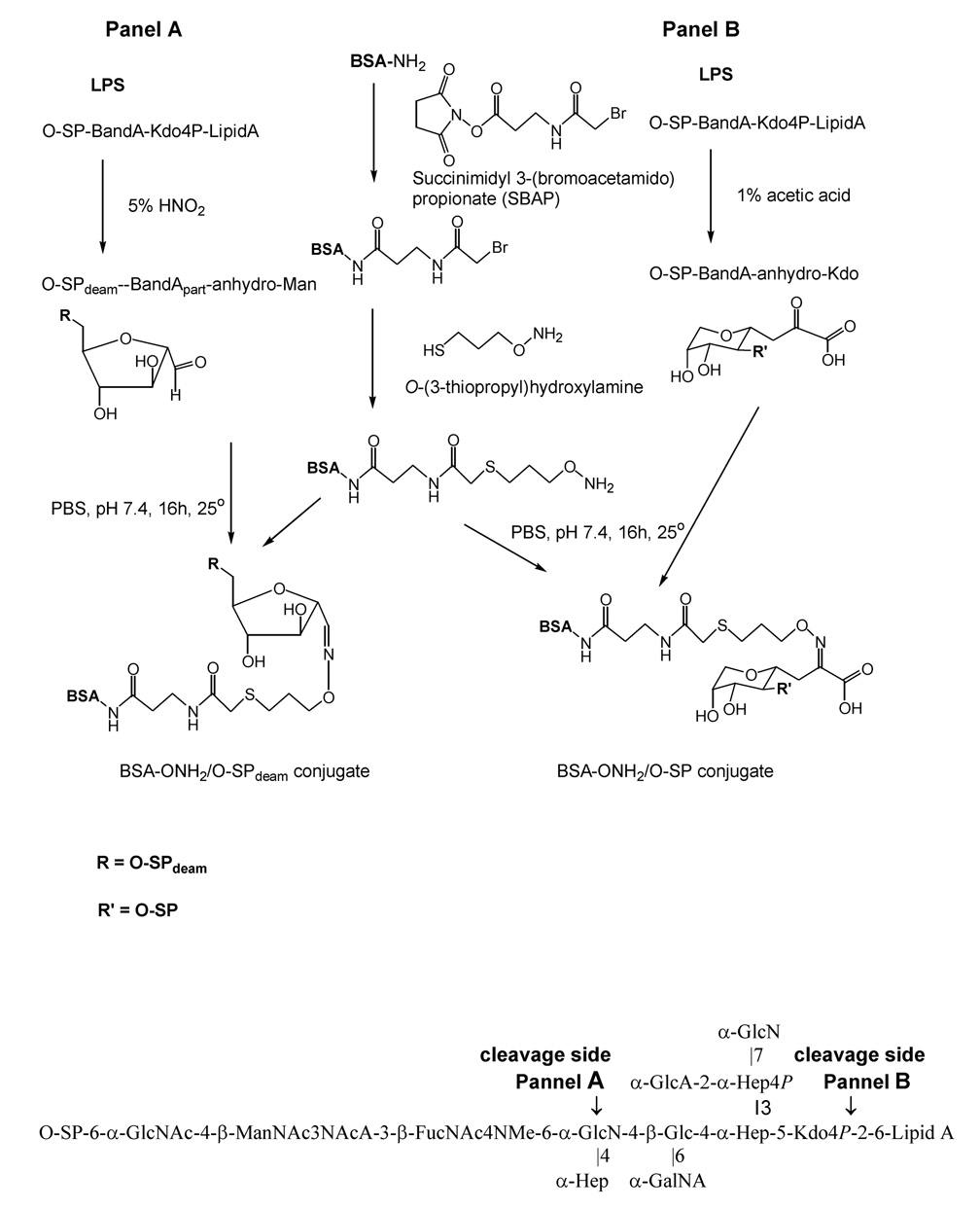
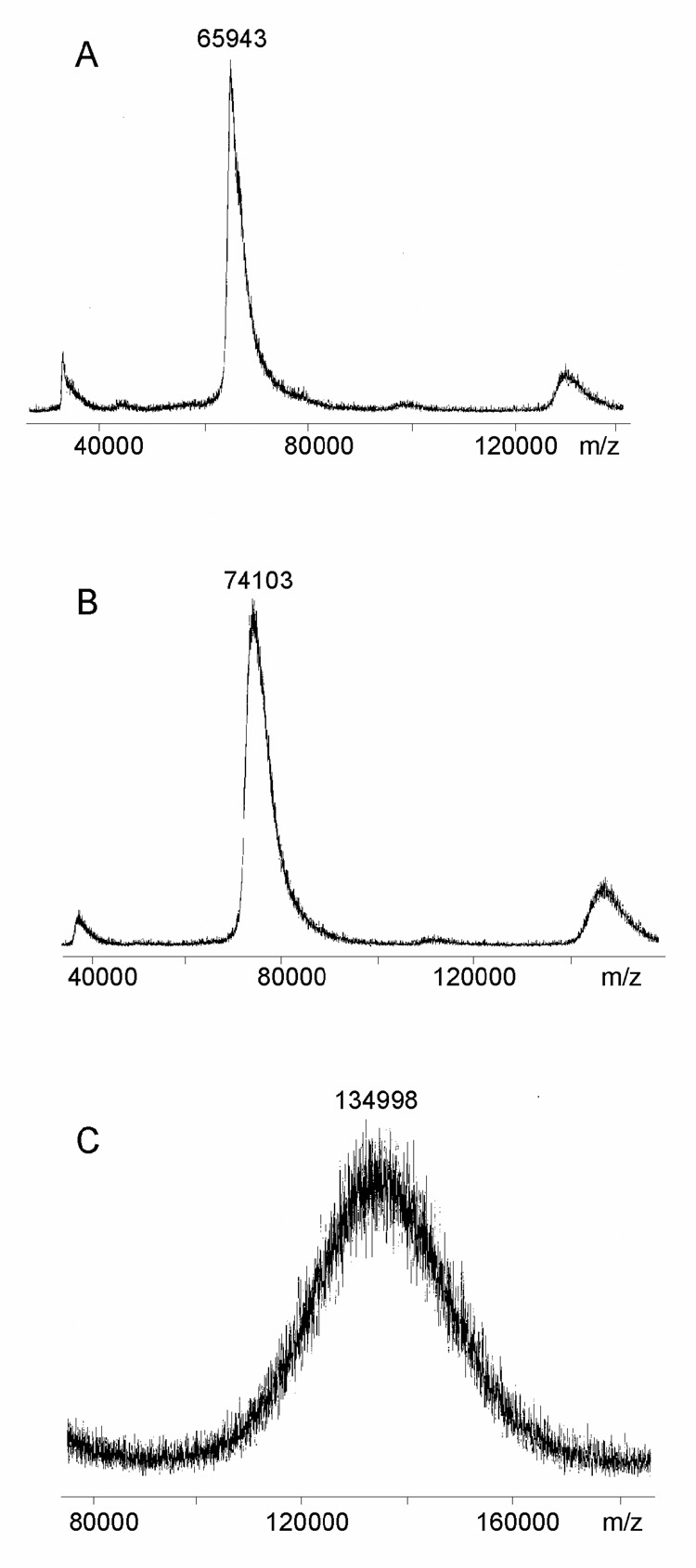
Schemes for preparing conjugates by derivatization of BSA with O-(3-thiopropyl)hydroxylamine in a two step procedure followed by a reaction with a carbonyl group of either acetic acid hydrolyzed (Conjugate #1,2,3) or deaminated (Conjugate #4,5,6) O-SP to form a stable oxime linkage are shown in Figure 3. Both procedures yielded high molecular mass conjugates, assayed by MALDI mass spectrometry (Table. 2). The number of O-SP chains per protein was calculated based on the known molecular mass of the aminoxylated BSA, the O-SP chains and the conjugate (Figure 4). For example, the average molecular mass of the aminooxylated BSA was 74.1 kDa, the average molecular mass of the O-SP from B. bronchiseptica strain 10580 was 6588 Da and that of the conjugate of 135 kDa (135 kDa - 74.1 kDa / 6.588 kDa) leading to an estimate of 9 O-SP chains per one BSA molecule (Conjugate #1). The average molecular mass of deaminated O-SP (O-SPdeam) from the same strain was 5108 Da, and that of the conjugate was 130 kDa. Therefore we estimated 11 O-SP chains per one BSA molecule (Conjugate #4).
Fig. 3.
Schemes of conjugation of B. bronchiseptica and B. parapertussis O-SP to protein Protein was first amiooxylated in a two step procedure and reacted with the carbonyl group on the terminal reducing end introduced into the O-SP either by deamidation of the GlcN, a first non-reducing end sugar of Band B (Panel A) or by acetic acid hydrolysis of the Kdo converting it to anhydro-Kdo (Panel B). Conjugates preserved the external nonreducing end of the O-SP. Below, the structure and the cleavage sides of B. bronchiseptica LPS.
Table 2.
Composition and GM of mouse IgG of anti-B. bronchiseptica 10580, Rb50 and B. parapertussis 15989 LPS induced by conjugates of O-SP and O-SPdeam bound to bovine serum albumin (BSA). Mice (10 per group) were injected with 2.5 µg of saccharide as a conjugate per mouse, s.c., 3 times, 2 weeks apart and bled one week after last injection‥
| # | Conjugate | Mol. mass1 [kDa] | Ratio protein:sugar (wt:wt) | Mol O-SP/ Mol Protein | ELISA Units after 3rd injection Coating antigen |
||
|---|---|---|---|---|---|---|---|
| 10580 LPS | Rb50 LPS | 15989 LPS | |||||
| 1 | BSA-ONH2/Bb10580 | 135 | 1 : 0.9 | 9 | 4.9 | 0.3 | 0.4 |
| 2 | BSA-ONH2/BbRb50 | 137 | 1 : 0.9 | 9 | 2.4 | 132.0 | 0.4 |
| 3 | BSA-ONH2/Bpp15989 | 165 | 1 : 1.4 | 14 | 0.3 | 0.3 | 1.2 |
| 4 | BSA-ONH2/Bb10580deam | 130 | 1 : 1.0 | 11 | 55.0 | 0.6 | 4.8 |
| 5 | BSA-ONH2/BbRb50deam | 105 | 1 : 0.5 | 8 | 0.1 | 3.5 | 0.3 |
| 6 | BSA-ONH2/ Bpp15989deam | 116 | 1 : 0.7 | 10 | 0.5 | 0.1 | 15.6 |
Molar mass assayed by Maldi , Mol mass of BSA-ONH2 was 74.1 kDa
Fig. 4.
MALDI-TOF of conjugates. (A) BSA, (B) aminooxylated BSA (BSA-ONH2), (C) BSA-ONH2/Bb10580 Conjugate #1 (C).
The amount of saccharide used for conjugation was in excess (twice the weight of protein) to ensure maximal binding. The conjugate yield was 65–75% for the protein and 30–35% for the sugar. All conjugates had < 5 EU/µg, endotoxin levels, compared to 15000 EU/µg of B. bronchiseptica 10580 LPS.
3.4. Antibodies to O-SP
The conjugates varied in their O-SP type (‘ala-type’ or ‘lac-type’), the chain length (O-SPdeam is shorter by about 1700 Da than the O-SP and in the density of the O-SP/O-SPdeam per protein (9–14 chains per BSA molecule), Table 2. All conjugates reacted with the hyperimmune sera raised against the homologues strains and with the anti-protein serum by a line of identity. All conjugates were immunogenic in mice (Table 2) and the following observations were made: (1) antibodies to ‘ala-type’: Conjugate #4 of B. bronchiseptica 10580, made with O-SPdeam was more immunogenic than Conjugate #1 made with B. bronchiseptica 10580 O-SP (GM=55 vs 4.9; P=0.01); Conjugate #6 of B. parapertussis 15989 made with O-SPdeam also induced higher antibody levels than Conjugate #3, prepared with B. parapertussis 15989 O-SP but the difference was not statistically significant (GM=15.6 vs 1.2; P =NS); (2) antibodies to ‘lac-type’: conjugate #2 of B. bronchiseptica Rb50 made with O-SP was more immunogenic than Conjugate #5 made with B. bronchiseptica Rb50 O-SPdeam (GM = 132 vs 3.5; P< 0.001); (3) only one of six conjugates, #4 of B. bronchiseptica 10580deam cross-reacted with B. parapertussis 15989 LPS (both ‘ala-type’).
4. Discussion
Immunization with whole cell and subunit vaccines has been used to protect humans against B. pertussis and animals against B. bronchiseptica. The licensed pertussis vaccines confer incomplete efficacy on an individual basis, probably because pertussis toxin antibodies do not kill the organism directly, however herd immunity contributes to the almost complete protection with the wide vaccine usage [46,1]. An additional component inducing bactericidal antibodies, such as anti-LPS, could improve pertussis vaccine individual effectiveness. Veterinary vaccines against B. bronchiseptica are available but their efficacy is limited and challenge studies were performed with the homologues strains only [47,5]. Several formulations are licensed, for instance Bronchi-Shield (Fort Dodge, Overland Park, KS), a Canine Adenovirus Type 2-Parainfluenza-Bordetella Bronchiseptica Vaccine, composed of modified live viruses and avirulent live bacteria, used for vaccination of healthy dogs and puppies eight weeks of age or older; Bronchicine (Pfeizer, NY), prepared from B. bronchiseptica extracts; or Protex-Bb (Intervet, Millsboro, DE), an intranasal vaccine containing avirulent live B. bronchiseptica, recommended for vaccination of healthy kittens and cats. Our studies are predicated on the assumption that LPS-based Bordetella vaccines could be of benefit.
O-SP-based vaccines were immunogenic in adults and in children [11,27]. Shigella sonnei O-SP conjugates were effective in adults [10]. Monoclonal antibodies to the core oligosaccharide of B. pertussis LOS exhibited bactericidal activity and reduced colonization with these bacteria in the lungs and tracheas of mice [13]. We hypothesize that the O-SP from B. parapertussis and B. bronchiseptica conjugated to an immunogenic carrier protein will stimulate production of protective levels of LPS antibodies. Vaccine of B. pertussis LPS prepared according to our scheme could add to protection afforded by current pertussis vaccine.
In this work we provided immunological verification to previously described structural variations at the terminal non-reducing end of B. bronchiseptica O-SP [18] and expanded the studies. A similar serospecifity attributed to the differences in methylation of the non-reducing end sugar of the O-SP was observed in V. cholerae O1 Ogawa and Inaba [19]. The terminal residues of B. bronchiseptica O-SP were shown to be immunodominant as no cross reaction between the two types: the ‘ala-type’, and the ‘lac-type’, was observed. B. parapertussis O-SP was described previously as structurally identical to that of B. bronchiseptica, a homopolymer of 1,4-linked 2,3-diacetamido-2,3-dideoxy-α-galacturonic acid (GalNAc3NAcA) [16]. B. parapertussis strains analyzed here expressed this homopolymer chain and belonged exclusively to the ‘ala-type’. B. parapertussis O-SP differed however from some B. bronchiseptica O-SP in the amidation of the penultimate non-reducing end residue. Immunodiffusion and competitive inhibition ELISA showed that this difference influences serospecificity. Immunogenicity of several types of O-SP-protein conjugate corroborated this assertion. High levels of antibodies were induced only to the homologues strains. Conjugates of ‘lac-type’ O-SP did not induce antibodies to ‘ala-type’ O-SP of the same species and conjugates prepared with B. parapertussis O-SP did not induce antibodies to B. bronchiseptica O-SP, though both belonged to the same ‘ala-type’ but differed in the amidation of the penultimate non-reducing end sugar of the O-SP. Survey of disease isolates will determine the number of O-SP types to be included in a comprehensive B. bronchiseptica vaccine.
We report new methods for binding O-SP to a carrier protein by one-point attachment at their reducing end. Many protocols have been proposed for synthesizing this type of conjugates with reductive amidation being the most popular [30,31,32,33,34]. The choice of conjugation methods is restricted by the pH and temperature sensitivities of both vaccine components. The types of chemical linkages between the O-SP and the protein as well as the saccharide chain length and the chain density on the protein were shown to influence the serum antibody responses to both vaccine components [35,36,37,38,39,40]. Here we studied two conjugation methods of O-SP either by their Kdo or their glucosamine residues. Conjugation was performed between the carbonyl group on the terminal reducing end of the O-SP and an amiooxy group of a bifunctional linker bound to the protein [23,41,42,43]. The carbonyl group was introduced into the O-SP either by deamidation of the GlcN, a first non-reducing end sugar of Band B or by the Kdo released by acetic acid hydrolysis, proposed to undergo degradation to carbonyl containing anhydro-Kdo [44,45,46]. This is an efficient reaction carried out under mild conditions and in a short time. Conjugates thus prepared preserved the external non-reducing end of the O-SP and induced antibodies to both conjugate components. This method can be used for preparing O-SP-based clinical vaccines.
Acknowledgment
This research was supported by the Intramural Research Program of the NIH, NICHD. We thank Chris Mocca and Chunyan Guo (NICHD, Bethesda) for the technical assistance.
Abbreviations
- GM
geometric mean
- SBAP
N-succinimidyl 3-(bromoacetamido) propionate
- GalNAc3NAcA
2,3-diacetamido-2,3-dideoxy-L-galacturonic acid
- Buffer A, PBS
0.1% glycerol, 5 mM EDTA, pH 7.4
- s.c.
subcutaneously
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Referances
- 1.Brinig MM, Register KB, Ackermann MR, Relman DA. Genomic features of Bordetella parapertussis clades with distinct host species specificity. Genome Biol. 2006;7:R81. doi: 10.1186/gb-2006-7-9-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taranger J, Trollfors B, Lagergard T, Zackrisson G. Parapertussis infection followed by pertussis infection. Lancet. 1994;344:1703. doi: 10.1016/s0140-6736(94)90485-5. [DOI] [PubMed] [Google Scholar]
- 3.Burns EH, Jr, Norman JM, Hatcher MD, Bemis DA. Fimbriae and determination of host species specificity of Bordetella bronchiseptica. J Clin Microbiol. 1993;31:1838–1844. doi: 10.1128/jcm.31.7.1838-1844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodnow RA. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCandlish IA, Thompson H, Wright NG. Vaccination against Bordetella bronchiseptica infection in dogs using a heat-killed bacterial vaccine. Res Vet Sci. 1978;25:45–50. [PubMed] [Google Scholar]
- 6.Matherne CM, Steffen EK, Wagner JE. Efficacy of commercial vaccines for protecting guinea pigs against Bordetella bronchiseptica pneumonia. Lab Anim Sci. 1987;37:191–194. [PubMed] [Google Scholar]
- 7.Ellis JA, Krakowka GS, Dayton D, Konoby C. Comparative efficacy of an injectable vaccine and an intranasal vaccine in stimulating Bordetella bronchiseptica-reactive antibody responses in seropositive dogs. J Am Vet Med Assoc. 2002;220:43–48. doi: 10.2460/javma.2002.220.43. [DOI] [PubMed] [Google Scholar]
- 8.Marchitto KS, Smith SG, Locht C, Keith JM. Nucleotide sequence homology to pertussis toxin gene in Bordetella bronchiseptica and Bordetella parapertussis. Infect Immun. 1987;55:497–501. doi: 10.1128/iai.55.3.497-501.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arico B, Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987;169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor DN, Hale TL, Sadoff JC, Pavliakova D, Schneerson R, Robbins JB. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RK, Taylor DN, Bryla DA, Robbins JB, Szu SC. Phase 1 evaluation of Vibrio cholerae O1, serotype Inaba, polysaccharide-cholera toxin conjugates in adult volunteers. Infect Immun. 1998;66:3095–3099. doi: 10.1128/iai.66.7.3095-3099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins JB, Chu CY, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 13.Mountzouros KT, Kimura A, Cowell JL. A bactericidal monoclonal antibody specific for the lipooligosaccharide of Bordetella pertussis reduces colonization of the respiratory tract of mice after aerosol infection with B. pertussis. Infect Immun. 1992;60:5316–5318. doi: 10.1128/iai.60.12.5316-5318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caroff ML, Aussel L, Zarrouk H, Martin A, Richards JC, Therisod H, Perry MB, Karibian D. Structural variability and originality of the Bordetella endotoxins. J. Endotoxin Res. 2001;7:63–68. [PubMed] [Google Scholar]
- 15.Vinogradov E. The structure of the carbohydrate backbone of the lipopolysaccharides from Bordetella hinzii and Bordetella bronchiseptica. Eur J Biochem. 2000;267:4577–4582. doi: 10.1046/j.1432-1327.2000.01512.x. [DOI] [PubMed] [Google Scholar]
- 16.Di Fabio JL, Caroff M, Karibian D, Richards JC, Perry MB. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol Lett. 1992;97:275–282. doi: 10.1016/0378-1097(92)90348-r. [DOI] [PubMed] [Google Scholar]
- 17.Preston A, Petersen BO, Duus JO, Kubler-Kielb J, Ben-Menachem G, Li J, Vinogradov E. Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J Biol Chem. 2006;281:18135–18144. doi: 10.1074/jbc.M513904200. [DOI] [PubMed] [Google Scholar]
- 18.Vinogradov E, Peppler MS, Perry MB. The structure of the nonreducing terminal groups in the O-specific polysaccharides from two strains of Bordetella bronchiseptica. Eur J Biochem. 2000;276:7230–7236. doi: 10.1046/j.1432-1327.2000.01835.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Villeneuve S, Zhang J, Lei P, Miller CE, Lafaye P, Nato F, Szu SC, Karpas A, Bystricky S, Robbins JB, Kovac P, Fournier JM, Glaudemans CP. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. Biol Chem. 1998;273:2777–2783. doi: 10.1074/jbc.273.5.2777. [DOI] [PubMed] [Google Scholar]
- 20.Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 21.Westphal O, Jann K. Extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 22.Le Blay K, Caroff M, Blanchard F, Perry MB, Chaby R. Epitopes of Bordetella pertussis lipopolysaccharides as potential markers for typing of isolates with monoclonal antibodies. Microbiology. 1996;142:971–978. doi: 10.1099/00221287-142-4-971. [DOI] [PubMed] [Google Scholar]
- 23.Kubler-Kielb J, Pozsgay V. A new method for conjugation of carbohydrates to proteins using an aminooxy-thiol heterobifunctional linker. J Org Chem. 2005;70:6987–6990. doi: 10.1021/jo050934b. [DOI] [PubMed] [Google Scholar]
- 24.Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu CY, Liu BK, Watson D, Szu SC, Bryla D, Shiloach J, Schneerson R, Robbins JB. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga's bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry JO, Rosenbrough NJ, Farr AL, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Taylor DN, Trofa AC, Sadoff J, Chu C, Bryla D, Shiloach J, Cohen D, Ashkenazi S, Lerman Y, Egan W, Schneerson R, Robbins JB. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993;61:3678–3687. doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy E, Ohlin M, Llano M. Enhanced ELISA sensitivity using TCA for efficient coating of biologically active lipopolysaccharides or lipid A to the solid phase. J Immunol Methods. 1994;176:111–116. doi: 10.1016/0022-1759(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 29.Plikaytis BD, Carlone GM. Program ELISA for Windows User’s Manual. Version 2. Atlanta, Ga: Centers for Disease Control; 2005. [Google Scholar]
- 30.Mieszala M, Kogan G, Jennings HJ. Conjugation of meningococcal lipooligosaccharides through their lipid A terminus conserves their inner epitopes and results in conjugate vaccines having improved immunological properties. Carbohydr Res. 2003;338:167–175. doi: 10.1016/s0008-6215(02)00395-6. [DOI] [PubMed] [Google Scholar]
- 31.Cox AD, Zou W, Gidney MA, Lacelle S, Plested JS, Makepeace V, Wright JC, Coull PA, Moxon ER, Richards JC. Candidacy of LPS-based glycoconjugates to prevent invasive meningococcal disease: developmental chemistry and investigation of immunological responses following immunization of mice and rabbits. Vaccine. 2005;23:5045–5054. doi: 10.1016/j.vaccine.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz BA, Gray GR. Proteins containing reductively aminated disaccharides. Synthesis and chemical characterization. Arch Biochem Biophys. 1977;181:542–549. doi: 10.1016/0003-9861(77)90261-2. [DOI] [PubMed] [Google Scholar]
- 33.Niedziela T, Letowska I, Lukasiewicz J, Kaszowska M, Czarnecka A, Kenne L, Lugowski C. Epitope of the vaccine-type Bordetella pertussis strain 186 lipooligosaccharide and antiendotoxin activity of antibodies directed against the terminal pentasaccharide-tetanus toxoid conjugate. Infect Immun. 2005;73:7381–7389. doi: 10.1128/IAI.73.11.7381-7389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller-Loennies S, Grimmecke D, Brade L, Lindner B, Kosma P, Brade H. A novel strategy for the synthesis of neoglycoconjugates from deacylated deep rough lipopolysaccharides. J Endotoxin Res. 2002;8:295–305. doi: 10.1179/096805102125000506. [DOI] [PubMed] [Google Scholar]
- 35.Kubler-Kielb J, Liu TY, Mocca C, Majadly F, Robbins JB, Schneerson R. Additional conjugation methods and immunogenicity of B. anthracis poly-γ-D-glutamic acid-proteins conjugates. Infect Immun. 2006;74:4744–4749. doi: 10.1128/IAI.00315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneerson R, Kubler-Kielb J, Liu TY, Dai ZD, Yergey A, Backlund P, Shiloach J, Leppla SH, Majadly F, Robbins JB. Poly-γ-D-glutamic acid protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc Natl Acad Sci USA. 2003;100:8945–8950. doi: 10.1073/pnas.1633512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pozsgay V, Chu C, Pannell L, Wolfe J, Robbins JB, Schneerson R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc Natl Acad Sci U S A. 1999;96:5194–5197. doi: 10.1073/pnas.96.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mawas F, Niggemann J, Jones C, Corbel MJ, Kamerling JP, Vliegenthart JF. Immunogenicity in a mouse model of a conjugate vaccine made with a synthetic single repeating unit of type 14 pneumococcal polysaccharide coupled to CRM197. Infect Immun. 2002;70:5107–5114. doi: 10.1128/IAI.70.9.5107-5114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laferriere CA, Sood RK, de Muys JM, Michon F, Jennings HJ. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect Immun. 1998;66:2441–2446. doi: 10.1128/iai.66.6.2441-2446.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dick WE, Beurret M., Jr Glycoconjugates of bacterial carbohydrate antigens. A survey and consideration of design and preparation factors. Contrib Microbiol Immunol. 1989;10:48–114. [PubMed] [Google Scholar]
- 41.Pozsgay V, Kubler-Kielb J. Synthesis of carbohydrate antigens related to Shigella dysenteriae type 1 and their protein conjugates. In: Demchenko AV, editor. Frontiers in Modern Carbohydrate Synthesis. Vol. 960. 2006. pp. 238–252. ACS Symposium Series. [Google Scholar]
- 42.Lees A, Sen G, LopezAcosta A. Versatile and efficient synthesis of protein-polysaccharide conjugate vaccines using aminooxy reagents and oxime chemistry. Vaccine. 2006;24:716–729. doi: 10.1016/j.vaccine.2005.08.096. [DOI] [PubMed] [Google Scholar]
- 43.Blixt O, Hoffmann J, Svenson S, Norberg T. Pathogen specific carbohydrate antigen microarrays: a chip for detection of Salmonella O-antigen specific antibodies. Glycoconj J. 2008;25:27–36. doi: 10.1007/s10719-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 44.Bock K, Vinogradov EV, Holst O, Brade H. Isolation and structural analysis of oligosaccharide phosphates containing the complete carbohydrate chain of the lipopolysaccharide from Vibrio cholerae strain H11 (non-O1) Eur J Biochem. 1994;225:1029–1039. doi: 10.1111/j.1432-1033.1994.1029b.x. [DOI] [PubMed] [Google Scholar]
- 45.Volk W, Salomonsky NL, Hunt D. Xanthomonas sinensis cell wall lipopolysaccharide. J Biol Chem. 1972;24:3881–3887. [PubMed] [Google Scholar]
- 46.Auzanneau FI, Charon D, Szabo L. Phosphorylated sugars. Part 27. Synthesis and reactions, in acid medium, of 5-O-substituted methyl 3-deoxy-α-D-manno-oct-2-ulopyranosidonic acid 4-phosphates. J Chem So Perkin Trans I. 1991:509–517. [Google Scholar]
- 47.Sato H, Ito A, Chiba J, Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984;46:422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris DL, Switzer WP. Nasal and tracheal resistance of swine against reinfection by Bordetella bronchiseptica. Am. J. Vet. Res. 1969;30:1161–1166. [PubMed] [Google Scholar]