Abstract
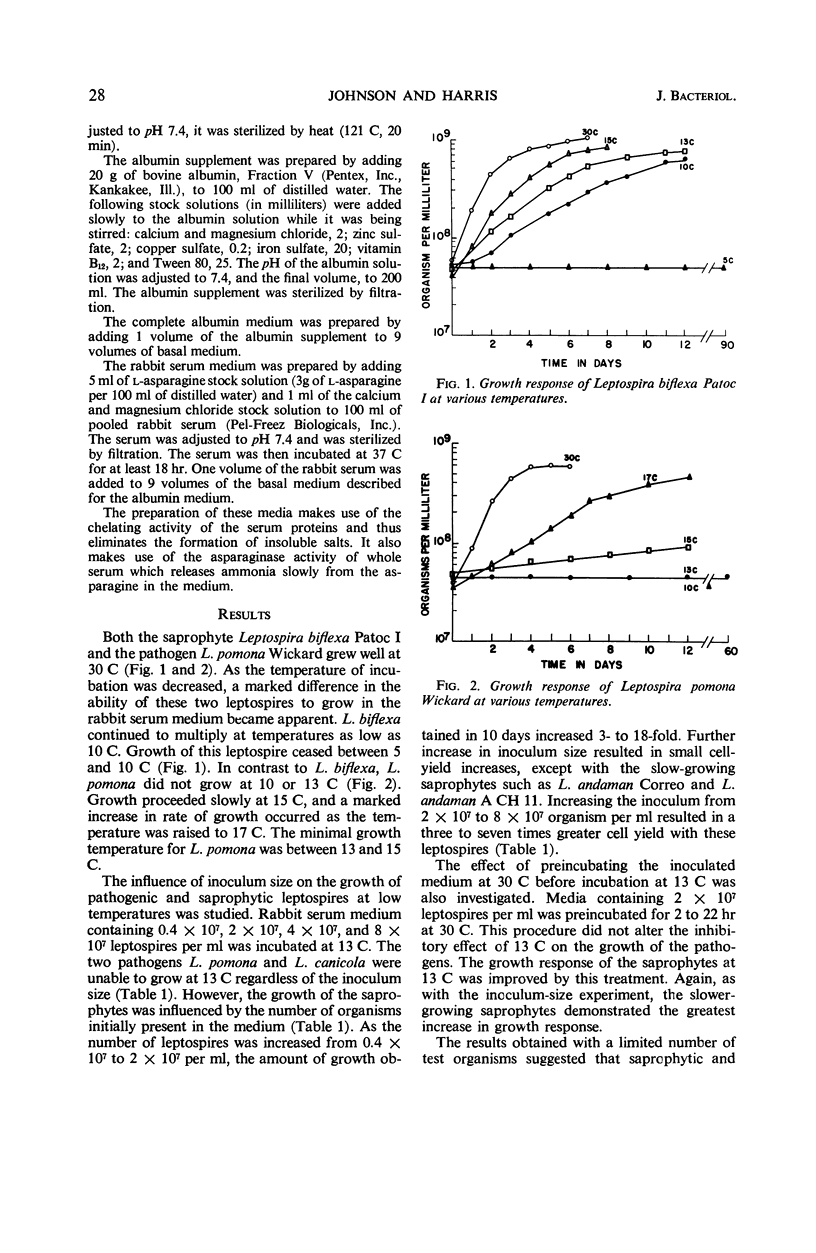
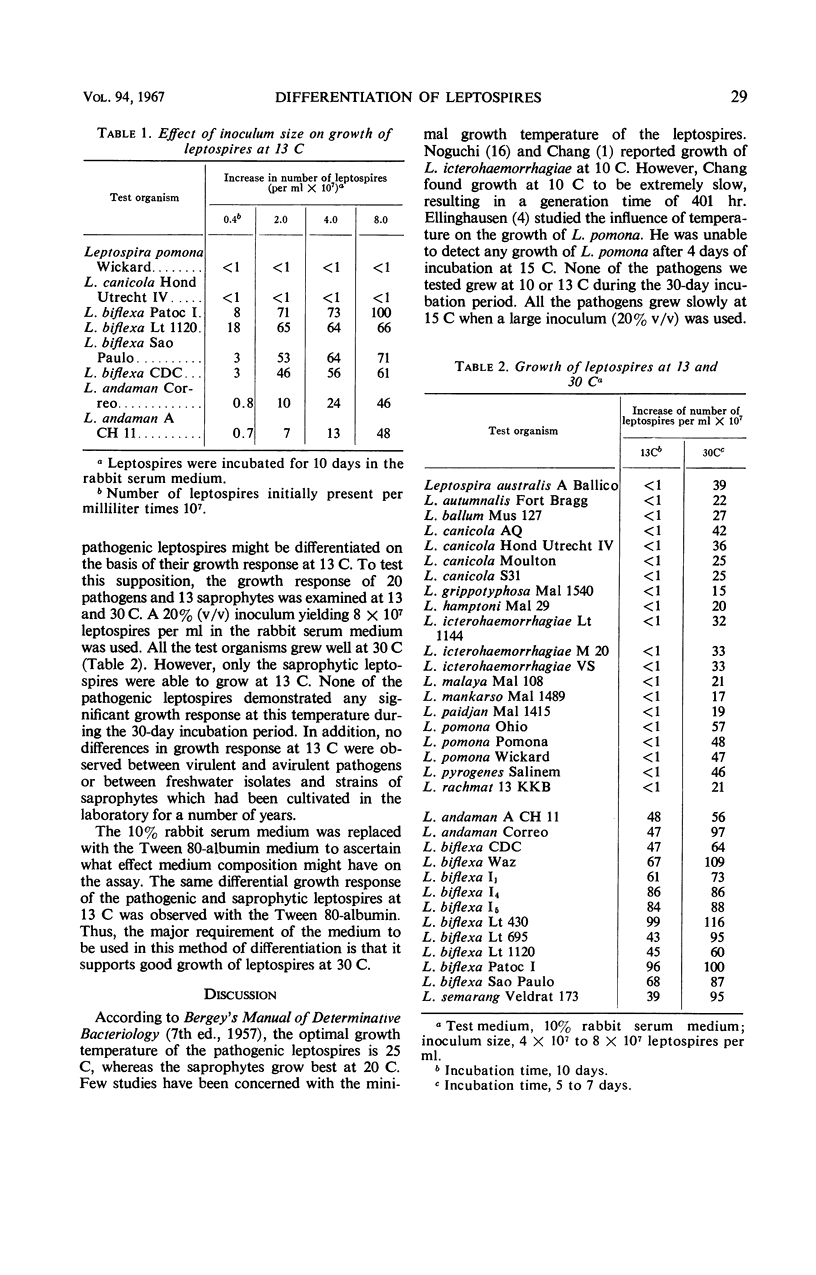
The minimal growth temperature of the pathogenic leptospires is between 13 and 15 C. The saprophytic leptospires have a minimal growth temperature between 5 and 10 C, or approximately 5 C below that of the pathogens. The capability of the saprophytic leptospires to grow at temperatures below those which allow the growth of the pathogenic leptospires provides a simple method of discrimination. With an inoculum yielding approximately 8 × 107 cells per ml in the test medium and an incubation temperature of 13 C, the saprophytic leptospires were easily differentiated from the pathogenic leptospires. All 13 saprophytic leptospires tested grew in the 10% rabbit serum medium at 13 C, whereas none of the 20 pathogens grew during the 30-day incubation period.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COX C. D., LARSON A. D. Colonial growth of leptospirae. J Bacteriol. 1957 Apr;73(4):587–589. doi: 10.1128/jb.73.4.587-589.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Studies on the isolation and growth of Leptospira from surface waters. Ann Soc Belges Med Trop Parasitol Mycol. 1966;46(2):193–202. [PubMed] [Google Scholar]
- ELLINGHAUSEN H. C., Jr, MCCULLOUGH W. G. NUTRITION OF LEPTOSPIRA POMONA AND GROWTH OF 13 OTHER SEROTYPES: FRACTIONATION OF OLEIC ALBUMIN COMPLEX AND A MEDIUM OF BOVINE ALBUMIN AND POLYSORBATE 80. Am J Vet Res. 1965 Jan;26:45–51. [PubMed] [Google Scholar]
- ELLINGHAUSEN H. C., Jr Some observations on cultural and biochemical characteristics of Leptospira pomona. J Infect Dis. 1960 May-Jun;106:237–244. doi: 10.1093/infdis/106.3.237. [DOI] [PubMed] [Google Scholar]
- FUZI M., CSOKA R. An egg-yolk reaction test for the differentiation of Leptospirae. J Pathol Bacteriol. 1961 Jul;82:208–212. doi: 10.1002/path.1700820129. [DOI] [PubMed] [Google Scholar]
- FUZI M., CSOKA R. Rapid method for the differentiation of parasitic and saprophytic leptospirae. J Bacteriol. 1961 Jun;81:1008–1008. doi: 10.1128/jb.81.6.1008-1008.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUZI M., CSOKA R. [Differentiation of pathogenic and saprophytic Leptospira with a copper sulfate test]. Zentralbl Bakteriol. 1960 Jun;179:231–237. [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. 5-FLUOROURACIL AS A SELECTIVE AGENT FOR GROWTH OF LEPTOSPIRAE. J Bacteriol. 1964 Feb;87:422–426. doi: 10.1128/jb.87.2.422-426.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. DIFFERENTIATION OF PATHOGENIC AND SAPROPHYTIC LEPTOSPIRES WITH 8-AZAGUANINE. J Bacteriol. 1964 Dec;88:1618–1623. doi: 10.1128/jb.88.6.1618-1623.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Antileptospiral activity of serum. II. Leptospiral virulence factor. J Bacteriol. 1967 Feb;93(2):513–519. doi: 10.1128/jb.93.2.513-519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Muschel L. H. Antileptospiral activity of serum. I. Normal and immune serum. J Bacteriol. 1966 Apr;91(4):1403–1409. doi: 10.1128/jb.91.4.1403-1409.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmety E., Plesko I., Bakoss P., Chorvath B. Evaluation of methods for differentiating pathogenic and saprophytic leptospira strains. Ann Soc Belges Med Trop Parasitol Mycol. 1966;46(1):111–122. [PubMed] [Google Scholar]
- LARSON A. D., TREICK R. W., EDWARDS C. L., COX C. D. Growth studies and plate counting of leptospires. J Bacteriol. 1959 Mar;77(3):361–366. doi: 10.1128/jb.77.3.361-366.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANAGAWA R., HIRAMUNE T., AKAIKE Y. GROWTH OF SAPROPHYTIC AND PATHOGENIC LEPTOSPIRAE ON SOLID MEDIUM IN CARBON DIOXIDE-FREE AIR. J Bacteriol. 1963 Apr;85:953–954. doi: 10.1128/jb.85.4.953-954.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


