Abstract
In β cells, both glucose and hormones, such as GLP-1, stimulate production of the second messenger cAMP, but glucose and GLP-1 elicit distinct cellular responses. We now show in INS-1E insulinoma cells that glucose and GLP-1 produce cAMP with distinct kinetics via different adenylyl cyclases. GLP-1 induces a rapid cAMP signal mediated by G protein–responsive transmembrane adenylyl cyclases (tmAC). In contrast, glucose elicits a delayed cAMP rise mediated by bicarbonate, calcium, and ATP-sensitive soluble adenylyl cyclase (sAC). This glucose-induced, sAC-dependent cAMP rise is dependent upon calcium influx and is responsible for the glucose-induced activation of the mitogen-activated protein kinase (ERK1/2) pathway. These results demonstrate that sAC-generated and tmAC-generated cAMP define distinct signaling cascades.
INTRODUCTION
In mammals, glucose homeostasis is regulated by pancreatic β cells. When serum glucose is elevated, β cells release insulin, which signals muscle, liver, and fat to take up and store glucose. While it is clear that Ca2+ influx is a key factor in the regulation of glucose-induced insulin secretion, the role of other second messengers, such as cAMP, remain unclear. Studies dating back three decades suggest that glucose itself elevates cAMP levels (Charles et al., 1973; Grill and Cerasi, 1973; Rutter, 2001); however, in the absence of a mechanism for glucose-induced cAMP generation, the significance of these findings was questioned (Montrose-Rafizadeh et al., 1997; Rutter, 2001; Delmeire et al., 2003).
In β cells, metabolism of glucose leads to an increase in intracellular ATP, which in turn induces the depolarization of the plasma membrane through the closure of ATP-sensitive potassium (KATP) channels (Ashcroft and Rorsman, 1989). Subsequently, membrane depolarization causes the opening of voltage-dependent calcium channels and elevation of cytosolic calcium (Rutter et al., 1993). The resultant rise in intracellular calcium is required for insulin release. This rise is also linked to an increase in the second messenger cAMP (Charles et al., 1975; Rutter, 2001; Landa et al., 2005). Coordinated release from calcium stores results in calcium waves, which are temporally and spatially linked to fluctuations of cAMP (Landa et al., 2005; Dyachok et al., 2006). These studies convincingly demonstrated that cAMP is generated consequent to glucose metabolism, but the specific source of the second messenger remained unclear.
In contrast to the controversy surrounding glucose-induced cAMP generation, it is well accepted that cAMP is produced in β cells in response to incretins released by the intestine in response to food intake. The incretin glucagon-like peptide-1 (GLP-1) increases cAMP levels by activating a specific G protein–coupled receptor (GLP-1 receptor), resulting in stimulation of one of a family of G protein–responsive, transmembrane adenylyl cyclases (tmACs) (Thorens, 1992; Usdin et al., 1993; Moens et al., 1996). Although unable to elicit insulin secretion on its own, GLP-1–induced cAMP potentiates glucose-induced insulin secretion (Holz et al., 1995; Yang et al., 1999; Delmeire et al., 2003; Dyachok et al., 2006).
A recent study examining glucose-induced cAMP generation in the Min6 β-cell line using a FRET-based cAMP sensor revealed a temporal distinction between glucose-induced and GLP-1–induced cAMP production (Landa et al., 2005). In response to exendin-4, a GLP-1 receptor agonist, or to the pharmacological stimulator of tmACs, forskolin, cAMP production was stimulated within the first minutes of agonist addition. In contrast, the cAMP rise observed in response to high glucose media required more than 6 min. These different kinetics are consistent with distinct mechanisms of cAMP generation.
Cellular cAMP levels are determined by the relative activities of adenylyl cyclases, which synthesize the second messenger, and phosphodiesterases (PDEs), which catabolize it. Various studies have demonstrated that the glucose-induced cAMP rise occurs in the presence of PDE inhibitors (Charles et al., 1973; Landa et al., 2005), revealing the actions of glucose must be mediated by changes in the rate of cAMP synthesis by adenylyl cyclases. In mammals, there are two families of cAMP-producing adenylyl cyclases, tmACs and “soluble” adenylyl cyclase (sAC). Both families of adenylyl cyclase include members whose activity can be stimulated by calcium (Kamenetsky et al., 2006). The tmAC family consists of nine G protein–responsive cyclases, of which two (tmAC types I and VIII) can be activated by calcium (Willoughby and Cooper, 2007) and are found in β cells (Yang et al., 1999; Delmeire et al., 2003; Landa et al., 2005). Our laboratory identified a distinct source of cAMP in mammalian cells, sAC, which is insensitive to G proteins and forskolin (Buck et al., 1999). Unlike tmACs, sAC is not exclusively linked to the plasma membrane but distributed to several subcellular compartments, such as the mitochondria, the nucleus, and the centrioles (Zippin et al., 2003, 2004; Bundey and Insel, 2004). sAC activity has been extensively characterized in vitro, and its activity can be modulated by bicarbonate anions (Chen et al., 2000), calcium (Jaiswal and Conti, 2003; Litvin et al., 2003), or synergistically by calcium and bicarbonate (Litvin et al., 2003). Additionally, because its affinity for substrate, ATP, is in the millimolar range, cellular sAC activity is predicted to be sensitive to cellular levels of ATP (Litvin et al., 2003). Bicarbonate regulation of sAC was shown to be physiologically relevant in sperm (Esposito et al., 2004; Hess et al., 2005), bronchii (Schmid et al., 2007), and epididymis (Pastor-Soler et al., 2003), while calcium stimulation of sAC was found to be essential for cellular responses to nerve growth factor in PC12 cells (Stessin et al., 2006) and tumor necrosis factor in neutrophils (Han et al., 2005).
The two classes of adenylyl cyclases can be distinguished by pharmacological inhibitors. The P site ligand 2′5′-dideoxyadenosine (2′5′ ddAdo) potently inhibits tmACs (IC50 = 3–16 μM) and inhibits sAC only at high concentrations (Johnson et al., 1997; Gille et al., 2004); in contrast, KH7, a molecule identified in our laboratory by a chemical library screen, is a potent inhibitor of sAC (IC50 = 2–5 μM) and inert toward tmACs (Han et al., 2005; Hess et al., 2005; Stessin et al., 2006; Wu et al., 2006; Schmid et al., 2007). Here we use these pharmacological inhibitors, along with genetic knockdown of sAC, to identify the sources of GLP-1–induced and glucose-induced cAMP in INS-1E cells. INS-1E cells are an extensively characterized cell line used for β-cell studies, and in particular, studies of glucose-induced responses, including insulin secretion (Kennedy et al., 1996; Scheenen et al., 1998; Maechler et al., 1999; Hohmeier et al., 2000; Kang and Holz, 2003), and they are a stable subline (Merglen et al., 2004) of the cell line used most extensively to study the cAMP-mediated, glucose-induced activation of the mitogen-activated protein (MAP) kinase ERK (extracellular signal–regulated kinase) (Frodin et al., 1995; Khoo and Cobb, 1997). We now show that sAC-generated cAMP and tmAC-generated cAMP play distinct roles in both glucose-induced cAMP generation and activation of the MAP kinases ERK1/2. While both tmACs and sAC contribute to the total intracellular cAMP level in the presence of high glucose, tmACs are mainly responsible for basal cAMP production while sAC is responsible for the glucose-induced component. We further find that glucose-induced, sAC-generated cAMP is uniquely responsible for the ERK1/2 activation observed subsequent to glucose metabolism.
MATERIALS AND METHODS
Reagents
INS-1E cells were a gift from Claus Wollheim (University Medical Center, Geneva, Switzerland). RPMI media and HEPES were purchased from CellGrow, FBS from Gemini Bio-Products, and β-mercaptoethanol from JT Baker. Opti-MEM was from Invitrogen. Phospho-ERK and total ERK rabbit polyclonal antibodies were obtained from Cell Signaling Technology, and actin rabbit polyclonal antibody was from Santa Cruz Biotechnology, Inc. Horseradish peroxidase–linked anti-mouse and anti-rabbit antibodies and SuperSignal West Pico Chemiluminescent Substrate were from Pierce Chemical Co. GLP-1 (7-36) was obtained from Bachem. Verapamil, 2′5′ dideoxyadenosine, and EGTA-AM were from Calbiochem, Sp-8Br-cAMP was from Biolog, diazoxide was from Biomol, and 3-isobutyl-1-methylxanthine (IBMX) was from Sigma-Aldrich. KH7 was synthesized by ChemDiv, Inc. or by the Milstein Chemical Core Facility of Weill Medical College of Cornell University. The anti-sAC mAbs R21 and R37 were developed in our laboratory; R21 is directed against amino acids 203–216 and R37 against amino acids 436–466 of human sAC. Antibodies were conjugated to biotin for use in Western blotting following immunoprecipitation.
Cell Culture
INS-1E cells (passage 150–175) were cultured as previously described (Asfari et al., 1992). The cells were cultured under 5% CO2 in RPMI media containing 10% heat-inactivated FBS, 10 mM HEPES, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol, and passaged every 2–3 d.
Western Blot
100 μl of Laemmli sample buffer was added directly to the cells in the well of a 24-well plate. 10–15 μl of sample was resolved on an SDS-PAGE, transferred to a PVDF membrane, and probed with specific antibodies (i.e., R21, actin, phospho-ERK, or ERK). Where indicated, quantification of bands was performed using a Fluorochem 8800 image station (Alpha Innotech).
Immunoprecipitation
Cells were lysed in NP-40 buffer (150 mM NaCl, 1.0% NP-40 detergent, 50 mM Tris, pH 8.0) in the presence of 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM PMSF for 30 min, and spun for 15 min at 10,000 g. The supernatant was precleared overnight with 50 μl of 70% protein G slurry (Amersham Pharmacia). Precleared sample was incubated for 4 h or overnight with 50 μg of R37 or 50 μg of mouse IgG, followed by incubation for 1 h with protein G slurry. The immune complexes on beads were washed five times with lysis buffer, and resuspended in Laemmli buffer for Western blot analysis.
Immunocytochemistry
INS-1E cells were fixed for 15 min in 4% paraformaldehyde, washed, permeabilized in 0.1% Triton X-100, washed, blocked in 2% BSA, and then probed with R21 overnight. Sections and coverslips were then washed and probed with FITC-conjugated goat anti-mouse secondary antibody (1:200, Alexafluor, Molecular Probes) and DAPI. Controls included no primary antibody and R21 primary antibody preincubated with blocking peptide (amino acids 203–216 of human sACfl). Images were recorded on a Nikon microscope.
cAMP Accumulation
On day one, 2.5 × 105 INS-1E cells were plated in each well of a 24-well plate. On day three, cells were incubated in 2.5 mM glucose Krebs-Ringer buffer (pH 7.5) supplemented with 2 mM sodium bicarbonate, 10 mM HEPES, and 0.1% BSA for 1–2 h before start of the experiment. At time zero for each experiment, media was switched to Krebs-Ringer buffer containing 2.5 mM glucose, 16 mM glucose, or 2.5 mM glucose plus 60 mM KCl Krebs-Ringer buffer containing 500 μM IBMX in the presence of the different inhibitors (i.e., DMSO vehicle, 30 μM KH7, or 50 μM 2′5′ dideoxyadenosine, 100 μM EGTA-AM, 300 μM diazoxide, or 60 μM verapamil) or additional reagents (i.e., 30 nM GLP-1, 0.5 mM Sp-8Br-cAMP) as indicated. In experiments using EGTA-AM, diazoxide and verapamil, cells were pretreated with inhibitor for 15 min before media change. After media change, cells were incubated for the indicated time at 37°C, media was aspirated, and cells lysed in 200 μl 0.1 M HCl. Intracellular cAMP content was determined using Correlate-EIA Direct Assay (Assay Designs, Inc).
RNAi Silencing
RNAi oligonucleotides were purchased from QIAGEN and reconstituted as per manufacturer's instructions. sAC RNAi was targeted to the following sequence in the rat sAC mRNA: TCGGAGCATGATTGAAATCGA. Controls included untransfected cells, cells transfected with no RNA (mock transfected), and cells transfected with QIAGEN AllStars Negative Control siRNA (negative control). For transfection, INS-1E cells were split into 24-well plates or 10-mm dishes and transfection was performed on the same day. After 72–80 h, cells were assayed for cAMP and sAC content as above.
RNA Production and RT-PCR Amplification of sAC Products
Total RNA was harvested from rat pancreas or INS-1E cells using Trizol according to manufacturer's protocol. Total RNA was quantified spectrophotometrically, and at least 2 mg of total RNA was used to generate polyA+ RNA using the Micro Poly(A) Purist Kit according to manufacturer's protocol (Ambion). Purified polyA+ RNA was resuspended in DEPC-treated water and treated with amplification grade DNase I according to the manufacturer's protocol (Invitrogen). Approximately 500 ng of polyA+ RNA was used to generate first strand cDNA using Invitrogen's Platinum Taq PCR kit according to manufacturer's instructions. Polymerase chain reactions used a three-step standard protocol with an initial denaturation step at 93°C for 3 min, followed by 40 cycles of 93°C for 20 s, 60°C for 20 s, and 68°C for 1–3 min, followed by a final step at 68°C for 10 min.
Online Supplemental Material
For data presented in Fig. 3, ANOVA statistical analyses were performed with the Bonferroni comparison test and are provided in Table S1 (available at http://www.jgp.org/cgi/content/full/jgp.200810044/DC1).
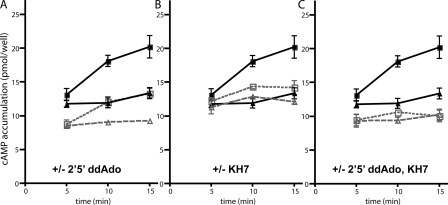
Figure 3.
Individual contribution of tmACs and sAC to glucose-induced cAMP. Total cellular cAMP was measured in INS-1E cells at the indicated times in Krebs Ringer buffer with 2.5 mM glucose (triangle symbols) or 16 mM glucose (square symbols) in the presence of IBMX, with either vehicle control (A, B, and C, closed symbols and solid lines: ▴ and ▪), or in the presence of the inhibitors (open symbols with dotted lines: ▵ and □); 50 μM 2′5′ ddAdo (A), 30 μM KH7 (B), or both ddAdo and KH7 (C). Values represent means ± SEM (n = 4) of total cAMP content per well, with each well containing equivalent number of cells. ANOVA statistical analyses were performed with the Bonferroni comparison test and provided in Table S1 (available at http://www.jgp.org/cgi/content/full/jgp.200810044/DC1).
RESULTS
Temporal Difference in cAMP Production
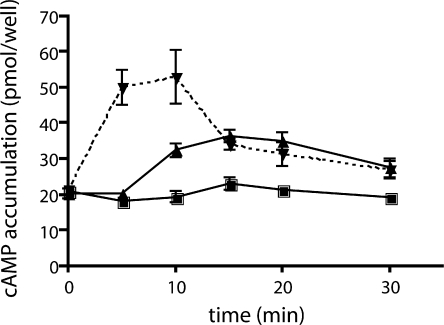
Addition of GLP-1 to INS-1E cells elicited the expected rise in cellular cAMP levels (Fig. 1). Consistent with previous observations (Landa et al., 2005), GLP-1 stimulation of cAMP production was rapid; the maximum 2.5-fold increase above basal levels was observed at the first time point measured (5 min). We included the broad specificity PDE inhibitor IBMX to facilitate cAMP measurements and to focus on cAMP synthesis by adenylyl cyclases. Despite the presence of IBMX, cellular levels of cAMP were greatly reduced by 15 min. There are several possible reasons for this decline in cAMP level, including the potential presence of IBMX-insensitive PDEs, IBMX inactivation, or cAMP extrusion or leak out of the cell.
Figure 1.
Effects of GLP-1 and glucose on cAMP levels in INS-1E cells. Total cellular cAMP was measured in INS-1E cells at the indicated times after incubation in Krebs Ringer buffer with 2.5 mM (▪) glucose, 16 mM (▴) glucose, or 2.5 mM glucose with 30 nM GLP-1 (▾) in the presence of 0.5 mM IBMX. Values represent means ± SEM (n = 4) of total cAMP content per well, with each well containing equivalent number of cells (2.5 × 105 cells were plated into each well two days before the assay).
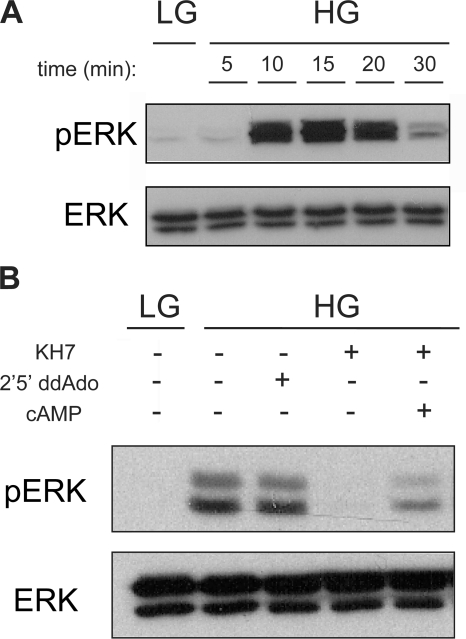
Incubation in high glucose (16 mM) induced a 1.7-fold increase above basal cAMP levels. However, in contrast to the rapid and transient cAMP rise following GLP-1, glucose-induced cAMP required more than 5 min to accumulate to detectable levels. This delayed elevation peaked at 15 min and was sustained for the duration of the experiment (30 min). Thus, our whole cell measurements replicate the distinct time courses for hormonally stimulated versus glucose-induced cAMP generation previously seen in studies using intracellular cAMP sensors (Landa et al., 2005). Because our cAMP measurements were obtained in the presence of the broad specificity PDE inhibitor IBMX, the distinct kinetics induced by hormonal stimulation and nutrient sensing likely reflect differential activation of the cell's adenylyl cyclases.
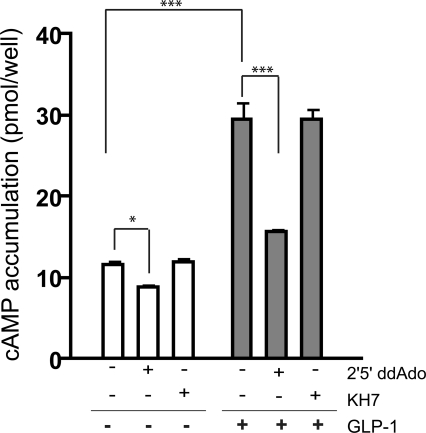
GLP-1 stimulates a G protein–coupled receptor (Thorens, 1992; Usdin et al., 1993), and as expected, the increase in cAMP due to GLP-1, measured at 5 min, was blocked by inhibition of G protein–responsive tmACs using 2′5′ dideoxyadenosine (ddAdo) (Fig. 2). Inclusion of KH7, which inhibits sAC, had no effect on GLP-1–stimulated cAMP levels, confirming both the specificity of KH7 and the lack of sAC contribution to GLP-1–induced cAMP levels. Thus, GLP-1 increases cAMP levels in INS-1E cells exclusively via G protein–coupled receptor (GPCR) stimulation of tmAC activity. Notably, P site inhibitors, but not KH7, reproducibly diminished the “basal” cAMP accumulation seen in Krebs-Ringer Buffer with low glucose, implying that the majority of cAMP “turnover” (i.e., the consistent production of cAMP that is balanced by intrinsic PDE activity) in Krebs-Ringer buffer with 2.5 mM glucose is due to tmACs.
Figure 2.
GLP-1-induced cAMP production is mediated via tmACs. Cyclic AMP was measured in INS-1E cells after incubation for 5 min in 2.5 mM glucose in the presence of 0.5 mM IBMX, with either vehicle control, 30 μM KH7, 50 μM 2′5′ ddAdo, 30 nM GLP-1, GLP-1 and KH7, or GLP-1 and 2′5′ ddAdo. 2 d before the assay, 2.5 × 105 cells were plated into each well. Values represent means ± SEM (n = 8). ANOVA statistical analyses were performed with the Newman-Keuls post-hoc test. ***, indicates P < 0.001; *, P < 0.05.
We next explored whether tmACs contribute to the cAMP induced by high glucose. Because the glucose-induced elevation of cAMP required more than 5 min to reach detectable levels (Landa et al., 2005; Fig. 1) and plateaued after 15 min (Fig. 1), we measured total cAMP accumulation in INS-1E cells after 5, 10, and 15 min. Glucose still induced cAMP production in the presence of the tmAC-selective P-site inhibitor 2′5′ddAdo (Fig. 3 A). While inclusion of 2′5′ ddAdo did diminish the total cellular content of cAMP in high glucose, this effect was primarily due to lowered basal cAMP accumulation in Krebs-Ringer buffer (Fig. 2 and Fig. 3 A). In fact, INS-1E cells retained almost normal cAMP responsiveness to high glucose in the absence of any contribution by tmACs: high glucose stimulated cAMP production 51% in the absence of P-site inhibitors and 43% in their presence.
Soluble Adenylyl Cyclase Is the Predominant Source of Glucose-induced cAMP in INS-1E Cells
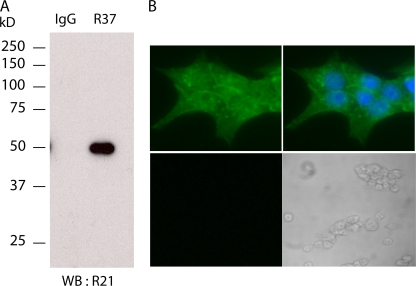
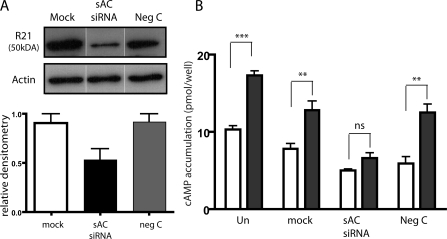
Because glucose-induced cAMP accumulation appears to be mostly independent of tmAC activity, we explored the possibility that soluble adenylyl cyclase plays a role. We demonstrated the presence of sAC mRNA in pancreas (Chen, Y., personal communication; unpublished data) and INS-1E cells by reverse transcription polymerase chain reaction (unpublished data). Interestingly the sAC mRNA in INS-1E cells seems to arise from a somatic cell-specific promoter (Farrell, 2008). We confirmed the presence of sAC protein in INS-1E cells using two distinct monoclonal antibodies specific for sAC (Fig. 4 A). Immunoprecipitation of sAC from INS-1E cells with a monoclonal antibody (R37) followed by Western blotting using a second mAb (R21) detecting a nonoverlapping epitope identified a 50-kD protein. This 50-kD protein, which can also be detected by Western blotting whole cell extracts using R21, is diminished by 40% after transfection with sAC-specific siRNA oligonucleotides (Fig. 5 A). Thus, INS-1E cells express a 50-kD isoform of sAC, which most likely corresponds to the alternatively spliced somatic sAC isoform recently characterized in brain and kidney (Farrell, 2008). Immunocytochemistry using R21 revealed sAC protein is distributed throughout the cell (Fig. 4 B).
Figure 4.
sAC is present in pancreatic β cells. (A) Western blot (WB) using biotinylated anti-sAC mAb R21 of an immunoprecipitation using anti-sAC mAb R37 or nonspecific mouse IgG from INS-1E cells. Specificity of the Western blot was confirmed using streptavidin alone (not depicted). (B) Immunocytochemistry of INS-1E cells using anti-sAC mAb R21 (green) alone (left top) or in combination with the nuclear stain, DAPI (blue) (right top). All specific staining was lost when the sAC mAb was preincubated with excess peptide antigen (bottom; left panel is fluorescence; right panel is bright field). Immunostains with secondary antibodies alone were also negative (not depicted).
Figure 5.
RNAi confirms the role of sAC in glucose-induced cAMP production. (A) Western blot, along with quantitation, using sAC-specific mAb R21 (top) or anti–β-actin mAb (middle) of INS-1E cells transfected with no RNA (mock; white bar), sAC-specific RNAi oligonucleotide (sAC-siRNA; black bar), or nonspecific oligonucleotide (Neg C; gray bar). sAC bands from two independent transfections were quantified by densitometry and normalized to β-actin (bottom). (B) Total cellular cAMP in untransfected INS-1E cells (Un) or INS-1E cells transfected with no RNA (mock), sAC-specific RNAi (sAC siRNA), or nonspecific oligonucleotide (neg C) after incubation for 15 min in Krebs Ringer buffer with low (2.5 mM; white bars) or high (16 mM; black bars) glucose. Values represent means ± SEM (n = 6) of total cAMP content per well, where each well contained equivalent number of cells upon RNAi transfection. High glucose stimulated cAMP production 1.78-fold in controls (untransfected, mock-transfected, and negative control–transfected cells) and 1.34-fold in sAC-siRNA-transfected INS-1E cells. ANOVA statistical analysis was performed with the Newman-Keuls post-hoc test. ns, not significant; **, P < 0.01; ***, P < 0.001.
Using pharmacological inhibition and genetic knockdown, we found that sAC was primarily responsible for the glucose-induced cAMP generation. Incubation with KH7 blocked the cAMP rise between 5 and 15 min (Fig. 3 B; Table S1, available at http://www.jgp.org/cgi/content/full/jgp.200810044/DC1, LG KH7 vs. HG KH7). As expected, cells incubated in the presence of both sAC-specific KH7 and tmAC-specific P-site inhibitors accumulated minimal cAMP, which did not change over time (Fig. 3 C). The different effects of KH7 and P-site inhibitor (Fig. 3, A and B), plus the fact that they are additive (Fig. 3 C), confirms their selectivity.
Glucose-induced cAMP production was also inhibited by transfection with sAC-specific siRNA. sAC-specific siRNA reduced sAC protein levels by ∼40% (Fig. 5 A), which resulted in a 56% reduction in cAMP accumulation due to glucose (Fig. 5 B). sAC-siRNA treatment may also be blunting basal cAMP accumulation (i.e., in low glucose). However, if true, this could be ascribed to either a specific function of sAC protein, off-target effects of the sAC-siRNA, or other nonspecific effects caused by the transfection procedure.
Glucose Activation of ERK1/2 (p44/42 MAP Kinases) Is Dependent on sAC-generated cAMP
Among the consequences of glucose-elicited cAMP generation is phosphorylation of the MAP kinases, ERK1/2 (Frodin et al., 1995; Khoo and Cobb, 1997; Benes et al., 1999; Arnette et al., 2003; Briaud et al., 2003). Western blotting with phospho-ERK–specific antibody revealed that the kinetics of glucose-induced ERK1/2 activation, in the absence of IBMX, matched the kinetics of glucose-induced cAMP accumulation. Both ERK activation (Fig. 6 A) and cAMP accumulation (Fig. 1) peaked after 15 min in high glucose. Because both sAC and tmACs contribute to the total cellular cAMP content of β cells in high glucose, we asked which source of cAMP, sAC or tmACs, is responsible for glucose-induced MAP kinase activation. Consistent with the fact that ERK activation is a direct consequence of glucose stimulation, glucose-induced ERK1/2 phosphorylation was exclusively dependent upon sAC-generated cAMP. Inhibiting glucose-dependent cAMP generation with the sAC inhibitor, KH7, prevented phosphorylation of ERK1/2 (Fig. 6 B), and KH7 inhibition could be rescued by adding membrane-permeable cAMP (Sp-8Br-cAMP). Blocking basal cAMP generation via tmACs with P-site ligands had no effect.
Figure 6.
Glucose leads to ERK (p42/44) activation in a sAC-dependent manner. Western blots using anti-phosphoERK (pERK) or total ERK (ERK) of INS-1 cells incubated in Krebs Ringer buffer with 2.5 mM glucose (LG) or 16 mM glucose (HG) (in the absence of IBMX) (A) for 5, 10, 15, 20, and 30 min or (B) for 15 min in the presence or absence of 30 μM KH7 or 50 μM 2′5′ ddAdo or KH7 and 0.5 mM Sp-8Br-cAMP. Western blot shows phosphorylation and total protein for both ERK1 and ERK2, p44 and p42. Shown are representative experiments repeated multiple times (for A, n = 4; for B, n = 7).
Calcium Mediates the Glucose-induced cAMP Production via sAC
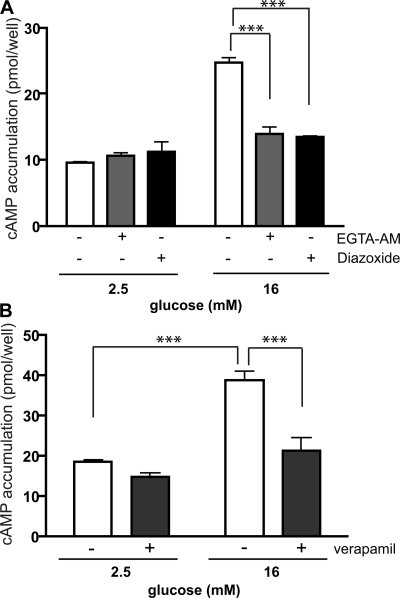
A rise in intracellular calcium was previously shown to be necessary for glucose stimulation of cAMP (Charles et al., 1975; Landa et al., 2005), and calcium influx due to glucose-dependent membrane depolarization is concomitant with the cAMP rise (Landa et al., 2005). We confirmed that the glucose-dependent calcium influx stimulates sAC activity by (A) chelating the rise of intracellular calcium using EGTA-AM (Fig. 7 A); (B) preventing ATP-dependent closure of KATP channels using diazoxide (Fig. 7 A); and (C) blocking calcium influx through L-type voltage-dependent calcium channels using verapamil (Fig. 7 B). All three inhibitors prevented glucose-induced cAMP generation in INS-1E cells in the presence of 16 mM glucose. Consistently, these methods for blocking calcium influx were previously shown to impair glucose-induced ERK activation (Benes et al., 1998; Arnette et al., 2003; Briaud et al., 2003).
Figure 7.
Glucose-induced calcium entry is necessary for sAC activation. Cyclic AMP was measured in INS-1E cells after incubation for 15 min in 2.5 or 16 mM glucose in the presence of 0.5 mM IBMX, with either vehicle control (white bars) or (A) 100 μM EGTA-AM (gray bars), 300 μM diazoxide (black bars) or (B) 60 μM verapamil (black bars). Values represent means ± SEM (n ≥ 4). ANOVA statistical analyses were performed with the Newman-Keuls post-hoc test. ***, P < 0.001.
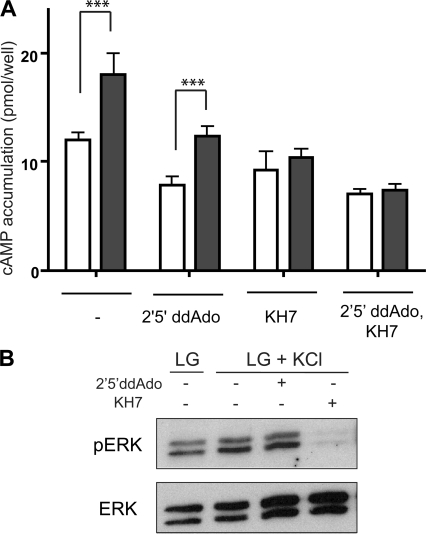
We next tested whether depolarization-induced calcium influx was sufficient to stimulate cAMP production via sAC. Incubation of INS-1E cells in depolarizing concentration of KCl, in low glucose, increased cAMP production, which was blocked by KH7 but not by P-site inhibitor (Fig. 8 A). Furthermore, this depolarization-induced, sAC-dependent cAMP rise was responsible for activation of ERK1/2 (Fig. 8 B); thus, depolarization-induced calcium influx effectively mimics the situation in high glucose.
Figure 8.
Depolarization-induced calcium entry is sufficient to induce functional sAC-generated cAMP. (A) Cyclic AMP was measured in INS-1E cells after incubation for 15 min in 2.5 mM glucose (white bars) or 2.5 mM glucose plus 60 mM KCl (gray bars) in the presence of 0.5 mM IBMX, with either vehicle control, 50 μM 2′5′ ddAdo, and/or 30 μM KH7. Values represent means ± SEM (n ≥ 4). ANOVA statistical analyses were performed with the Bonferroni comparison test. ***, P < 0.001. (B) Western blots using anti-phosphoERK (pERK) or total ERK (ERK) of INS-1E cells incubated in Krebs-Ringer buffer with 2.5 mM glucose (LG) or 2.5 mM glucose with 60 mM KCl (LG+KCl), in the absence of IBMX, for 15 min in the presence or absence of 50 μM 2′5′ ddAdo or 30 μM KH7. Shown are representative experiments repeated four times.
DISCUSSION
Signal transduction specificity is dependent upon spatial restriction of the signaling machinery. For second messengers, such as cAMP, this is achieved by compartmentalization of the second messenger and its effectors (Buxton and Brunton, 1983; Zaccolo et al., 2002; Zaccolo and Pozzan, 2002; Zippin et al., 2003; Baillie et al., 2005; Willoughby and Cooper, 2007). Compartmentalization of a cell into multiple cAMP signaling microdomains permits this second messenger to mediate various, independent cellular processes. Here, we identify two temporally and mechanistically separate cAMP signaling cascades in INS-1E cells, a model for pancreatic β cells. GLP-1 induces a rapid elevation of cAMP via stimulation of membrane-anchored tmACs, while glucose induces a delayed elevation of cAMP via stimulation of sAC. Because sAC isoforms are not tethered to the plasma membrane, like tmACs, but are distributed throughout the cytoplasm and in organelles (Fig. 4 B; Zippin et al., 2003, 2004), we presume GLP-1– and glucose-regulated cAMP microdomains are spatially distinct as well. However, determination of their subcellular localization requires further study using targeted, intracellular cAMP sensors.
A rapid and transient response induced by the incretin, GLP-1, is consistent with it being mediated via GPCR stimulation of a tmAC (Thorens, 1992; Doyle and Egan, 2007). Each step of a GPCR signaling cascade, from receptor desensitization to intrinsic timing of the G protein activation cycle, ensures its transient nature. Furthermore, the functions of an incretin may be well served by a pulse of cAMP. Incretins represent tonic signals that need to be interpreted once by β cells, for example, by a spike of cAMP, to prepare them for coming nutrients.
The INS-1E cell response to glucose was different; cAMP production was delayed when the cells were moved into high glucose. In contrast to the incretin-induced pulse of cAMP, which primes the β-cell for coming nutrients, the slow increase in second messenger would be an appropriate response to a chronic signal, like elevated glucose. Our cAMP accumulation experiments were performed in the presence of IBMX, to enable in vitro measurements of accumulated cAMP; therefore, it is unclear whether the sustained cAMP elevation observed here would be reproduced in vivo. Such a study awaits identification of a correctly targeted intracellular cAMP sensor. We speculate that the sAC-dependent elevation of cAMP due to glucose will be maintained for as long as glucose remains elevated; in this way, the glucose-dependent regulation would resemble a thermostat.
A delayed response is consistent with sAC biochemistry. sAC is directly modulated by ATP, bicarbonate, and calcium, all three of which are elevated downstream of glucose metabolism in β cells. We have shown calcium influx due to glucose is both necessary and sufficient for sAC activation, but calcium stimulation may not be the sole modulator of sAC. Because its Km for substrate ATP of 1 mM matches predicted cytosolic levels, sAC would also serve as a cellular ATP sensor. In addition, cellular bicarbonate levels fluctuate in direct proportion to the rate of CO2 synthesis. Thus, all three sAC modulators would increase due to glucose metabolism, and sAC activity would only be stimulated after glucose is metabolized. Furthermore, as long as glucose is being metabolized, all three sAC modulators would remain elevated, resulting in sustained stimulation of sAC activity.
Two recent studies explored cAMP generation in β-cell lines using intracellular, FRET-based sensors. Dyachok et al. (2006) focused on the interrelationship between calcium influx and cAMP generation in INS-1E cells. Their data demonstrating cAMP induction by GLP-1 agrees with our studies; i.e., physiological levels of the incretin induce rapid and transient cAMP induction. However, this study did not detect a glucose-induced cAMP rise. Our data suggests this is likely due to the fact that cAMP was measured for short times (<5 min) after glucose addition. The kinetics due to GLP-1 and glucose observed in the second study, by Landa et al. (2005), agree with our data using whole cell cAMP measurements. However, they observed nearly complete inhibition of the glucose-induced cAMP elevation by 50 μM P-site inhibitors. There are a number of differences in the experimental design of our study compared with theirs. Landa et al. use a cytosolic FRET-based probe in mouse Min6 cells, while we are measuring whole cell cAMP in rat INS-1E cells. We found sAC to be distributed throughout INS-1E cells (Fig. 4 B), and its localization is consistent with other proliferating cells, where sAC is found inside the nucleus, at mitochondria, in the centrosome, and throughout the cytoplasm (Zippin et al., 2003). We do not know which subset of sAC is responsible for glucose-induced cAMP. Suppose, for example, that the major elevation of cAMP is due to intramitochondrial-localized sAC, then the major changes we are studying here would not be identified by a cytosolic cAMP sensor. For these reasons, our measurements of whole cell cAMP accumulation are complementary to the previously described cytosolic FRET-based sensor.
In INS-1E cells, glucose metabolism leads to activation of the MAP kinases ERK1 and ERK2 (Frodin et al., 1995; Khoo and Cobb, 1997; Benes et al., 1999; Arnette et al., 2003; Briaud et al., 2003). The glucose-induced ERK1/2 phosphorylation was shown to be mediated via the cAMP-induced activation of PKA. We now show that the glucose-induced cAMP generation by sAC is responsible for ERK activation, which plays a role in insulin gene expression (Khoo et al., 2003). Thus, glucose-induced cAMP generation by sAC is functionally relevant in β cells. It remains to be seen whether glucose-induced, sAC-dependent cAMP generation also plays a role in insulin release.
The distinct kinetics of GLP-1–induced and glucose-induced cAMP generation may underlie the reason for evolution of two types of cAMP signaling cascades in mammalian cells: tmACs, which respond with a spike to a tonic signal like hormones and neurotransmitters, versus sAC, which responds with a sustained signal to prolonged cues, such as glucose sensing by β cells, bicarbonate sensing by sperm (Esposito et al., 2004; Hess et al., 2005), CO2 sensing in bronchii (Schmid et al., 2007), pH sensing in epididymis (Pastor-Soler et al., 2003), migration toward guidance cues like Netrin-1 in developing neurons (Wu et al., 2006), and responses to growth factors like TNF (Han et al., 2005) and NGF (Stessin et al., 2006) in neutrophils and neurons, respectively.
Supplementary Material
Acknowledgments
We would like to thank the members of the Levin/Buck Laboratory, especially Eric Salazar and Dr. Martin Tresguerres for critical comments on the manuscript, Dr. Martin Tresguerres for microscopy assistance, and P. Antinozzi and C. Wollheim for the kind gift of INS-1E cells.
This work has been funded by the Ellison Medical Foundation (J. Buck), American Diabetes Association (L.R. Levin), Hirschl-Weil Cauler Trust (L.R. Levin), National Institutes of Health (GM62328 to J. Buck; DA007274 to M. Kamenetsky), Tri-Institutional MSTP (J.H. Zippin), and Barbara and Stephen Friedman Fellowship (J.H. Zippin).
Olaf S. Andersen served as editor.
Note added in proof. Our assumption that Dyachok et al. (2006) did not observe a glucose-induced elevation of cAMP because they only measured short times after glucose addition proved to be true. More recent work from Dyachok et al. (Dyachok, O., O. Idevall-Hagren, J. Sågetorp, G. Tian, A. Wuttke, C. Arrieumerlou, G. Akusjärvi, E. Gylfe, and A. Tengholm. 2008. Cell Metab. 8:26–37) describes a delayed, glucose-induced rise in cAMP in Min6 cells.
L.S. Ramos and J.H. Zippin contributed equally to this paper.
J.H. Zippin's present address is Department of Dermatology, Weill Medical College of Cornell University, 1300 York Avenue, New York, NY 10065.
Abbreviations used in this paper: 2′5′ ddAdo, 2′5′ dideoxyadenosine; ERK1/2, extracellular signal-regulated kinases 1 and 2; GPCR, G protein–coupled receptor; IBMX, 3-isobutyl-1-methylxanthine; MAP, mitogen-activated protein; PDE, phosphodiesterase; sAC, soluble adenylyl cyclase; tmAC, transmembrane adenylyl cyclase.
References
- Arnette, D., T.B. Gibson, M.C. Lawrence, B. January, S. Khoo, K. McGlynn, C.A. Vanderbilt, and M.H. Cobb. 2003. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic β cells. J. Biol. Chem. 278:32517–32525. [DOI] [PubMed] [Google Scholar]
- Asfari, M., D. Janjic, P. Meda, G. Li, P.A. Halban, and C.B. Wollheim. 1992. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 130:167–178. [DOI] [PubMed] [Google Scholar]
- Ashcroft, F.M., and P. Rorsman. 1989. Electrophysiology of the pancreatic β-cell. Prog. Biophys. Mol. Biol. 54:87–143. [DOI] [PubMed] [Google Scholar]
- Baillie, G.S., J.D. Scott, and M.D. Houslay. 2005. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 579:3264–3270. [DOI] [PubMed] [Google Scholar]
- Benes, C., M.P. Roisin, H.V. Tan, C. Creuzet, J. Miyazaki, and R. Fagard. 1998. Rapid activation and nuclear translocation of mitogen-activated protein kinases in response to physiological concentration of glucose in the MIN6 pancreatic β cell line. J. Biol. Chem. 273:15507–15513. [DOI] [PubMed] [Google Scholar]
- Benes, C., V. Poitout, J.C. Marie, J. Martin-Perez, M.P. Roisin, and R. Fagard. 1999. Mode of regulation of the extracellular signal-regulated kinases in the pancreatic β-cell line MIN6 and their implication in the regulation of insulin gene transcription. Biochem. J. 340:219–225. [PMC free article] [PubMed] [Google Scholar]
- Briaud, I., M.K. Lingohr, L.M. Dickson, C.E. Wrede, and C.J. Rhodes. 2003. Differential activation mechanisms of Erk-1/2 and p70(S6K) by glucose in pancreatic β-cells. Diabetes. 52:974–983. [DOI] [PubMed] [Google Scholar]
- Buck, J., M.L. Sinclair, L. Schapal, M.J. Cann, and L.R. Levin. 1999. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA. 96:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundey, R.A., and P.A. Insel. 2004. Discrete intracellular signaling domains of soluble adenylyl cyclase: camps of cAMP? Sci. STKE. pe19. [DOI] [PubMed]
- Buxton, I.L., and L.L. Brunton. 1983. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J. Biol. Chem. 258:10233–10239. [PubMed] [Google Scholar]
- Charles, M.A., R. Fanska, F.G. Schmid, P.H. Forsham, and G.M. Grodsky. 1973. Adenosine 3′,5′-monophosphate in pancreatic islets: glucose-induced insulin release. Science. 179:569–571. [DOI] [PubMed] [Google Scholar]
- Charles, M.A., J. Lawecki, R. Pictet, and G.M. Grodsky. 1975. Insulin secretion. Interrelationships of glucose, cyclic adenosine 3:5-monophosphate, and calcium. J. Biol. Chem. 250:6134–6140. [PubMed] [Google Scholar]
- Chen, Y., M.J. Cann, T.N. Litvin, V. Iourgenko, M.L. Sinclair, L.R. Levin, and J. Buck. 2000. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 289:625–628. [DOI] [PubMed] [Google Scholar]
- Delmeire, D., D. Flamez, S.A. Hinke, J.J. Cali, D. Pipeleers, and F. Schuit. 2003. Type VIII adenylyl cyclase in rat β cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia. 46:1383–1393. [DOI] [PubMed] [Google Scholar]
- Doyle, M.E., and J.M. Egan. 2007. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 113:546–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachok, O., Y. Isakov, J. Sagetorp, and A. Tengholm. 2006. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells. Nature. 439:349–352. [DOI] [PubMed] [Google Scholar]
- Esposito, G., B.S. Jaiswal, F. Xie, M.A. Krajnc-Franken, T.J. Robben, A.M. Strik, C. Kuil, R.L. Philipsen, M. Van Duin, M. Conti, and J.A. Gossen. 2004. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. USA. 101:2993–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell, J. 2008. The molecular identity of soluble adenylyl cyclase. Weill Medical College of Cornell University, New York.
- Frodin, M., N. Sekine, E. Roche, C. Filloux, M. Prentki, C.B. Wollheim, and E. Van Obberghen. 1995. Glucose, other secretagogues, and nerve growth factor stimulate mitogen-activated protein kinase in the insulin-secreting β-cell line, INS-1. J. Biol. Chem. 270:7882–7889. [DOI] [PubMed] [Google Scholar]
- Gille, A., G.H. Lushington, T.C. Mou, M.B. Doughty, R.A. Johnson, and R. Seifert. 2004. Differential inhibition of adenylyl cyclase isoforms and soluble guanylyl cyclase by purine and pyrimidine nucleotides. J. Biol. Chem. 279:19955–19969. [DOI] [PubMed] [Google Scholar]
- Grill, V., and E. Cerasi. 1973. Activation by glucose of adenylyl cyclase in pancreatic islets of the rat. FEBS Lett. 33:311–314. [DOI] [PubMed] [Google Scholar]
- Han, H., A. Stessin, J. Roberts, K. Hess, N. Gautam, M. Kamenetsky, O. Lou, E. Hyde, N. Nathan, W.A. Muller, J. Buck, L.R. Levin, and C. Nathan. 2005. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J. Exp. Med. 202:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, K.C., B.H. Jones, B. Marquez, Y. Chen, T.S. Ord, M. Kamenetsky, C. Miyamoto, J.H. Zippin, G.S. Kopf, S.S. Suarez, et al. 2005. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell. 9:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmeier, H.E., H. Mulder, G. Chen, R. Henkel-Reiger, M. Prentki, and C.B. Newgard. 2000. Isolation of INS-1 derived-cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 49:424–430. [DOI] [PubMed] [Google Scholar]
- Holz, G.G., C.A. Leech, and J.F. Haberner. 1995. Activation of a cAMP-regulated Ca2+-signaling pathway in pancreatic B-cells by the insulinotropic hormone glucagon-like peptide-1. J. Biol. Chem. 270:17749–17757. [PMC free article] [PubMed] [Google Scholar]
- Jaiswal, B.S., and M. Conti. 2003. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl. Acad. Sci. USA. 100:10676–10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R.A., L. Desaubry, G. Bianchi, I. Shoshani, E. Lyons Jr., R. Taussig, P.A. Watson, J.J. Cali, J. Krupinski, J.P. Pieroni, and R. Iyengar. 1997. Isozyme-dependent sensitivity of adenylyl cyclases to P-site-mediated inhibition by adenine nucleosides and nucleoside 3′-polyphosphates. J. Biol. Chem. 272:8962–8966. [DOI] [PubMed] [Google Scholar]
- Kamenetsky, M., S. Middelhaufe, E.M. Bank, L.R. Levin, J. Buck, and C. Steegborn. 2006. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J. Mol. Biol. 362:623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, G., and G.G. Holz. 2003. Amplification of exocytosis by Ca2+-induced Ca2+ release in INS-1 pancreatic β cells. J. Physiol. 546:175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, E.D., R. Rizzuto, J.M. Theler, W.F. Pralong, C. Bastianutto, T. Pozzan, and C.B. Wollheim. 1996. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J. Clin. Invest. 98:2524–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo, S., and M.H. Cobb. 1997. Activation of mitogen-activating protein kinase by glucose is not required for insulin secretion. Proc. Natl. Acad. Sci. USA. 94:5599–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo, S., S.C. Griffen, Y. Xia, R.J. Baer, M.S. German, and M.H. Cobb. 2003. Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic β cells. J. Biol. Chem. 278:32969–32977. [DOI] [PubMed] [Google Scholar]
- Landa, L.R. Jr., M. Harbeck, K. Kaihara, O. Chepurny, K. Kitiphongspattana, O. Graf, V.O. Nikolaev, M.J. Lohse, G.G. Holz, and M.W. Roe. 2005. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 β-cell line. J. Biol. Chem. 280:31294–31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin, T.N., M. Kamenetsky, A. Zarifyan, J. Buck, and L.R. Levin. 2003. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 278:15922–15926. [DOI] [PubMed] [Google Scholar]
- Maechler, P., E.D. Kennedy, E. Sebo, A. Valeva, T. Pozzan, and C.B. Wollheim. 1999. Secretagogues modulate the calcium concentration in the endoplasmic reticulum of insulin-secreting cells. Studies in aequorin-expressing intact and permeabilized INS-1 cells. J. Biol. Chem. 274:12583–12592. [DOI] [PubMed] [Google Scholar]
- Merglen, A., S. Theander, B. Rubi, G. Chaffard, C.B. Wollheim, and P. Maechler. 2004. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 145:667–678. [DOI] [PubMed] [Google Scholar]
- Moens, K., H. Heimberg, D. Flamez, P. Huypens, E. Quartier, Z. Ling, D. Pipeleers, S. Gremlich, B. Thorens, and F. Schuit. 1996. Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes. 45:257–261. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh, C., Y. Wang, A.M. Janczewski, T.E. Henderson, and J.M. Egan. 1997. Overexpression of glucagon-like peptide-1 receptor in an insulin-secreting cell line enhances glucose responsiveness. Mol. Cell. Endocrinol. 130:109–117. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler, N., V. Beaulieu, T.N. Litvin, N. Da Silva, Y. Chen, D. Brown, J. Buck, L.R. Levin, and S. Breton. 2003. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 278:49523–49529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter, G.A. 2001. Nutrient-secretion coupling in the pancreatic islet β-cell: recent advances. Mol. Aspects Med. 22:247–284. [DOI] [PubMed] [Google Scholar]
- Rutter, G.A., J.M. Theler, M. Murgia, C.B. Wollheim, T. Pozzan, and R. Rizzuto. 1993. Stimulated Ca2+ influx raises mitochondrial free Ca2+ to supramicromolar levels in a pancreatic β-cell line. Possible role in glucose and agonist-induced insulin secretion. J. Biol. Chem. 268:22385–22390. [PubMed] [Google Scholar]
- Scheenen, W.J., C.B. Wollheim, T. Pozzan, and C. Fasolato. 1998. Ca2+ depletion from granules ihibits exocytosis. A study with insulin-secreting cells. J. Biol. Chem. 273:19002–19008. [DOI] [PubMed] [Google Scholar]
- Schmid, A., Z. Sutto, M.C. Nlend, G. Horvath, N. Schmid, J. Buck, L.R. Levin, G.E. Conner, N. Fregien, and M. Salathe. 2007. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J. Gen. Physiol. 130:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessin, A.M., J.H. Zippin, M. Kamenetsky, K.C. Hess, J. Buck, and L.R. Levin. 2006. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J. Biol. Chem. 281:17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens, B. 1992. Expression cloning of the pancreatic B cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. USA. 89:8641–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin, T., E. Mezey, D.C. Button, M.J. Brownstein, and D.C. Bonner. 1993. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral oragans and the brain. Endocrinology. 133:2861–2870. [DOI] [PubMed] [Google Scholar]
- Willoughby, D., and D.M. Cooper. 2007. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 87:965–1010. [DOI] [PubMed] [Google Scholar]
- Wu, K.Y., J.H. Zippin, D.R. Huron, M. Kamenetsky, U. Hengst, J. Buck, L.R. Levin, and S.R. Jaffrey. 2006. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat. Neurosci. 9:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B., B. He, S.M. Abdel-Halim, A. Tibell, M.D. Brendel, R.G. Bretzel, S. Efendic, and J. Hillert. 1999. Molecular cloning of a full-length cDNA for human type 3 adenylyl cyclase and its expression in human islets. Biochem. Biophys. Res. Commun. 254:548–551. [DOI] [PubMed] [Google Scholar]
- Zaccolo, M., and T. Pozzan. 2002. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 295:1711–1715. [DOI] [PubMed] [Google Scholar]
- Zaccolo, M., P. Magalhaes, and T. Pozzan. 2002. Compartmentalisation of cAMP and Ca2+ signals. Curr. Opin. Cell Biol. 14:160–166. [DOI] [PubMed] [Google Scholar]
- Zippin, J.H., Y. Chen, P. Nahirney, M. Kamenetsky, M.S. Wuttke, D.A. Fischman, L.R. Levin, and J. Buck. 2003. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 17:82–84. [DOI] [PubMed] [Google Scholar]
- Zippin, J.H., J. Farrell, D. Huron, M. Kamenetsky, K.C. Hess, D.A. Fischman, L.R. Levin, and J. Buck. 2004. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell Biol. 164:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.