Abstract
Ors-binding activity (OBA) was previously semipurified from HeLa cells through its ability to interact specifically with the 186-basepair (bp) minimal replication origin of ors8 and support ors8 replication in vitro. Here, through competition band-shift analyses, using as competitors various subfragments of the 186-bp minimal ori, we identified an internal region of 59 bp that competed for OBA binding as efficiently as the full 186-bp fragment. The 59-bp fragment has homology to a 36-bp sequence (A3/4) generated by comparing various mammalian replication origins, including the ors. A3/4 is, by itself, capable of competing most efficiently for OBA binding to the 186-bp fragment. Band-shift elution of the A3/4–OBA complex, followed by Southwestern analysis using the A3/4 sequence as probe, revealed a major band of ∼92 kDa involved in the DNA binding activity of OBA. Microsequencing analysis revealed that the 92-kDa polypeptide is identical to the 86-kDa subunit of human Ku antigen. The affinity-purified OBA fraction obtained using an A3/4 affinity column also contained the 70-kDa subunit of Ku and the DNA-dependent protein kinase catalytic subunit. In vitro DNA replication experiments in the presence of A3/4 oligonucleotide or anti-Ku70 and anti-Ku86 antibodies implicate Ku in mammalian DNA replication.
INTRODUCTION
Ku antigen (autoantigen) is a heterodimeric (p70/p86) DNA-binding protein recognized by autoantibodies from the sera of certain patients with systemic rheumatic diseases (Mimori et al., 1981; Reeves, 1985; Yaneva et al., 1985; Mimori and Hardin, 1986). It consists of two polypeptides of 86 and 70 kDa (Yaneva et al., 1985). Ku is identical to a DNA-dependent ATPase isolated from HeLa cells (Cao et al., 1994) that had been previously reported to cofractionate with a 21S multiprotein complex competent for DNA synthesis from HeLa cells (Vishwanatha and Baril, 1990). Furthermore, the interaction of Ku antigen with a human DNA region (B48) containing a replication origin was reported (Tóth et al., 1993), and a novel ATP-dependent DNA unwinding enzyme, DNA helicase II (HDH II), was identified as Ku (Tuteja et al., 1994). Recently, Ochem et al. (1997) reported that the Ku70 subunit is the one associated with the helicase activity in the Ku70/Ku86 heterodimer. Moreover, a role for Ku70 as a tumor suppressor for murine T cell lymphoma has been suggested, because Ku70 deficiency facilitates neoplastic growth (Li et al., 1998). Ku has been shown to be the DNA-binding subunit of the DNA-dependent protein kinase (DNA-PK) holoenzyme (Gottlieb and Jackson, 1993; Suwa et al., 1994), a nuclear component that phosphorylates a number of DNA-binding, regulatory proteins, including transcription factors (Sp1, p53), RNA polymerase II, topoisomerases I and II, Ku antigen, and SV-40 large T antigen (Anderson, 1993, and references therein). Although Ku has been characterized as a DNA end–binding protein, it was recently shown that it is also a sequence-specific DNA-binding protein, binding to negative regulatory element 1 (NRE1) in the long terminal repeat of mouse mammary tumor virus (Giffin et al., 1996). It has also been recently reported that a Ku-like protein from Saccharomyces cerevisiae is required for the in vitro assembly of a multiprotein complex at the ARS121 origin of replication (Shakibai et al., 1996).
Our laboratory has previously isolated and cloned early-replicating origin-enriched sequences (ors) from synchronized African Green monkey kidney (CV-1) cells (Kaufmann et al., 1985). The ors-containing plasmids are capable of transient autonomous replication in vivo, when transfected into monkey (CV-1 and COS-7) and human (HeLa) cells (Frappier and Zannis-Hadjopoulos, 1987; Landry and Zannis-Hadjopoulos, 1991) and in an in vitro replication system that uses HeLa cell extracts (Pearson et al., 1991). Both in vivo and in vitro, replication is semiconservative, bidirectional, depends on the presence of an ors-containing template, and initiates within the ors sequence (Frappier and Zannis-Hadjopoulos, 1987; Pearson et al., 1991; Zannis-Hadjopoulos et al., 1992; Pearson et al., 1994). We have recently shown that one of the functional ors, ors12, serves as a chromosomal origin of DNA replication in CV-1 cells (Pelletier, Price, and Zannis-Hadjopoulos, unpublished observations).
The fractionation of HeLa cell replication proteins with ors-binding activity (OBA) was reported previously (Ruiz et al., 1995). OBA sediments at ∼150 kDa in a glycerol gradient. The OBA-containing fraction is enriched for polymerases α and δ, topoisomerase II, and RP-A and can support the in vitro replication of ors8 plasmid (Ruiz et al., 1995). Partial purification of OBA was achieved through its sequence-specific binding to a 186-bp subfragment of ors8, which was previously identified as the minimal sequence required for ors8 function as a replication origin in vivo and in vitro (Todd et al., 1995).
In this study, we have identified the DNA binding activity of OBA as the 86-kDa subunit of Ku (Ku86) antigen. We have also affinity-purified OBA (apOBA) based on its ability to specifically bind to A3/4, a sequence derived by comparison of mammalian DNA replication origins. Sequence-specific binding of OBA/Ku was also supported by band-shift competition analysis using a supercoiled A3/4-containing plasmid. Furthermore, inhibition of in vitro DNA replication was observed in the presence of either increasing amounts of the A3/4 oligonucleotide or anti-Ku70 and anti-Ku86 antibodies. The results indicate an involvement of OBA/Ku antigen in mammalian DNA replication.
MATERIALS AND METHODS
Cells and Plasmids
pBR322, p186, pors8, and pors12 plasmids were propagated in Escherichia coli HB101, as described previously (Frappier and Zannis-Hadjopoulos, 1987; Landry and Zannis-Hadjopoulos, 1991). Ors8 (GenBank accession no. M26221) plasmid has been described previously (Kaufmann et al., 1985; Zannis-Hadjopoulos et al. 1985; Rao et al., 1990). p186 consists of the NdeI–RsaI fragment (186 bp) of ors8 inserted into the NruI site of pBR322 (Todd et al., 1995). Linearized A3/4/pBR322 and pBR322 plasmids were obtained by digestion with NruI enzyme.
Preparation of DNA Fragments, Oligonucleotides, and End-labeling
To obtain the 186-bp fragment for band-shift experiments, pors8 plasmid was used as template in PCR reactions for amplification of the ors8 insert, which was then digested with NdeI and RsaI, as described previously (Ruiz et al., 1995). A nonspecific competitor fragment, pBRfg (206 bp), was prepared by PCR amplification of pBR322 DNA, as described previously (Ruiz et al., 1995). Oligonucleotides containing the A3/4 sequence (36 nucleotides in length; 5′-CCTCAAATGGTCTCCAATTTTCCTTTGGCAAATTCC-3′) and a nonspecific competitor derived from pBR322 (16 nucleotides in length; 5′-TTCCGAATACCGCAAG-3′) were synthesized (Sheldon Biotechnology Center, McGill University, Montreal, Canada), further purified by denaturing PAGE, and annealed as described in Wall et al. (1988). 5′ end-labeling of the 186-bp fragment and A3/4 double-stranded oligonucleotide were performed as described previously (Ruiz et al., 1995).
Fractionation of HeLa Cell Extracts
HeLa S3 nuclei and cytosol (Cellex Biosciences, Minneapolis, MN) were used to prepare nuclear and cytosolic extracts as described previously (Pearson et al., 1991). Nuclear and cytosolic extracts were mixed (total cell extracts) and dialyzed against buffer A (26 mM HEPES, pH 7.8, 82 mM potassium acetate, 5.0 mM MgCl2, 2.5 mM EDTA, 1.0 mM DTT, 1.0 mM PMSF, 1.0 μM pepstatin A, 1.0 μM leupeptin, 10% glycerol). The chromatographic steps for the purification of OBA were performed essentially as described before (Ruiz et al., 1995), except that a Sephacryl S-300 (Pharmacia AB Laboratory Separation Division, Uppsala, Sweden) step replaced the 10–40% glycerol gradient and an affinity-purification step was added at the end. Briefly, total cell extracts were applied to a DEAE Sephadex A-50 column previously equilibrated in buffer A. The flowthrough (FT) was collected, and the bound protein was eluted with a linear salt gradient of potassium acetate (0.082–1.0 M) in buffer A. The fractions collected from the elution gradient were pooled into four different pools (A, B, C, and D) on the basis of their salt concentration. Pool B was then dialyzed against buffer B (0.01 M KHPO4, pH 7.4, 0.15 M NaCl, 2.5 mM EDTA, 1.0 mM DTT, 1.0 mM PMSF, 1.0 μM pepstatin A, 1.0 μM leupeptin, 10% glycerol) and loaded onto an Affi-Gel Heparin column (Bio-Rad, Hercules, CA). The FT was collected, and the proteins bound to the matrix were eluted with a linear salt gradient of NaCl (0.15–1.0 M) in buffer B. The fractions were monitored for OBA activity by band-shift analyses, and those that were positive (300 mM NaCl) were concentrated, dialyzed, and labeled as pool E. Pool E was subsequently loaded onto a Sephacryl S-300 column in buffer B, and fractions were collected and monitored for OBA activity as above. The OBA-positive fractions were pooled (pool F), concentrated, and loaded onto an A3/4 DNA affinity column (see below), equilibrated with buffer B. The FT was recovered, and the protein bound to the matrix was eluted with a linear salt gradient of NaCl (0.15–1 M) in buffer B. OBA-positive fractions were pooled (apOBA), concentrated, dialyzed against buffer B, and frozen in aliquots at −70°C.
DNA Affinity Column
Oligonucleotides complementary (antisense) to the A3/4 sequence oligonucleotide (sense; see above) were synthesized, and 5′ directional overhangs (5′-GATC) were added, yielding a 40-mer oligonucleotide (Sheldon Biotechnology Center, McGill University). The oligonucleotides were further purified by denaturing PAGE. Equal amounts (220 μg) of antisense and sense oligonucleotides were mixed, annealed, phosphorylated, and ligated, as described previously (Kadonaga and Tjian, 1986). The multimers were then coupled to 10 ml of Sepharose CL-2B (Pharmacia Biotech) freshly activated by cyanogen bromide (Kadonaga and Tjian, 1986).
Gel Mobility-Shift Assays and Competition Experiments
Gel mobility-shift (band-shift) assays were typically performed as described previously (Ruiz et al., 1995) in 20 μl volume, by incubating 0.1 ng (0.81 fmol) of end-labeled 186-bp fragment or 0.5 ng (21 fmol), or other indicated amount, of double-stranded, end-labeled oligonucleotide containing the A3/4 sequence, with 200 ng of protein from pool F (Sephacryl S-300). Reactions were performed in binding buffer (10 mM Tris-HCL, pH 7.5, 80 mM NaCl, 1 mM EDTA, 10 mM 2-mercaptoethanol, 0.1% Triton X-100, 4% glycerol) in the presence of 1 μg of double-stranded (ds) poly (dI-dC) (Amersham Pharmacia, Baie d’Urfé, Québec, Canada), used as nonspecific competitor. After incubation on ice for 30 min, the reaction mixture was analyzed by 4% PAGE; the gel was then dried and exposed for autoradiography. For band-shift competition experiments, a constant amount (0.1 ng) of radioactively labeled 186-bp fragment was mixed with increasing molar excess amounts of either the 186-bp fragment or various subfragments (75, 69, 59, and 42 bp, respectively) generated from it, as well as the A3/4 oligonucleotide, used as cold competitors. pBRfg (see above) was also used as nonspecific competitor. A constant amount of protein (200 ng) from pool F (Sephacryl S-300) was then added to the mixture, and the reaction was left to proceed as described above. The shifted complexes were quantitated by densitometry performed using a Phosphoimager (Fuji BAS 2000; Fuji Medical Systems, Stamford, CT), and the results were expressed as percentage reduction in complex formation with increasing amounts of competitor. In competition reactions using the A3/4 oligonucleotide as probe, increasing molar amounts of either the double- or single-stranded A3/4 oligonucleotides were used as cold competitors. Competition reactions were also performed using either supercoiled or linearized plasmid containing the A3/4 sequence (A3/4/pBR322), or the vector (pBR322) alone; 260 ng of affinity-purified OBA (apOBA) were incubated for 1 h with 50 and 500× molar excess amounts of either the supercoiled or the linearized plasmid, relative to the radioactive A3/4 sequence (0.25 ng; 10.5 fmol), which was added last, and the reaction was left to proceed as described above.
When the mobility-shift assays were followed by Western blotting analyses, the reactions were performed using 0.25 ng/reaction of the probe and increasing amounts of apOBA (100, 250, and 350 ng, respectively), using the conditions described above. Half of the reactions were performed using radioactively labeled DNA, and the other half were performed using cold A3/4 as probe. A control reaction was also carried out in the absence of DNA. The reactions were analyzed using the mini-protean II slab cell electrophoresis system (Bio-Rad, Richmond, CA). After electrophoresis (free probe was run out of the gel), and the radioactive part of the gel was dried and exposed for autoradiography, whereas the equivalent “cold” part of the gel was prepared for Western blotting as described below.
In Vitro DNA Replication
In vitro replication reactions were performed as described previously (Pearson et al., 1991), with modifications according to Matheos et al. (1998). In the experiments involving the addition of the A3/4 oligonucleotide competitor, increasing molar excess amounts (relative to the input 200 ng p186 or pors12 template DNA) of either the A3/4 or the nonspecific oligonucleotide (see above) were preincubated with the HeLa cell extracts on ice for 20 min.
Experiments involving the addition of the anti-Ku antibodies were performed in a similar manner as described previously (Lin et al., 1997). Anti-Ku70 (Santa Cruz Biotechnology, Santa Cruz, CA; sc-1486) and anti-Ku86 (Santa Cruz; sc-1484) antibodies, 3.5, 7.0, and 14.0 μg of a 1.5 mg/ml antibody stock, concentrated using Microcon-10 microconcentrators (Amicon, Beverly, MA), were preincubated, respectively, with the HeLa cell extracts, on ice for 20 min. A goat IgG antibody (Sigma, St. Louis, MO) was used as a control.
The antibodies were neutralized as recommended by the manufacturer by reacting 14.0 μg of either the anti-Ku70 or anti-Ku86 antibody with a sevenfold (by weight) excess of the Ku70 (Santa Cruz; sc-1486P) or the Ku86 (Santa Cruz; sc-1484P) blocking peptides or with a mixture of GATA-1 (Santa Cruz; sc-1233P), GATA-2 (Santa Cruz; sc-1235P), and DNA-PK (Santa Cruz; sc-1552P) blocking peptides, the latter three serving as nonspecific blocking peptides. The incubations were carried out overnight at 4°C. Subsequently, the neutralized antibodies were preincubated with the extracts and finally added to the in vitro replication reaction, performed as described above.
The in vitro replication products were divided into three aliquots: one-third was digested with 1 U of DpnI (New England Biolabs, Beverly, MA) for 60 min at 37°C, as described previously (Matheos et al., 1998). The DpnI-digested and one-third of the undigested products were subjected to electrophoresis on 1% agarose gel in 1× Tris-acetate (0.04 M Tris-acetate, 0.001 M EDTA) buffer (16–20 h, 50–55 V). The gels were dried and exposed to Kodak X-Omat Blue XB-1 autoradiographic film (Eastman Kodak, Rochester, NY). Quantification was performed by densitometric measurements of the DpnI-digested products, as described previously (Diaz-Perez et al., 1996; Matheos et al., 1998), using a phosphoimager analyzer (Fuji BAS 2000). The amount of radioactive precursor incorporated into the DNA was expressed as a percentage of the control p186 reaction (100%).
Band-Shift Elution of OBA–A3/4 Complexes Followed by Southwestern Analysis
Ten band-shift reactions were performed with radioactively labeled A3/4 DNA (10 ng/reaction) and 3.75 μg/reaction of protein from pool F (from the Sephacryl-S300 column; see MATERIALS AND METHODS), using the conditions described above. As a control, similar reactions were performed in the absence of DNA. The band-shifts were analyzed by electrophoresis in a native 4% polyacrylamide gel, and the wet gel was exposed for 5 h at 4°C for autoradiography. The OBA–DNA complexes were then excised from the gel, and the proteins and the DNA were eluted from the gel by isotachophoresis (Ofverstedt et al., 1984) and then subjected to electrophoresis on 8% SDS-polyacrylamide gel under reducing conditions. The proteins were then transferred electrically to an Immobilon-P membrane (Millipore, Bedford, MA) and subjected to Southwestern analysis, following the protocol described in Philippe (1994), with some modifications. Briefly, the membrane was incubated overnight (14–16 h) in blocking solution (buffer S: 25 mM HEPES-KOH, pH 7.7, 25 mM NaCl, 5 mM MgCl2, 1 mM DTT, containing 5% skim milk and 0.05% NP-40). The next day the membrane was subjected to a process of denaturation–renaturation, as follows: it was incubated for 10 min in a denaturing solution of 6 M guanidine hydrochloride in buffer S, followed by 10 min incubations in 3, 1.5, 0.75, 0.375, and 0.187 M guanidine hydrochloride, respectively, diluted in buffer S; it was then washed twice for 10 min with buffer S, and incubated for 2 h in blocking buffer, followed by 1 h incubation in buffer S + 1% skim milk. The membrane was then incubated overnight in hybridization solution (20 mM HEPES, pH 7.7, 75 mM KCl, 0.1 mM EDTA, 2.5 mM MgCl2, 1% skim milk, 0.05% NP-40) containing radioactively labeled A3/4 oligonucleotide (5.2 ng/ml, 2.6 × 106 cpm/ml) in the presence of poly (dI-dC) (50 μg/ml) and pBRfg DNA (454 ng/ml) as nonspecific competitors. Finally, the membrane was washed three times with hybridization solution and subsequently exposed for autoradiography. The entire procedure was carried out at 4°C, and the incubations were performed on a rocking platform.
Western Blotting Experiments and SDS-PAGE
Denaturing PAGE was performed as described previously (Laemmli, 1970) using the mini-protean II slab cell electrophoresis system (Bio-Rad). Western blot analysis was performed essentially as previously described (Burnette, 1981), using the ECL detection kit (Amersham, Arlington Heights, IL). All of the antibodies were purchased from Santa Cruz Biotechnology. For immunodetection of Ku autoantigen subunits (Ku86 and Ku70), 10 and 20 μg of total cell extracts (NC) and 1 and 3 μg of affinity-purified OBA were subjected to electrophoresis on 8% SDS-PAGE and electrically transferred to Immobilon-P (Millipore). The membrane was first probed with anti-Ku86 (C-20) antibody (1 μg/ml); it was then stripped and reprobed with anti-Ku70 (C-19) antibody (2 μg/ml) using the ECL detection kit protocol. For DNA-PK catalytic subunit (DNA-PKcs) detection, 20 and 40 μg of total cell extracts (NC), and 3 and 6 μg of affinity-purified OBA were run on a 6% SDS-PAGE and transferred onto Immobilon-P membrane (Millipore), as described above. The membrane was then probed with anti–DNA-PKcs (C-19) antibody (4 μg/ml). An anti-goat IgG–horseradish peroxidase-conjugated secondary antibody (1:2000) was used in the immunoblots. A similar procedure was used for the Western analysis performed on the membranes that had been previously used for Southwestern or band-shift analyses. Visualization of the proteins in the gels was performed using the Rapid Silver Staining Kit (Sigma).
Microsequencing Analysis of OBA
Protein concentration was determined by the method of Bradford (Bradford, 1976) and the Nucleic Acid Soft-Pack module from a DU-65 Spectrophotometer (Beckman, Mississauga, Ontario, Canada). Twenty-nine micrograms of affinity-purified OBA fraction were subjected to electrophoresis on an 8% SDS-PAGE under reducing conditions. Proteins were blotted onto a Problott membrane (Applied Biosystems, Foster City, CA) and visualized by staining with Ponceau S (Sigma) (0.2% wt/vol in 1% vol/vol acetic acid). The excess of dye was washed off with 1% acetic acid, and a protein band estimated to be ∼92 kDa was subsequently excised from the membrane and sent for internal sequencing analysis (Harvard Microchemistry Facility, Cambridge, MA).
RESULTS
Specific Interaction between OBA and the A3/4 DNA Consensus Sequence
The map and sequence characteristics of the 186-bp fragment of ors8 have previously been reported (Ruiz et al., 1995; Todd et al., 1995). In the present study, band-shift competition experiments were performed to localize the OBA binding site within the 186-bp minimal origin of ors8 (Figure 1A). In contrast to the nonspecific competitor pBRfg, which did not compete, the different subfragments of the 186 bp competed to different extents for OBA binding. The most efficient competitor was the internal 59-bp fragment (Figure 1A), generated from the digestion of the 186 bp with MslI and FokI (Figure 1B), which competed as efficiently as, or better than, the 186-bp fragment itself. The 59-bp fragment (Figure 1C) contains two 7-bp stretches with 85% identity to a 36-bp DNA sequence, A3/4 (Figure 1C), deduced from different ors (Kaufmann et al., 1985; Rao et al., 1990) and human replication origins isolated in our laboratory (Bell et al., 1991; Wu et al., 1993; Nielsen et al., 1994). When the A3/4 sequence was tested as competitor for OBA binding to the 186-bp fragment in the band-shift assay, it was able to compete just as well as the 59-bp fragment for OBA binding (Figure 1A). Furthermore, the specificity of OBA binding to the A3/4 sequence was tested in a series of competition band-shift assays, using the A3/4 oligonucleotide as specific competitor, in increasing (50–2000×) molar-fold amounts (Figure 2). Formation of the OBA–A3/4 complex decreased in the presence of 50-fold molar excess of cold A3/4 oligonucleotide competitor, and it was 95% abolished at 500-fold molar excess of A3/4 (Figure 2, A3/4 ds). In contrast, when similar competition reactions were carried out using the two single-stranded oligonucleotides of the A3/4 sequence as competitors, neither was able to compete the OBA–A3/4 complex (Figure 2; leading and complementary).
Figure 1.
Competition band-shift analysis of OBA binding. (A) Band-shift reactions were performed by incubating constant amounts of both protein (pool F; 200 ng) and radioactively labeled DNA (186-bp fragment; 0.1 ng). The various subfragments of the 186-bp and the A3/4 (36-bp) sequence were used as cold competitors at the molar-fold excess level indicated. The shifted complexes were quantitated by densitometry, and the results are expressed as percentage reduction in complex formation. (B) Restriction map of the 186-bp sequence of ors8 indicating the fragments used as competitors. The FokI site (arrowhead) bisects the inverted repeat (→←). The position of the A3/4 homologous sequence is indicated (*). (C) Sequence of the 59-bp fragment of ors8. The nucleotides that are identical to the A3/4 36-bp sequence are indicated (*).
Figure 2.
Specificity of OBA binding to the A3/4 sequence. Band-shift reactions were performed by incubating constant amounts of radioactively labeled A3/4 DNA (0.5 ng) and pool F (200 ng). Increasing molar excess (50, 500, 1000, and 2000×) amounts of the A3/4 sequence, either ds or leading and complementary A3/4 single strands, were used as cold competitors.
A3/4 Oligonucleotide Inhibits the In Vitro DNA Replication of p186
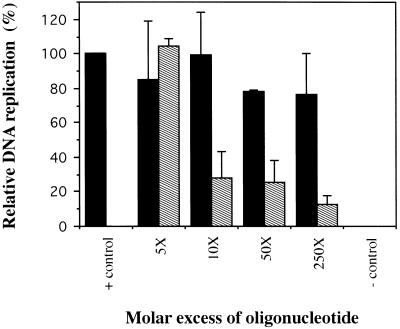
To investigate the effect of the A3/4 sequence on the replication of p186, we performed in vitro DNA replication assays (Pearson et al., 1991, 1994; Matheos et al., 1998) in which the HeLa cell extracts were preincubated with increasing molar excess amounts of either the A3/4 oligonucleotide or a nonspecific oligonucleotide used as a competitor (Figure 3). Addition of increasing amounts of the A3/4 oligonucleotide strongly inhibited p186 replication in vitro, decreasing it by approximately fourfold at 10× molar excess and by approximately 10-fold at 250× molar excess amounts relative to the control (Figure 3). In contrast, addition of the nonspecific oligonucleotide did not affect the replication of p186 (Figure 3), nor did that of a 29-bp random oligonucleotide (our unpublished results). Addition of the A3/4 oligonucleotide similarly inhibited the in vitro replication reaction of pors12, a plasmid containing ors12, a functional ors that serves as a chromosomal origin of DNA replication, whereas the nonspecific oligonucleotide did not have an inhibitory effect (our unpublished results). The products of the in vitro replication reaction included open circular (form II), linear (III), and supercoiled (I) forms of the plasmid DNA. In addition, replicative intermediates and topoisomeric forms of the plasmid DNA were also obtained, in agreement with previous observations (Pearson et al., 1991; Zannis-Hadjopoulos et al., 1994; and Matheos et al., 1998). As shown previously, the in vitro replication system mimics in vivo replication in that replication initiates specifically within the ors and is bidirectional, semiconservative, and sensitive to aphidicolin (Pearson et al., 1991, 1994).
Figure 3.
Effect of the A3/4 oligonucleotide sequence on the in vitro replication of p186. A p186 plasmid (200 ng), containing the 186-bp fragment of ors8, was used as template for in vitro replication. Increasing molar excess amounts (indicated) of the specific A3/4 (dashed bars) or nonspecific pBR322 (black bars) oligonucleotides, relative to the p186 template, were used.
Identification of the Polypeptide Involved in the DNA Binding Activity of OBA
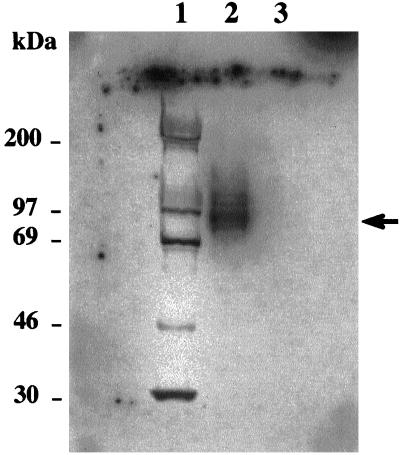
To analyze the peptide(s) involved in the DNA binding activity of OBA, band-shift elution (Ruiz et al., 1995) of the OBA–A3/4 complex followed by Southwestern blot analysis was performed (Figure 4). Several band-shift reactions were performed simultaneously, and the OBA–A3/4 complex was excised and eluted from the gel by isotachophoresis. The OBA proteins were then subjected to Southwestern analysis (see MATERIALS AND METHODS) using the A3/4 oligonucleotide as radioactive probe. A major band of ∼92 kDa was revealed as the one primarily involved in the DNA binding activity of OBA (Figure 4, arrow).
Figure 4.
Southwestern analysis of OBA–A3/4 complex. OBA–A3/4 band-shifted complex (see Figure 2) was eluted from the gel by isotachophoresis and subjected to 8% SDS-PAGE under reducing conditions. A control area from the same gel containing protein, but not DNA, was excised and similarly treated. The gel was blotted onto an Immobilon membrane and probed with radioactively labeled A3/4 DNA, in the presence of ds poly (dI-dC) and pBRfg as nonspecific competitors. Lane 1, molecular size markers (molecular sizes are indicated); lane 2, OBA from OBA–A3/4 eluted complex; lane 3, protein control lane.
OBA Purification on an A3/4 Affinity Column
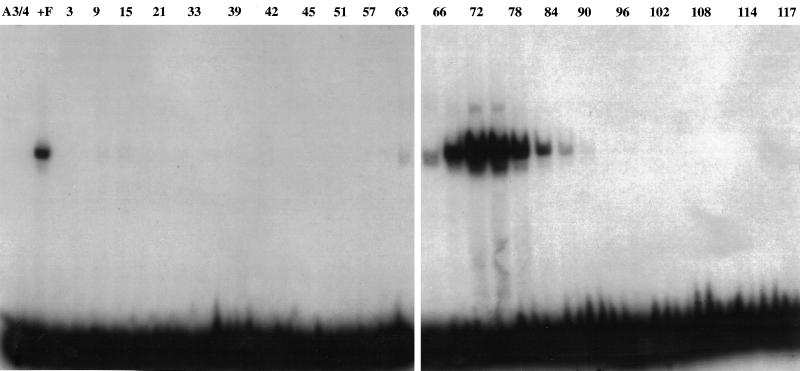
An A3/4 DNA sequence-specific affinity column was prepared to enrich for the DNA binding activity of OBA, as determined by Southwestern analysis. Pool F (from Sephacryl-S300 column) was loaded onto the column, the FT was recovered, and the proteins bound to the column were eluted with a linear salt (NaCl) gradient (see MATERIALS AND METHODS). The apOBA, localized by band-shift analyses using the A3/4 oligonucleotide as probe, was eluted at 0.45 M NaCl as a single peak around fraction 72 (Figure 5) and generated the same characteristic complexes as those that had been obtained with OBA in the previous chromatographic steps (Ruiz et al., 1995). PAGE analysis of apOBA (Figure 6A) revealed three predominant bands with estimated relative molecular mass of 78,000 (78 kDa), 92,000 (92 kDa), and >200,000 (>200 kDa). In addition, a band with molecular mass of 130,000 (130 kDa) (Figure 6A, lane apOBA, asterisk), as well as a doublet of lesser abundance with estimated molecular mass of 104,000 (104 kDa) and 110,000 (110 kDa), respectively, were observed.
Figure 5.
Elution profile of OBA from the A3/4 affinity column. A DNA sequence-specific affinity column was prepared by binding of oligomers of the A3/4 oligonucleotide sequence to Sepharose CL-2B, freshly activated with cyanogen bromide. Pool F (see MATERIALS AND METHODS) was loaded onto the column, the FT was recovered, and the proteins bound to the matrix were eluted with a linear salt gradient (150 mM-1.0 M) of NaCl in buffer B. The elution of OBA (0.45 M NaCl) was localized by band-shift reactions, incubating 16 μl of every second fraction with the A3/4 DNA as probe.
Figure 6.
Ku antigen is present in the affinity-purified OBA fraction. (A) OBA enrichment profile in the nuclear and cytoplasmic (NC) HeLa cell extracts and in pools B, E, F, and affinity-purified OBA (apOBA) through the purification steps; 2.5 μg of pools NC, B, E, and F as well as 1.0 μg of affinity-purified OBA were subjected to 8% SDS-PAGE and silver-stained. The position of the DNA-PKcs band is indicated (arrow). Western blot analyses of the affinity-purified OBA fraction were performed using (B) anti-Ku86 antibody (1 μg/ml), (C) anti-Ku70 antibody (2 μg/ml), (D) anti–DNA-PKcs antibody (4 μg/ml). The DNA-PKcs band is indicated (D, arrow); the faster migrating band is a degradation product of DNA-PKcs (*). Western analyses were done on 8% SDS-PAGE for Ku86 and Ku70, and on 6% SDS-PAGE for DNA-PKcs.
DNA-binding Polypeptide of OBA Is Ku86
apOBA was electroblotted onto Problott membrane (Perkin Elmer-Cetus, Emeryville, CA) and stained with Ponceau-S. The 92 kDa exhibiting DNA binding (Figure 4) was excised from the membrane and sequenced at the Harvard Microchemistry Facility by collisionally activated dissociation mass spectroscopy on a Finnigan TSQ 700 triple quadrupole mass spectrometer. The three peptide sequences obtained (Table 1) were subjected to homology searches using the BLAST program (Altschul et al., 1990), and all three were found to have 100% identity to the Ku86 subunit of human Ku autoantigen (Table 1).
Table 1.
Microsequencing analysis of tryptic peptides of the OBA band
| Peptides | Amino acid sequence | Homology | Position |
|---|---|---|---|
| Aff92-PK160 | SQIPLSK | Ku86 (100%) | 526–532 |
| Aff92-PK312 | TLFPLIEAK | Ku86 (100%) | 535–543 |
| Aff92-PK296 | TDTLEDLFPTTK | Ku86 (100%) | 470–481 |
The enrichment of OBA throughout the purification scheme was visualized by 8% denaturing PAGE of the different pools (Figure 6A, lanes NC to apOBA). To verify the presence of Ku antigen in the affinity-purified OBA fractions, Western blotting analysis was performed, using antibodies raised against the Ku86 and Ku70 subunits (Santa Cruz). The results (Figure 6, B and C) show that the two major OBA bands, estimated as migrating as 92 kDa and 78 kDa, correspond to the Ku86 and Ku70 subunits of Ku autoantigen, respectively. Similar Western blotting analyses using anti–DNA-PKcs antibodies revealed that the high molecular weight predominant band (>200 kDa) present in the affinity-purified OBA (Figure 6A, arrow) corresponded to DNA-PKcs (p465) (Figure 6D, arrow). The 130-kDa band present in apOBA (Figure 6A, asterisk) and in the Western blot is a degradation product of DNA-PKcs (Figure 6D, asterisk). Western analysis performed on the same membrane that had been used for Southwestern analysis (Figure 4) confirmed that the OBA band with DNA binding activity, which was also sequenced, is the Ku86 subunit of Ku autoantigen (Figure 7A). Although the Ku70 subunit could also be detected (Figure 7B), no DNA binding activity was associated with it, in contrast to previous reports (Griffith et al., 1992; Zhang and Yaneva, 1992; Wu and Lieber, 1996).
Figure 7.
OBA corresponds to the Ku86 subunit of Ku antigen. The membrane used for Southwestern analysis (Figure 4) was probed with (A) anti-Ku86 (1 μg/ml) and (B) anti-Ku70 (2 μg/ml) antibodies, respectively.
Band-Shift Analysis of OBA
When apOBA is used in band-shift assays with A3/4 DNA (Figure 8), a faster migrating band (Figure 8, arrow) is detected below the regular OBA complex (Figure 8, asterisk). To investigate the nature of this band, band-shift reactions were performed in duplicate, using radioactively labeled and unlabeled A3/4 oligonucleotide as probe. The radioactive part of the gel was used for band-shift analysis, where the expected band-shift pattern was obtained with increasing amounts of apOBA (Figure 8, I). The nonradioactive part of the gel was transferred after electrophoresis to a membrane and subjected to Western blotting analysis, as described above. The results (Figure 8, II and III) showed that Ku86 is present only in the upper OBA complex (Figure 8, II), together with Ku70 (Figure 8, III), whereas in the faster migrating band only the p70 subunit of Ku was detected (Figure 8, III).
Figure 8.
Western blot analysis of the band-shifted complexes generated with apOBA. (I) Band-shift reactions were performed under regular conditions, using 0.25 ng/reaction of A3/4 radioactive probe and increasing amounts of apOBA (100, 250, and 350 ng, respectively). A control reaction was performed in which the DNA was absent. A duplicate set of reactions was also performed using nonradioactive A3/4 as probe. All reactions were subjected to electrophoresis in a minigel apparatus so that the DNA probe ran out of the gel. The nonradioactive half of the gel was transferred to a membrane and subjected to Western Blotting analysis with (II) anti-Ku86 antibody and (III) anti-Ku70 antibody. The position of the main OBA–A3/4 (asterisk) and of the faster migrating (arrow) complexes is indicated.
OBA/Ku86 Binds the DNA in a Sequence-specific Manner
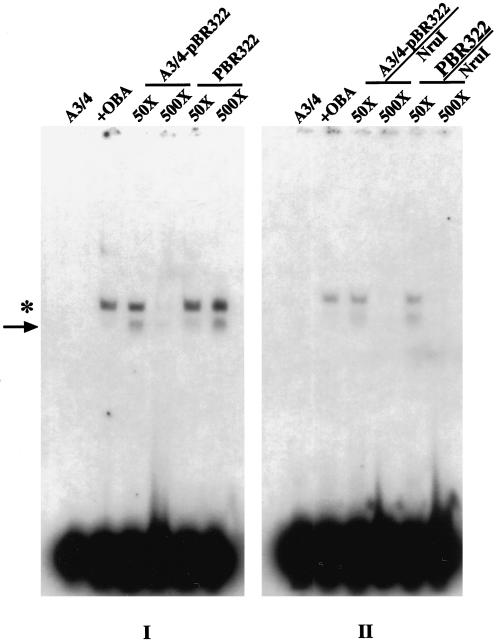
To investigate whether OBA/Ku interacts with the A3/4 DNA in a sequence-specific manner or whether it binds to the ends of double-stranded linear DNA without any sequence preference, competition band-shift assays were carried out using as competitor a supercoiled double- stranded plasmid containing the A3/4 sequence A3/4/pBR322. Similar control reactions were carried out using the supercoiled vector plasmid pBR322, lacking the A3/4 sequence. The results (Figure 9, I) show that at 50× molar excess, the supercoiled A3/4/pBR322 plasmid competed to a small extent (2.9%) for the OBA binding to the A3/4 oligonucleotide, whereas at 500× molar excess it competed very efficiently, reducing the formation of the OBA–A3/4 complex by 97% (Figure 9, I, A3/4/pBR322); the nonspecific supercoiled competitor (pBR322), on the other hand, was not able to affect at all the OBA–A3/4 complex formation (Figure 9, I). When the same plasmids (A3/4/pBR322 and pBR322) were linearized and used as competitors (A3/4/pBR322/Nru I and pBR322/Nru I, respectively) (Figure 9, II), at 50× molar excess only the A3/4/pBR322 plasmid competed to a small extend (4.4%), whereas at 500× molar excess both linear plasmids competed for OBA binding to A3/4 with equal efficiencies (98%) (Figure 9, II).
Figure 9.
Sequence-specific binding of apOBA to A3/4. Band-shift reactions were performed by incubating radioactively labeled A3/4 DNA (0.25 ng) and affinity-purified OBA (260 ng). I, The supercoiled plasmids A3/4/pBR322 and pBR322 (see MATERIALS AND METHODS) were used as competitors at 50 and 500× molar excess, respectively. II, Similar experiments were performed in the presence of linearized A3/4/pBR322 (A3/4/pBR322/Nru I) and pBR322 (pBR322/Nru I) plasmids. The position of the main OBA–A3/4 (asterisk) and of the faster migrating (arrow) complexes is indicated.
Ku70 and Ku86 Antibodies Inhibit the In Vitro DNA Replication of p186
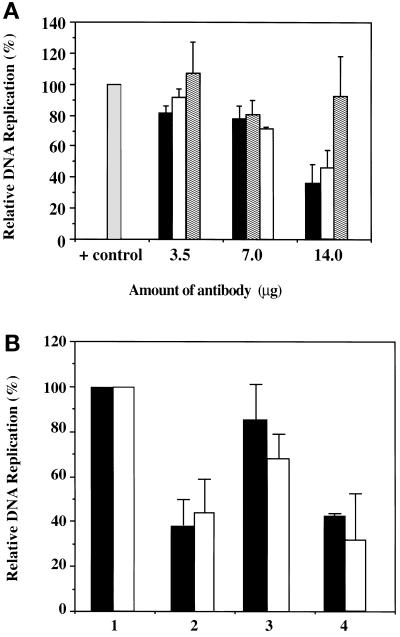
To investigate whether Ku is involved in the replication of p186, we performed in vitro replication reactions preincubating the HeLa cell extracts with increasing (3.5, 7, and 14 μg) amounts of the anti-Ku70 and anti-Ku86 antibodies, as well as a control, goat IgG antibody (Figure 10A). Addition of 14 μg of either the anti-Ku70 or anti-Ku86 antibodies inhibited the relative in vitro replication of p186 by 62 and 56%, respectively. In contrast, addition of the nonspecific goat IgG antibody had no effect over the entire range of concentrations assayed, indicating that the observed inhibition is specific to the Ku antibodies.
Figure 10.
(A) Effect of anti-Ku70 and anti-Ku86 antibodies on the in vitro replication of p186. Increasing amounts (3.5, 7.0, and 14 μg) of anti-Ku70 (black bars), anti-Ku86 (white bars), and goat IgG (dashed bars) antibodies were preincubated with HeLa cell extracts, before the addition of the plasmid DNA template (p186, 200 ng). (B) Neutralization of the anti-Ku70 and anti-Ku86 antibodies. A constant amount (14 μg) of the anti-Ku70 (black bars) and anti-Ku86 (white bars) antibodies was neutralized using specific (lane 3) or nonspecific (lane 4) peptides before being added to the reaction. Lane 1 represents the p186 control reaction, and lane 2 represents the reaction inhibited with 14 μg of antibodies. In vitro reactions were performed, and the data were processed as described in MATERIALS AND METHODS.
To confirm that the inhibition of p186 in vitro replication was in fact due to specific interaction of the antibodies with Ku antigen in the reaction, the respective antibodies were neutralized with either the Ku-specific or nonspecific blocking peptides before their addition to the reaction (Figure 10B). Addition of anti-Ku70 and anti-Ku86 antibodies that had been neutralized with the Ku70- and Ku86-specific peptides, respectively, reversed the inhibition of p186 replication in vitro (Figure 10B, lane 3) when compared with the reaction with the untreated antibodies (Figure 10B, lane 2). Neutralization of the anti-Ku70 antibody (black bars) restored replication by 47% and of the anti-Ku86 antibody (white bars) by 30%, respectively, bringing replication levels close to the control reaction (Figure 10B, lane 1). In contrast, when the Ku antibodies were blocked with a nonspecific peptide mixture, and then added to the reaction, they failed to restore replication (Figure 10B, lane 4).
DISCUSSION
Ku is a nuclear protein originally identified as an autoantigen associated with human lupus erythematosus and related overlap syndromes (Mimori et al. 1981). Ku has been reported to be part of a family of related proteins (Griffith et al., 1992), conserved in organisms that range from yeast (Feldmann and Winnacker, 1993; Jacoby and Wensink, 1996) to humans (Mimori et al., 1981). Ku (p70/p86) serves as the DNA-binding subunit for the DNA-PK holoenzyme (Dvir et al., 1992; Gottlieb and Jackson, 1993). It mediates the recruitment of DNA-PKcs to DNA, and although it binds to the ends of naked DNA and is translocated along the DNA in an ATP-independent manner (de Vries et al., 1989), it was recently reported as a transcription factor that recruits DNA-PK directly to specific DNA sequences (Giffin et al., 1996, 1997). DNA-PK holoenzyme, composed of the DNA-PKcs (465 kDa) and Ku antigen, has been implicated in multiple nuclear processes, including transcription (Finnie et al., 1993; Genersch et al., 1995; Kuhn et al., 1995;), double-stranded DNA break repair (Getts and Stamato, 1994; Jeggo et al., 1995; Weaver, 1995), V(D)J recombination (Smider et al., 1994; Taccioli et al., 1994; Blunt et al., 1995; Kirchgessner et al., 1995; McConnell and Dynan, 1996, and references therein; Han et al., 1997), and DNA replication (Anderson and Lees-Miller, 1992; Anderson, 1993).
In this study, we identified a 59-bp region within the 186-bp fragment of ors8, to which OBA is specifically binding. OBA, a DNA binding activity from HeLa cells, was previously identified as a protein activity that specifically interacts with the 186-bp fragment of ors8 (Ruiz et al., 1995), the minimal sequence required for the replication function of ors8 (Todd et al., 1995). The 59-bp subfragment of ors8 contains two stretches of 7 bp each with 85% identity to a 36-bp sequence (A3/4) deduced from mammalian replication origins, among them the ors. Band-shift competition analyses confirmed that OBA binds the double-stranded A3/4 sequence specifically. Furthermore, addition of increasing amounts of an oligonucleotide derivative of the A3/4 sequence inhibited the in vitro replication of p186, a plasmid containing the 186-bp minimal ori, as well as that of pors12, a plasmid containing a chromosomal origin of DNA replication. Southwestern analyses of the OBA–A3/4 band-shifted complex revealed a major peptide of ∼92 kDa responsible for the DNA binding activity of OBA. Microsequencing analyses of this band revealed identity to the 86-kDa subunit of Ku autoantigen (Mimori et al., 1981; Reeves, 1985; Yaneva et al., 1985). OBA was enriched by affinity purification (apOBA) on a column (Kadonaga and Tjian, 1986) constructed using multimers of the A3/4 sequence.
A supercoiled circular plasmid containing the A3/4 sequence was able to compete for the binding of apOBA to A3/4, as opposed to the vector alone (Figure 9, I), indicating that the interaction of OBA with A3/4 is sequence-specific; however, OBA is also capable of binding to DNA termini, because the same plasmids (A3/4-pBR322 and pBR322), when linearized, competed for OBA binding as efficiently as the supercoiled specific competitor (Figure 9, II), in agreement with previous reports regarding Ku binding (Paillard and Strauss, 1991; Gottlieb and Jackson, 1993; Rathmell and Chu, 1994). Thus, the data indicate that, in addition to binding to DNA ends, as expected, OBA/Ku also exhibits efficient sequence-specific binding to the internal A3/4 sequence. These data support recent reports describing sequence-specific binding of Ku to an internal DNA sequence, NRE1 (Giffin et al., 1996, 1997); the direct binding of Ku/DNA-PK to NRE1 represses glucocorticoid-induced mouse mammary tumor virus transcription (Giffin et al., 1996, 1997). The 26-bp NRE1 sequence contains a seven-nucleotide stretch match to the 59-bp fragment of ors8, overlapping A3/4. Interestingly, a 207-bp fragment from the origin of bidirectional replication, oriβ, associated with the dihydrofolate reductase gene, which contains a region of homology (∼70%) to the A3/4 sequence, is also able to form a complex with apOBA, which is efficiently competed by increasing amounts of the A3/4 oligonucleotide (our unpublished results).
A Ku-like protein from S. cerevisiae was demonstrated to have affinity for ssDNA (Shakibai et al., 1996), whereas previous reports identified Ku70 as the DNA binding activity of the Ku(p70/p86) heterodimer, with affinity for DNA ends (Zhang and Yaneva, 1992; Wu and Lieber, 1996). Recently, the sequence-specific binding of Ku autoantigen to the single, upper strand of NRE1 was shown to be mediated by both Ku subunits (Torrance et al., 1998). The binding of Ku to double-stranded NRE1, however, although it also requires the two subunits, occurs in a two-step manner. The first step involves binding of the Ku70 subunit to the DNA and is followed by a Mg+2-dependent step that leads to the contact of the Ku86 subunit to DNA. In our study, band-shift competition analyses demonstrated that OBA binding cannot be competed by single-stranded DNA (Figure 2). Microsequencing analyses revealed that apOBA is identical to the p86 subunit of Ku antigen, whereas Western blotting showed that both Ku subunits (70 and 86 kDa) were present in the affinity-purified OBA fraction (Figure 6). Furthermore, Western analysis performed on the same membrane that was used in the Southwestern analysis confirmed that although both Ku antigen subunits (Ku86 and Ku70) were present, the DNA binding activity was associated only with the Ku86 subunit (Figures 4 and 7). Feldmann et al. (1996) reported the cloning of HDF2 (high-affinity DNA-binding factor), the S. cerevisiae gene encoding the second subunit of the HDF heterodimer, the yeast Ku homologue. HDF2 is homologous to the Ku86 subunit of Ku antigen and can bind DNA on its own. Because the DNA binding activity of HDF2 is much weaker than the binding activity of the HDF heterodimer, the authors argued that HDF2 is the one involved in DNA binding, whereas HDF1 (p70 subunit) increases the affinity of the heterodimer for the DNA. On the other hand, other reports argued that both subunits are directly involved in DNA end binding (Milne et al., 1996) and that only the heterodimeric form of recombinant Ku antigen is able to bind DNA ends (Ochem et al., 1997). Interestingly, the identification of Ku antigen isolated from HeLa cells in a two-dimensional–gel database of transformed human amnion cell proteins revealed that Ku86 consists of at least three charge variants, whose relative abundance is a function of cell proliferation (Stuiver et al., 1991). Different charge variants of this subunit could explain the discrepancy in the results regarding the DNA binding activity of the polypeptide.
It was recently reported (Bliss and Lane, 1997; Klug, 1997) that in band-shift analyses involving Ku antigen, the order of addition of reagents to the band-shift reaction is crucial in avoiding artifactual bands. In light of this, we performed band-shift reactions both under conditions in which all the components of the band-shift assay were present at the same time in the reaction, and by step incubation, in which the competitor DNA (A3/4) and the protein (apOBA) were preincubated together and the radioactive probe was added last (our unpublished results). These studies further confirmed that, regardless of the order of addition, the interaction between A3/4 and OBA/Ku is sequence-specific and revealed that preincubation of the A3/4 competitor with apOBA resulted in a higher level of inhibition of formation of the apOBA complex than was obtained otherwise. Interestingly, in band-shift reactions in which the nonspecific competitor (pBRfg), the 186 bp radioactive probe, and the protein fraction were simultaneously incubated, no complex competition was observed (Figure 1, pBRfg). These results indicate that, when the reagents are present simultaneously, OBA/Ku preferentially binds to its specific internal binding site (contained in the 186-bp fragment of ors 8) and not to the DNA termini presented by the linear nonspecific pBRfg (Figure 1). In contrast, in band-shift reactions in which the linear A3/4pBR322 (specific) or pBR322 (nonspecific) plasmids were initially incubated with the protein fraction and the probe was subsequently added, both plasmids were able to compete for OBA/Ku binding, indicating that under these conditions the protein interacted with DNA termini (Figure 9, II).
Although both subunits of Ku antigen (p70 and p86) were detected in the main (slower migrating) OBA-shifted complex (Figure 8, asterisk) as expected of a heterodimeric (p70/p86) DNA-binding protein (Figure 8, arrow), only the p70 subunit was detected in the faster-migrating complex (Figure 8, compare II and III). This is due to the fact that the Ku86 antibody used in these analyses was raised against the C-terminal end of the protein (Ku86 [C-20], Santa Cruz) and thus is unable to recognize the Ku86 subunit in the faster-migrating complex, which arises by the specific in vitro endoproteolysis of Ku86 at the C-terminus region (Paillard and Strauss, 1993). This proteolytic degradation of the Ku86 subunit gives rise to a 69-kDa peptide that is able to associate with Ku70 to form a lower molecular weight Ku heterodimer, which is still capable of binding DNA (Paillard and Strauss, 1993).
The apOBA preparation is also enriched for a high molecular weight protein, which was shown by Western analysis to correspond to DNA-PKcs (Figure 6D). Although DNA-PKcs is present in the preparation, it is absent from the OBA–A3/4 complex (Figure 8). The role of OBA/Ku in p186 in vitro replication may be independent of the DNA-PKcs activity, because addition of increasing amounts of anti–DNA-PKcs antibodies to the in vitro reaction did not affect p186 replication. Interestingly, it was reported recently that DNA-PKcs is able to bind DNA by itself, independently of Ku antigen (Yaneva et al., 1997).
A role of Ku in DNA replication has been suggested before: it was reported as part of a multiprotein complex competent for T antigen-dependent SV40 in vitro replication (Vishwanatha and Baril, 1990; Cao et al., 1994). More recent studies also suggest the involvement of Ku in replication, either as an independent protein or through its association with DNA-PKcs. Henricksen et al. (1996) reported that the phosphorylation of replication protein A by DNA-PK is involved indirectly in the modulation of DNA replication. Shakibai et al. (1996) reported the purification of OBF2 (origin binding factor 2) from S. cerevisiae, which is identical to HDF, the yeast homologue of the mammalian Ku antigen. OBF2 binds to the ARS121 replication origin and supports the formation of a protein complex at the origin. In our study, the inhibition of p186 replication, observed in the presence of either the A3/4 oligonucleotide or the anti-Ku antibodies directed against either subunit of Ku, also suggests a role of OBA/Ku in mammalian DNA replication. The specificity of the inhibition of p186 replication, attributable to the sequestering of p70 and p86 proteins, was demonstrated by the neutralization of these antibodies with their specific peptides. Because in vitro DNA replication is not fully inhibited by the presence of the Ku antibodies (Figure 10), there may also be alternative pathways to carry out OBA(Ku) activity in the absence of this protein.
Recently, Ku86 and Ku70 knockouts were obtained (Nussenzweig et al., 1996; Gu et al., 1997), and both of them are viable. Although Ku86−/− mice have a growth defect, neither the Ku86−/− mice nor the murine embryonic stem cell line that lacks Ku70 expression has been investigated for defects in DNA replication. The hypersensitivity of Ku86-deficient cell lines and mice to DNA damage supports the role of Ku86 in growth regulation (Nussenzweig et al., 1997). It has been demonstrated that multiple genes encode a family of Ku70-related polypeptides (Griffith et al., 1992). This could explain in part the diversity of functions and contradictory data that have been reported for Ku antigen with regard to its DNA binding activity and the phenotype obtained in the knockout experiments.
ACKNOWLEDGMENTS
This research was supported by the Medical Research Council of Canada (MT-7965) (M.Z.-H.) and the Cancer Research Society Inc. (G.B.P.). M.R. and D.M. are recipients of studentships from the Cancer Research Society, Inc.
REFERENCES
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson CW. DNA damage and the DNA-activated protein kinase. Trends Biochem Sci. 1993;18:433–437. doi: 10.1016/0968-0004(93)90144-c. [DOI] [PubMed] [Google Scholar]
- Anderson CW, Lees-Miller SP. The nuclear serine/threonine protein kinase DNA-PK. Crit Rev Eukaryotic Gene Exp. 1992;2:283–314. [PubMed] [Google Scholar]
- Bell D, Sabloff M, Zannis-Hadjopoulos M, Price G. Anticruciform DNA affinity purification of active mammalian origins of replication. Biochim Biophys Acta. 1991;1089:299–308. doi: 10.1016/0167-4781(91)90169-m. [DOI] [PubMed] [Google Scholar]
- Bliss TM, Lane DP. Ku selectively transfers between DNA molecules with homologous ends. J Biol Chem. 1997;272:5765–5773. doi: 10.1074/jbc.272.9.5765. [DOI] [PubMed] [Google Scholar]
- Blunt T, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cao QP, Pitt S, Leszyk J, Baril EF. DNA-dependent ATPase from HeLa cells is related to human Ku autoantigen. Biochemistry. 1994;33:8548–8557. doi: 10.1021/bi00194a021. [DOI] [PubMed] [Google Scholar]
- de Vries E, van Driel W, Bergsma WG, Arnberg AC, van der Vliet PC. HeLa nuclear protein recognizing DNA termini and translocating on DNA forming a regular DNA-mutimeric protein complex. J Mol Biol. 1989;208:65–78. doi: 10.1016/0022-2836(89)90088-0. [DOI] [PubMed] [Google Scholar]
- Diaz-Perez MJ, Wainer IW, Zannis-Hadjopoulos M, Price GB. Effects of drugs on in vitro DNA replication. J Cell Biochem. 1996;61:444–451. doi: 10.1002/(sici)1097-4644(19960601)61:3<444::aid-jcb11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Dvir A, Peterson SR, Knuth MW, Lu H, Dynan WS. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci USA. 1992;89:11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Winnacker EL. A putative homologue of the human autoantigen Ku from Saccharomyces cerevisiae. J Biol Chem. 1993;268:12895–12900. [PubMed] [Google Scholar]
- Feldmann H, Driller L, Meier B, Mages G, Kellermann J, Winnacker E-L. HDF2 the second subunit of the Ku homologue from Saccharomyces cerevisiae. J Biol Chem. 1996;271:27765–27769. doi: 10.1074/jbc.271.44.27765. [DOI] [PubMed] [Google Scholar]
- Finnie N, Gottlieb T, Hartley K, Jackson SP. Transcription factor phosphorylation by the DNA-dependent protein kinase. Biochem Soc Trans. 1993;21:930–935. doi: 10.1042/bst0210930. [DOI] [PubMed] [Google Scholar]
- Frappier L, Zannis-Hadjopoulos M. Autonomous replication of plasmids bearing monkey DNA origin-enriched sequences. Proc Natl Acad Sci USA. 1987;84:6668–6672. doi: 10.1073/pnas.84.19.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genersch E, Eckerskorn C, Lottspeich F, Herzog C, Kühn K, Pöschl E. Purification of the sequence-specific transcription factor CTCBF, involved in the control of human collagen IV genes: subunits with homology to Ku antigen. EMBO J. 1995;14:791–800. doi: 10.1002/j.1460-2075.1995.tb07057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts RC, Stamato TD. Absence of a Ku-like DNA end binding activity in the xrs double-strand DNA repair-deficient mutant. J Biol Chem. 1994;269:15981–15984. [PubMed] [Google Scholar]
- Giffin W, Torrance H, Rodda DJ, Préfontaine GG, Pope L, Haché RJG. Sequence-specific DNA binding by Ku autoantigen and its effects on transcription. Nature. 1996;380:265–268. doi: 10.1038/380265a0. [DOI] [PubMed] [Google Scholar]
- Giffin W, Kwast-Welfeld J, Rodda DJ, Préfontaine GG, Traykova-Andonova M, Zhang Y, Weigel NL, Lefebvre YA, Haché RJG. Sequence-specific DNA binding and transcription factor phosphorylation by Ku autoantigen/DNA-dependent protein kinase. J Biol Chem. 1997;272:5647–5658. doi: 10.1074/jbc.272.9.5647. [DOI] [PubMed] [Google Scholar]
- Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Griffith AJ, Craft J, Evans J, Mimori T, Hardin JA. Nucleotide sequence and genomic structure analyses of the p70 subunit of the human Ku autoantigen: evidence for a family of genes encoding Ku(p70)-related polypeptides. Mol Biol Rep. 1992;16:91–97. doi: 10.1007/BF00419754. [DOI] [PubMed] [Google Scholar]
- Gu Y, Jin S, Gao Y, Weaver DT, Ah FW. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA-end binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci USA. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J-O, Steen SB, Roth DB. Ku86 is not required for protection of signal ends or for formation of nonstranded V(D)J recombination products. Mol Cell Biol. 1997;17:2226–2234. doi: 10.1128/mcb.17.4.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricksen LA, Carter T, Dutta A, Wold MS. Phosphorylation of human replication protein A by the DNA-dependent protein kinase is involved in the modulation of DNA replication. Nucleic Acids Res. 1996;24:3107–3112. doi: 10.1093/nar/24.15.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby DB, Wensink PC. DNA binding specificities of YPF1, a Drosophila homolog to the DNA binding subunit of human DNA-dependent protein kinase, Ku. J Biol Chem. 1996;271:16827–16832. doi: 10.1074/jbc.271.28.16827. [DOI] [PubMed] [Google Scholar]
- Jeggo PA, Taccioli GG, Jackson SP. Menage à trois, double-strand break, V(D)J recombination and DNA-PK. Bioessays. 1995;17:949–957. doi: 10.1002/bies.950171108. [DOI] [PubMed] [Google Scholar]
- Kadonaga JT, Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci USA. 1986;83:5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G, Zannis-Hadjopoulos M, Martin RG. Cloning of nascent monkey DNA synthesized early in the cell cycle. Mol Cell Biol. 1985;5:721–727. doi: 10.1128/mcb.5.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner CU, Patil CK, Evans JW, Cuomo CA, Fried LM, Carter T, Oettinger MA, Brown JM. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- Klug J. Ku autoantigen is a potential major cause of nonspecific bands in electrophoretic mobility shift assays. BioTechniques. 1997;22:212–216. doi: 10.2144/97222bm02. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Gottlieb TM, Jackson SP, Grummt I. DNA-dependent protein kinase: a potent inhibitor of transcription by RNA polymerase I. Genes Dev. 1995;9:193–203. doi: 10.1101/gad.9.2.193. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry S, Zannis-Hadjopoulos M. Classes of autonomously replicating sequences are found among early-replicating monkey DNA. Biochim Biophys Acta. 1991;1088:234–244. doi: 10.1016/0167-4781(91)90059-u. [DOI] [PubMed] [Google Scholar]
- Li GC, Ouyang H, Li X, Nagasawa H, Little JB, Chen DJ, Ling CC, Fuks Z, Cordon-Cardo C. Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol Cell Biol. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- Lin S, Hickey RJ, Malkas LH. The isolation of a DNA synthesome from human leukemia cells. Leuk Res. 1997;21:501–512. doi: 10.1016/s0145-2126(96)00103-8. [DOI] [PubMed] [Google Scholar]
- Matheos D, Ruiz MT, Price GB, Zannis-Hadjopoulos M. Oct-1 enhances the in vitro replication of a mammalian autonomously replicating DNA sequence. J Cell Biochem. 1998;68:309–327. [PubMed] [Google Scholar]
- McConnell K, Dynan WS. The DNA-dependent protein kinase: a matter of life and (cell) death. Curr Opin Cell Biol. 1996;8:325–330. doi: 10.1016/s0955-0674(96)80005-6. [DOI] [PubMed] [Google Scholar]
- Milne GT, Jin S, Shannon KB, Weaver DT. Mutations in two Ku homologs define a DNA end-joining repair pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4189–4198. doi: 10.1128/mcb.16.8.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T, Akizuki M, Yamagata H, Inada S, Yoshida S, Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981;68:611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T, Hardin JA. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986;261:10375–10379. [PubMed] [Google Scholar]
- Nielsen T, Bell D, Lamoureux C, Zannis-Hadjopoulos M, Price GB. A reproducible method for the identification of human genomic DNA autonomously replicating sequences. Mol Gen Genet. 1994;242:280–288. doi: 10.1007/BF00280417. [DOI] [PubMed] [Google Scholar]
- Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig MC, Li GC. Requirement for Ku80 in growth and Ig V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig A, Sokol K, Burgman P, Li L, Li GC. Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage: the effects of ionizing radiation on growth, survival, and development. Proc Natl Acad Sci USA. 1997;94:13588–13593. doi: 10.1073/pnas.94.25.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochem AE, Skopac D, Costa M, Rabilloud T, Vuillard L, Simoncsits A, Giacca M, Falaschi A. Functional properties of the separate subunits of human DNA helicase II/Ku autoantigen. J Biol Chem. 1997;272:29919–29926. doi: 10.1074/jbc.272.47.29919. [DOI] [PubMed] [Google Scholar]
- Ofverstedt L-G, Hammarstrom K, Bagobin N, Hjeten S, Peterson U, Chattopadhyaya J. Rapid and quantitative recovery of DNA fragments from gels by displacement electrophoresis (isotachophoresis) Biochim Biophys Acta. 1984;782:120–126. doi: 10.1016/0167-4781(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Paillard S, Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991;19:5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard S, Strauss F. Site-specific proteolytic cleavage of Ku protein bound to DNA. Proteins. 1993;15:330–337. doi: 10.1002/prot.340150310. [DOI] [PubMed] [Google Scholar]
- Pearson CE, Frappier L, Zannis-Hadjopoulos M. Plasmids bearing mammalian DNA-replication origin-enriched (ors) fragments initiate semiconservative replication in a cell-free system. Biochim Biophys Acta. 1991;1090:156–166. doi: 10.1016/0167-4781(91)90096-5. [DOI] [PubMed] [Google Scholar]
- Pearson CE, Shihab-El-Deen A, Price GB, Zannis-Hadjopoulos M. Electron microscopic analysis of in vitro replication products of ors8, a mammalian origin enriched sequence. Somatic Cell Mol Genet. 1994;20:147–152. doi: 10.1007/BF02254755. [DOI] [PubMed] [Google Scholar]
- Philippe J. In: Methods in Molecular Biology. Harwood AJ, editor. Vol. 31. Totowa, NJ: Humana Press; 1994. pp. 349–361. [Google Scholar]
- Rathmell WK, Chu G. A DNA end-binding factor involved in double-strand break repair and V(D)J recombination. Mol Cell Biol. 1994;14:4741–4748. doi: 10.1128/mcb.14.7.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao BS, Zannis-Hadjopoulos M, Price GB, Reitman M, Martin RG. Sequence similarities among monkey ori-enriched (ors) fragments. Gene. 1990;87:233–242. doi: 10.1016/0378-1119(90)90307-d. [DOI] [PubMed] [Google Scholar]
- Reeves WH. Use of monoclonal antibodies for the characterization of novel DNA-binding proteins recognized by human autoimmune sera. J Exp Med. 1985;161:18–39. doi: 10.1084/jem.161.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MT, Pearson CE, Nielsen T, Price GB, Zannis-Hadjopoulos M. Cofractionation of HeLa cell replication proteins with Ors-binding activity. J Cell Biochem. 1995;58:221–236. doi: 10.1002/jcb.240580211. [DOI] [PubMed] [Google Scholar]
- Shakibai N, Kumar V, Eisenberg S. The Ku-like protein from Saccharomyces cerevisiae is required in vitro for the assembly of a stable multiprotein complex at a eukaryotic origin of replication. Proc Natl Acad Sci USA. 1996;93:11569–11574. doi: 10.1073/pnas.93.21.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smider V, Rathmell WK, Leiber MR, Chu G. Restoration of x-ray resistance and V(D)J recombination in mutant cells by Ku cDNA. Science. 1994;266:288–291. doi: 10.1126/science.7939667. [DOI] [PubMed] [Google Scholar]
- Stuiver MH, Celis JE, van der Vliet PC. Identification of nuclear factor IV/Ku autoantigen in a human 2D-gel protein database. FEBS Lett. 1991;282:189–192. doi: 10.1016/0014-5793(91)80474-h. [DOI] [PubMed] [Google Scholar]
- Suwa A, Hirakata M, Takeda Y, Jesch SA, Mimori T, Hardin J. DNA-dependent protein kinase (Ku protein-p350 complex) assembles on double-stranded DNA. Proc Natl Acad Sci USA. 1994;91:6904–6908. doi: 10.1073/pnas.91.15.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccioli GE, et al. Ku80: product of the XRCC5 gene. Role in DNA repair and V(D)J recombination. Science. 1994;265:1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- Todd A, Landry S, Pearson CE, Khoury V, Zannis-Hadjopoulos M. Deletion analysis of minimal sequence requirements for autonomous replication of ors8, a monkey early-replicating DNA sequence. J Cell Biochem. 1995;57:280–289. doi: 10.1002/jcb.240570212. [DOI] [PubMed] [Google Scholar]
- Torrance H, Giffin W, Rodda DJ, Pope L, Haché RJG. Sequence-specific binding of Ku autoantigen to single-stranded DNA. J Biol Chem. 1998;273:20810–20819. doi: 10.1074/jbc.273.33.20810. [DOI] [PubMed] [Google Scholar]
- Tóth EC, Marusic L, Ochem A, Patthy A, Pongor S, Giacca M, Falaschi A. Interactions of USF and Ku antigen with a human DNA region containing a replication origin. Nucleic Acids Res. 1993;21:3257–3263. doi: 10.1093/nar/21.14.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, et al. Human DNA helicase II: a novel DNA unwinding enzyme identified as the autoantigen. EMBO J. 1994;13:4991–5001. doi: 10.1002/j.1460-2075.1994.tb06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanatha JK, Baril EF. Single-stranded DNA-dependent ATPase from HeLa cells that stimulates DNA polymerase α-primase activity: purification and characterization of the ATPase. Biochemistry. 1990;29:8753–8759. doi: 10.1021/bi00489a036. [DOI] [PubMed] [Google Scholar]
- Wall L, deBoer E, Grosveld F. The human β-globin gene 3′ enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988;2:1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Weaver DT. What to do at an end: DNA double-strand-break repair. Trends Genet. 1995;11:388–392. doi: 10.1016/s0168-9525(00)89121-0. [DOI] [PubMed] [Google Scholar]
- Wu C, Friedlander P, Lamoreaux C, Zannis-Hadjopoulos M, Price GB. cDNA clones contain autonomous replication activity. Biochem Biophys Acta. 1993;1174:241–257. doi: 10.1016/0167-4781(93)90193-h. [DOI] [PubMed] [Google Scholar]
- Wu X, Lieber M. Protein-protein and protein-DNA interaction regions within the DNA end-binding protein Ku70-Ku86. Mol Cell Biol. 1996;16:5186–5193. doi: 10.1128/mcb.16.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneva M, Ochs R, McRorie DK, Zweig S, Bush H. Purification of an 86–70 kDa nuclear DNA-associated protein complex. Biochim Biophys Acta. 1985;841:22–29. doi: 10.1016/0304-4165(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Yaneva M, Kowalewski T, Lieber MR. Interaction of DNA-dependent protein kinase with DNA and with Ku: biochemical and atomic-force microscopy studies. EMBO J. 1997;16:5098–5112. doi: 10.1093/emboj/16.16.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M, Kaufmann G, Wang S-S, Lechner RL, Karawya E, Hesse J, Martin RG. Properties of some monkey DNA sequences obtained by a procedure that enriches for DNA replication origins. Mol Cell Biol. 1985;5:1621–1629. doi: 10.1128/mcb.5.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M, Nielsen TO, Todd A, Price GB. Autonomous replication in vivo and in vitro of a clone spanning the region of the DHFR origin of bidirectional replication (oriβ) Gene. 1994;151:273–277. doi: 10.1016/0378-1119(94)90670-x. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M, Pearson CE, Bell D, Mah D, Price GB. Structural and functional characterization of autonomously replicating mammalian origin-enriched sequences (ors) In: Hughes P, Fanning E, Kohiyama M, editors. DNA Replication: The Regulatory Mechanisms. Berlin: Springer-Verlag; 1992. pp. 107–116. [Google Scholar]
- Zhang W-W, Yaneva M. On the mechanism of Ku protein binding to DNA. Biochim Biophys Res Commun. 1992;186:574–579. doi: 10.1016/s0006-291x(05)80847-2. [DOI] [PubMed] [Google Scholar]