Abstract
Elevated external solute stimulates a conserved MAPK cascade that elicits responses that maintain osmotic balance. The yeast high-osmolarity glycerol (HOG) pathway activates Hog1 MAPK (mammalian ortholog p38α/SAPKα), which enters the nucleus and induces expression of >50 genes, implying that transcriptional up-regulation is necessary to cope with hyperosmotic stress. Contrary to this expectation, we show here that cells lacking the karyopherin required for Hog1 nuclear import or in which Hog1 is anchored at the plasma membrane (or both) can withstand long-term hyperosmotic challenge by ionic and nonionic solutes without exhibiting the normal change in transcriptional program (comparable with hog1Δ cells), as judged by mRNA hybridization and microarray analysis. For such cells to survive hyperosmotic stress, systematic genetic analysis ruled out the need for any Hog1-dependent transcription factor, the Hog1-activated MAPKAP kinases, or ion, glycerol, and water channels. By contrast, enzymes needed for glycerol production were essential for viability. Thus, control of intracellular glycerol formation by Hog1 is critical for maintenance of osmotic balance but not transcriptional induction of any gene.
Keywords: hyperosmotic stress, mutants, protein kinase, signal transduction, yeast
In budding yeast (Saccharomyces cerevisiae) as in all other eukaryotic cells, responses to many external stimuli are elicited via mitogen-activated protein kinase (MAPK) cascades (1). In S. cerevisiae, hyperosmotic shock triggers two upstream inputs, one acting through a transmembrane protein (Sln1) that leads to stimulation of MAPKKKs Ssk2 and Ssk22, and the other acting through another transmembrane protein (Sho1) and MAPKKK Ste11 (2). These two pathways converge by phosphorylating and activating the MAPKK Pbs2, which, in turn, phosphorylates and activates the MAPK Hog1. Once activated, Hog1 undergoes rapid (≤5 min) translocation into the nucleus that requires the carrier Nmd5 (an importin-β family member) (3, 4). Nuclear Hog1 is found in complexes at the promoters and throughout the coding sequences of many genes (5, 6) and influences chromatin remodeling and transcription (7–9). Shortly thereafter, Hog1 is largely dephosphorylated and exported back into the cytosol (10); however, the active enzyme remaining is required to maintain the osmo-adapted state even hours after shift to hyperosmotic conditions (4). Overall, activation of Hog1 by hyperosmotic challenge leads to increased production of intracellular glycerol as a counterbalancing osmolyte and, hence, is called the high-osmolarity glycerol (HOG) response (2, 8).
In addition to preserving cell viability under hyperosmotic stress, Hog1 is also important for maintaining signal fidelity. In hog1Δ cells, hyperosmotic shock inappropriately triggers induction of genes normally activated by the MAPKs Fus3 and Kss1 during the pheromone-stimulated mating response and the nutrient limitation-induced invasive growth response, respectively (4, 11). We have shown that continuous Hog1 catalytic function is required to prevent such “cross-talk” by using an allele (hog1-as) sensitive to acute inhibition of its kinase activity upon addition of an adenine analog (4-amino-1-tert-butyl-3-(1-naphthylmethyl) phenylpyrazolo[3,4-d]pyrimidine) (1-NM-PP1) (4).
Hog1 could block cross-talk by phosphorylating and down-regulating a cytosolic target essential for the other two pathways but not for HOG response (e.g., MAPKK Ste7). Alternatively, Hog1 might phosphorylate and down-regulate a nuclear factor that is necessary for the transcriptional response in the other two pathways but not in the HOG response (e.g., DNA-binding transactivator Ste12). To distinguish between these two extremes, we devised various means to preclude nuclear entry of Hog1. We found that cross-talk does not occur in such cells, indicating that restraining Hog1 in this fashion does not compromise signaling fidelity. Strikingly and unexpectedly, we also found that cells in which nuclear entry of Hog1 was prevented were still resistant to hyperosmotic stress, suggesting that the Hog1-mediated events that occur in the nucleus that have been reported by others are not required for growth under hyperosmotic conditions. While our work was in its final stages, Mettetal et al. (12) reported that a rapid event in HOG response does not require new protein synthesis, and, on this basis alone, they deduced that gene expression might not be necessary. However, this speculation was made on the basis of modeling derived from monitoring the kinetics of Hog1 nuclear entry and exit (in the absence and presence of cycloheximide) and did not include any direct measurements of transcription or any other experimental test of their inference. As demonstrated here by several independent approaches, the hallmark Hog1-dependent transcriptional effects normally seen in response to hyperosmotic challenge are indeed abrogated when nuclear entry of Hog1 is restrained by the methods we used, yet the cells are still able to grow under conditions of markedly elevated osmolarity. Thus, our work establishes that Hog1-mediated transcriptional response is not necessary for hyperosmotic stress resistance. Moreover, our further comprehensive genetic analysis indicates that the only Hog1-dependent process that seems critical for cell survival and growth on hyperosmotic medium is the ability to produce an elevated intracellular concentration of glycerol.
Results and Discussion
Hog1 Nuclear Import Factor Nmd5 Is Not Required for Signal Fidelity or Hyperosmotic Stress Resistance.
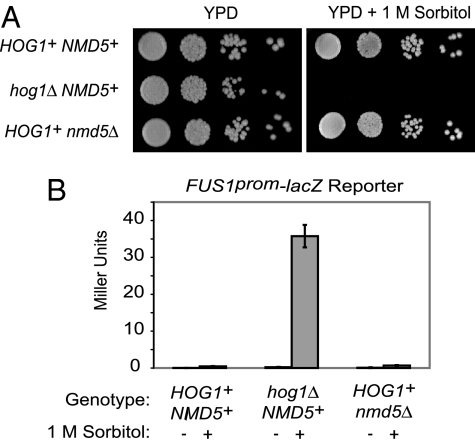
Prior work showed that the importin-β homolog Nmd5 is necessary for osmolyte-induced nuclear import of Hog1 (3); by using deconvolution fluorescence microscopy (4), we confirmed that activated Hog1-GFP fails to enter the nucleus in nmd5Δ cells. Nuclear entry of Hog1, its association with chromatin (5, 6), and ensuing transcriptional induction of numerous genes (7, 13–16) were thought necessary for cell survival upon hyperosmotic stress. Given the cumulative evidence for this inference, cells lacking Nmd5 should be osmosensitive. However, we found that HOG1+ NMD5+ and HOG1+ nmd5Δ cells grew equally well under hyperosmotic conditions (1 M sorbitol), unlike hog1Δ NMD5+ cells (Fig. 1A). Similarly, using the standard mating pathway-specific transcriptional reporter assay (FUS1prom-lacZ), we found that hog1Δ NMD5+ cells exhibited readily detectable cross-talk upon exposure to 1 M sorbitol, as expected, whereas HOG1+ nmd5Δ cells did not, just like HOG1+ NMD5 cells (Fig. 1B). Thus, Hog1 excluded from the nucleus in this manner is capable of both mounting an effective response to hyperosmotic challenge and preserving signaling specificity.
Fig. 1.
Absence of Nmd5 does not compromise osmoresistance or signaling fidelity. (A) Tenfold serial dilutions of HOG1+ NMD5+, hog1Δ NMD5+, and HOG1+ nmd5Δ cells were spotted onto plates containing YPD medium (Left) or YPD containing 1 M sorbitol (Right) and incubated for 48 h at 25°C. (B) HOG1+ NMD5+, hog1Δ NMD5+, and HOG1+ nmd5Δ cells carrying an integrated copy of a FUS1prom-lacZ reporter were grown to midexponential phase, collected, resuspended in either YPD (−) or YPD plus 1 M sorbitol (+) and, after 5 h, assayed for β-galactosidase activity.
Plasma Membrane-Tethered Hog1 Confers Hyperosmotic Stress Resistance and Maintains Signal Fidelity.
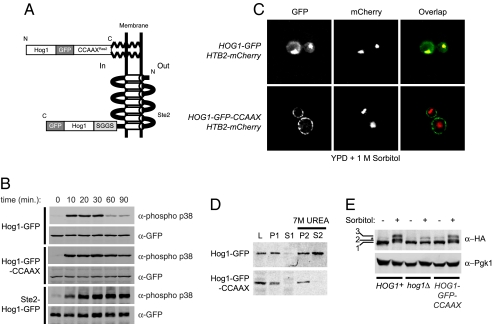
To corroborate the above results by an independent approach, we constructed two types of strains in which the only copy of Hog1 [or, where indicated, of an analog-sensitive derivative, Hog1(T100A) (hog1-as allele) (4)] was plasma membrane-tethered. In one, the nine C-terminal residues of Ras2 (abbreviated CCAAXRas2) were appended to the C terminus of Hog1-GFP and integrated in place of the endogenous HOG1 locus (Fig. 2A, Upper). In the other, Hog1-GFP was substituted for the C-terminal cytosolic tail of a polytopic integral membrane protein via fusion to residues 1–296 of the α-factor receptor (Ste2) (17) and integrated in place of the normal STE2 locus in hog1Δ cells (Fig. 2A, Bottom). Like wild-type Hog1, both fusion proteins were stably expressed and dually phosphorylated in response to hyperosmotic challenge (Fig. 2B). Consistent with their intended localization and the fact that the phosphatase mainly responsible for Hog1 deactivation (Ptp2) resides in the nucleus (18), the chimeric Hog1 alleles remained phosphorylated for a significantly longer period than wild-type Hog1 [Fig. 2B and supporting information (SI) Fig. S1].
Fig. 2.
Hog1-GFP-CCAAXRas2 is plasma membrane-associated and catalytically functional. (A) Schematic depiction of Hog1-GFP-CCAAXRas2 and Ste2 (1–296)-Hog1-GFP. S-palmitoyl and S-farnesyl substituents tethering the Hog1-GFP-CCAAXRas2 chimera to the plasma membrane are shown as bold zig-zag lines. (B) Cells expressing Hog1-GFP, Hog1-GFP-CCAAXRas2, or Ste2 (1–296)-Hog1-GFP were grown to midexponential phase in YPD and harvested at the indicated times after treatment with 1 M sorbitol. Extracts were immunoblotted with anti-phospho-p38 antibodies to detect the dually phosphorylated form of these Hog1 chimeras and with anti-GFP antibodies to detect the total amount of each chimera. (C) HOG1-GFP or HOG1-GFP-CCAAXRas2 cells expressing a histone H2B isoform (Htb2) tagged with mCherry (39) to mark the nucleus were grown to midexponential phase in YPD, treated with 1 M sorbitol for 10 min, and examined by deconvolution microscopy. (D) Samples of the cells shown in C were ruptured, clarified, fractionated, and immunoblotted with anti-GFP antibodies. (E) HOG1+, hog1Δ, or HOG1-GFP-CCAAXRas2 cells carrying an integrated copy of RCK2-(HA)3 were grown to midexponential phase in YPD and harvested 10 min after treatment with 1 M sorbitol. Extracts were immunoblotted with an anti-HA epitope mAb or with anti-Pgk1 antibodies as a control for equivalent loading. Row 1, unmodified Rck2; row 2, and row 3, hyperphosphorylated Rck2; asterisk, background band present in all lanes.
For simplicity and because they were expressed from the endogenous HOG1 promoter, we present here data obtained primarily with the Hog1-GFP-CCAAXRas2 chimera. However, all experiments were also carried out with the Ste2 (1–296)-Hog1-GFP chimeras and with the hog1-as alleles of each chimera with very similar results. As intended, and unlike wild-type Hog1-GFP, Hog1-GFP-CCAAXRas2 was membrane-localized both before (data not shown) and, most importantly, after hyperosmotic shock (Fig. 2C). Similarly, biochemical fractionation of cell extracts confirmed that Hog1-GFP-CCAAXRas2 remained firmly in the particulate plasma membrane-containing fraction (P2) even after extraction with 7 M urea, unlike wild-type Hog1-GFP (Fig. 2D). Despite its artificial localization, Hog1-GFP-CCAAXRas2 supported a normal level of phosphorylation of the Hog1-activated protein kinase Rck2, as assessed by gel mobility shift, indicating that it was catalytically active (Fig. 2E).
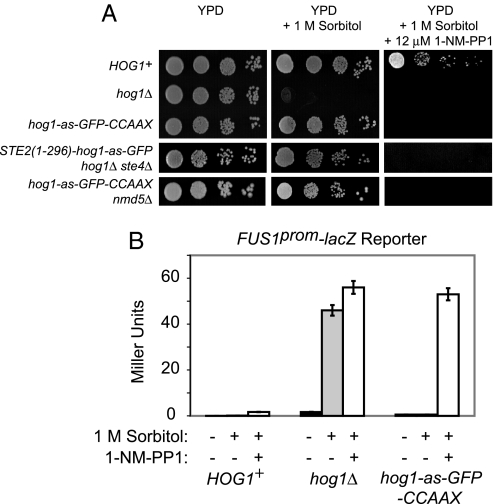
Most strikingly, either form of membrane-tethered Hog1 permitted robust growth under conditions of hyperosmotic stress (Fig. 3A), even in nmd5Δ cells (Fig. 3A Bottom). This survival required Hog1 catalytic activity because cells expressing either hog1-as-GFP-CCAAXRas2 or Ste2 (1–296)-hog1-as-GFP were unable to grow on 1 M sorbitol when 1-NM-PP1 was present (Fig. 3A Right). Moreover, cells expressing the Hog1-GFP-CCAAXRas2 chimeras (Fig. S2) or the Ste2 (1–296)-Hog1-GFP chimeras (data not shown) grew equivalently to HOG1+ cells when challenged with any of the standard solutes typically used to stimulate the HOG response: sorbitol (up to 2 M), KCl (up to 1 M), and NaCl (up to 1 M) (Fig. S2).
Fig. 3.
Membrane-tethered Hog1 confers osmoresistance and maintains signaling fidelity. (A) Ability of strains of the indicated genotypes to grow on YPD, YPD + 1 M sorbitol, or YPD + 1 M sorbitol containing 12 μM 1-NM-PP1 was assessed as in Fig. 1A. (B) HOG1+, hog1Δ, or hog1-as-GFP-CCAAXRas2 cells carrying an integrated copy of a FUS1prom-lacZ reporter were grown to midexponential phase, collected, and resuspended in either YPD (black bars), YPD + 1 M sorbitol (gray bars), or YPD + 1 M sorbitol containing 12 μM 1-NM-PP1 (open bars). After 5 h, samples were assayed for β-galactosidase activity.
Additionally, upon exposure to hyperosmotic conditions, cross-talk to the mating pheromone response pathway was fully squelched in both HOG1+ and hog1-as-GFP-CCAAXRas2 cells, unlike in hog1Δ cells (Fig. 3B). This result further confirms that the chimera is fully functional because, as we have shown elsewhere (4), cells that carry hog1 point mutations that compromise (but do not eliminate) catalytic activity display intermediate levels of cross-talk. As expected, addition of 1-NM-PP1 permitted cross-talk in hog1-as-GFP-CCAAXRas2 cells at a level equivalent to that in hog1Δ cells (Fig. 3B). Thus, plasma membrane-tethered Hog1 is able to maintain signal fidelity completely, indicating that the substrate on which Hog1 acts to block inadvertent activation of Fus3 and Kss1 is membrane-associated or cytosolic.
To control further for the efficacy of our membrane-tethering approach and for the specificity of our results, we asked whether another MAPK thought to induce a mandatory transcriptional response could also elicit an efficacious output when membrane-tethered. When restrained in the same way (by a C-terminal CCAAXRas2 box), a GFP-tagged version of Fus3 was unable to restore proficient mating to fus3Δ kss1Δ cells (Fig. S3A), even when markedly overexpressed and fully activated in response to pheromone (Fig. S3B), whereas normal Fus3-GFP or a nearly identical derivative (Fus3-GFP-ssAAX) that cannot undergo lipophilic modification supported efficient mating. Thus, unlike Hog1 in the HOG response (Fig. 3A), Fus3 nuclear entry and activation of Ste12-dependent genes (19) are required for the mating response.
Transcriptional Responses Are Abrogated by Membrane Tethering of Hog1.
Because cells lacking Nmd5 or in which Hog1 is plasma membrane-tethered (or both) are still osmoresistant, either nuclear entry and Hog1-regulated gene expression are not essential for osmoresistance, or some factor accessible to the tethered Hog1 is phosphorylated, enters the nucleus, and executes the necessary transcriptional program. As one approach to distinguish between these possibilities, we mutated in cells expressing HOG1-GFP-CCAAXRas2 known factors that might indirectly transmit the activated but membrane-restricted Hog1 signal to the nucleus. Hog1 phosphorylates and activates protein kinase Rck2 (20, 21). However, deletion of either RCK2 or its paralog RCK1 (or both) did not render HOG1-GFP-CCAAXRas2 cells osmosensitive (Fig. S4). It is well documented that certain eukaryotic transcription factors undergo nucleocytoplasmic shuttling. Transcriptional regulators reported to mediate Hog1-dependent gene regulation in response to hyperosmotic shock include: Hot1 (22) and its paralog Msn1 (16); Msn2 (23) and its paralog Msn4 (13); Smp1 (24); and Sko1 (25). However, deletion of each of these transcription factors, or even of related pairs, such as msn2Δ msn4Δ (Fig. S5) or hot1Δ msn1Δ (Table S1), did not render HOG1-GFP-CCAAXRas2 cells osmosensitive. It has also been reported (26) that rapid rebinding of regulatory proteins to chromatin and the resumption of transcription after hyperosmotic shock require action of the plasma membrane Na+/H+ antiporter, Nha1, working in conjunction with an outward-rectifying K+ channel, Tok1, to restore ionic balance. However, deletion of neither of these genes caused cells expressing Hog1-GFP-CCAAXRas2 to be osmosensitive (Table S1). Altogether, these results suggest that the ability of Hog1 to confer osmoresistance does not require its role in transcriptional regulation.
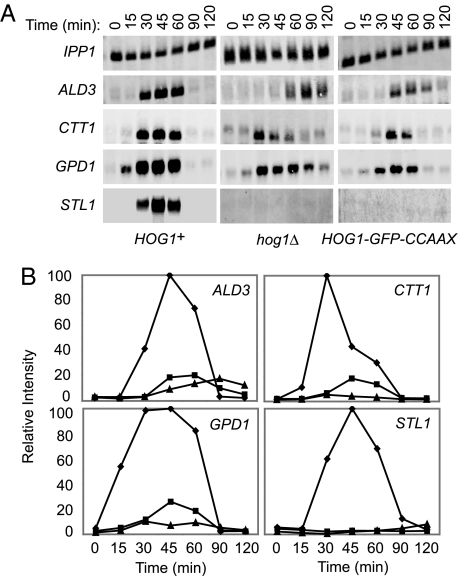
To confirm directly that the osmoresistance of cells containing membrane-anchored Hog1 occurs without the normal transcriptional response, we measured the transcript levels of four genes (ALD3, CTT1, GPD1, and STL1) known to be induced by hyperosmotic stress. As expected, all four transcripts were dramatically up-regulated in HOG1+ cells by 15–30 min after exposure to 1 M sorbitol, peaking at ≈45 min (Fig. 4). In contrast, HOG1-GFP-CCAAXRas2 cells did not express any of these genes significantly differently from the low level observed in hog1Δ cells. This was true for genes, like STL1, that are strictly Hog1-dependent (7) and for genes, like GPD1, that are expressed at a significant basal level but require Hog1 action for maximal induction after hyperosmotic shock (16) (Fig. 4).
Fig. 4.
Membrane tethering of Hog1 prevents gene induction by hyperosmotic stress. (A) HOG1+, hog1Δ, and Hog1-GFP-CCAAXRas2-expressing cells were grown to midexponential phase in YPD and harvested at the indicated times after exposure to 1 M sorbitol. mRNA was isolated and resolved by electrophoresis in a denaturing agarose gel, transferred to nylon membranes, and hybridized to biotinylated probes specific for the ALD3, CTT1, GPD1, and STL1 transcripts, which were detected by incubation with infrared dye-tagged streptavidin. A probe specific for the IPP1 transcript served as a control for mRNA recovery and equivalent loading and for normalization to quantify the relative intensities of the ALD3, CTT1, GPD1, and STL1 signals, plotted in B. Diamonds, HOG1+; triangles, hog1Δ; squares, HOG1-GFP-CCAAXRas2.
To corroborate these findings at the whole-genome level, we performed microarray analysis on the global transcriptome of HOG1-GFP, hog1Δ, and HOG1-GFP-CCAAXRas2 cells before and after treatment with 1 M sorbitol at 25°C for 60 min. We identified the 30 most Hog1-dependent genes in our dataset as those that showed a reproducible and statistically significant increase, as judged by the statistical analysis of microarrays method (27), of at least 3-fold after hyperosmotic challenge in HOG1-GFP cells and <3-fold in hog1Δ cells in three independent experiments (Fig. S6, Upper). Our gene set comprises many genes pinpointed in prior analyses (7, 14). The overall expression profile of HOG1-GFP-CCAAXRas2 cells was much more highly correlated with that of hog1Δ cells than with that of HOG1-GFP cells (Fig. S6b) (see SI Materials and Methods for analysis of the differences). Likewise, the response of HOG1-GFP-CCAAXRas2 nmd5Δ was well correlated with hog1Δ (and HOG1-GFP-CCAAXRas2 NMD5+) cells and not with HOG1-GFP cells, as judged by the Pearson correlation coefficient (data not shown).
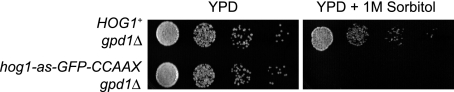
To verify further that Hog1-dependent transcriptional induction does not occur in HOG1-GFP-CCAAX cells, we also examined the effect of deleting GPD1, a cytosolic NADH-dependent oxidoreductase (sn-glycerol-3P dehydrogenase) essential for glycerol production (Fig. S7). In the absence of GPD1, cells rely on expression of GPD2, which encodes an alternative cytosolic isoform that is up-regulated under conditions of hyperosmotic stress (16). Indeed, HOG1+ cells lacking GPD1 retained osmoresistance because transcriptional induction of GPD2 compensates, whereas HOG1-GFP-CCAAX cells were rendered osmosensitive by deletion of GPD1 (Fig. 5). These findings provide dramatic confirmation that cells expressing HOG1-GFP-CCAAXRas2 are unable to induce gene expression at a physiologically significant level.
Fig. 5.
Transcriptional induction of GPD2 does not occur in cells expressing membrane-tethered Hog1. Ability of HOG1+ gpd1Δ and HOG1-GFP-CCAAXRas2 gpd1Δ cells to grow at 30°C on YPD, or YPD plus 1 M sorbitol, was assessed as in Fig. 1A.
Glycerol Production Is Critical for Survival Under Hyperosmotic Stress.
Our results show that nuclear translocation of Hog1 and the effects on transcription that it normally causes are not necessary for its role in hyperosmotic stress resistance. What cellular processes does Hog1 influence to mediate osmoresistance? If unable to counterbalance high external osmolarity, cells will plasmolyze because of loss of H2O through water channels (aquaporins) (28). However, lack of Hog1-dependent closure of such channels cannot be responsible for the inviability of hog1Δ cells under hyperosmotic stress conditions because deletion of either or both of the aquaporin genes (AQY1 and AQY2) (Fig. S7) neither permitted growth of hog1Δ cells on 1 M sorbitol (data not shown) nor enhanced osmoresistance of HOG1-GFP-CCAAXRas2 cells (Table S1). Similarly, glycerol passes in and out of cells via glyceroporins (FPS1 and FPS2 gene products) related to aquaporins (Fig. S7) (28). Two compounds toxic to yeast at high concentration, arsenite and acetate, also enter cells via the glyceroporins and, reportedly, Hog1-dependent phosphorylation of Fps1 at Thr-231 closes the channel and promotes its endocytosis, thereby conferring resistance to these compounds (29, 30). However, even when HOG1-GFP-CCAAXRas2 cells carried as the sole source of this channel a nonphosphorylatable allele, Fps1(T231A), which is recalcitrant to endocytosis (29) and thought to be constitutively “open” (31), they grew as well as wild-type cells in the presence of 1 M sorbitol (Table S1).
In contrast, the fact that a gpd1Δ mutation prevented survival of HOG1-GFP-CCAAXRas2 cells under hyperosmotic conditions (Fig. 5) provided a clue that one key process regulated by Hog1 must be glycerol production. The major route to produce glycerol involves triose-phosphate isomerase (Tpi1)-dependent conversion of glyceraldehyde 3-phosphate (GA3P) to dihydroxyacetone phosphate (DHAP), which is then reduced by the sn-glycerol-3-phosphate dehydrogenases (Gpd1 and Gpd2), generating glycerol 3-phosphate, followed by phosphate removal by two related phosphatases (Gpp1/Rhr2 and Gpp2/Hor2) (Fig. S7). As with gpd1Δ, a tpi1Δ mutation or a gpp1Δ gpp2Δ double mutation (Table S1) prevented growth of HOG1-GFP-CCAAXRas2 cells on medium with (but not without) 1 M sorbitol, just like mutations that eliminate central components of the Hog1 activation pathway itself (e.g., pbs2Δ and sho1Δ ssk1Δ) (Table S1). Thus, of many candidate genes tested (Table S1), only those whose products participate directly in glycerol production are necessary for HOG1-GFP-CCAAXRas2 cells to survive hyperosmotic stress.
At what level might Hog1-mediated phosphorylation promote glycerol production? Gpd1, Gpd2, Gpp1, and Gpp2 all lack consensus MAPK sites (SP or TP). Tpi1 contains one site in a solvent-exposed loop situated near the sites of mutations that influence the rate of interconversion of GA3P to DHAP (32). Phosphorylation here by Hog1 might enhance the rate of DHAP generation and, thus, glycerol synthesis. However, HOG1-GFP-CCAAXRas2 cells in which TPI1 was replaced with a fully functional, but nonphosphorylatable allele, Tpi1(T177A), were not osmosensitive (Table S1).
Another means to divert more carbon into glycerol would be to prevent further glycolytic breakdown of triose-P. Consistent with this reaction being an important control point in carbon flow, conversion of GA3P into 1,3-bisphosphoglycerate in S. cerevisiae (as in certain mammals, e.g., rat) is catalyzed by three glyceraldehyde-3-phosphate dehydrogenase isoforms (Tdh1, Tdh2, and Tdh3; 88–96% pairwise identity) of identical length (332 residues) (Fig. S7). Tdh1, Tdh2, and Tdh3 contain a single identical MAPK consensus site (QLSP) near their C termini (and Tdh1 has an additional site). Indeed, Tdh3 was among a few proteins identified in a recent global screen for in vitro Hog1 substrates conducted with the use of a different as allele, Hog1(T100G) (33); however, the nature and physiological relevance of the apparent modification were not explored, and whether Tdh1 and Tdh2 were also targets was not reported. Using purified recombinant Tdh1 and Tdh3, we have now confirmed and extended the observation that these enzymes are Hog1 substrates (data not shown), and we are presently examining whether Hog1-mediated phosphorylation affects their oligomeric state (they are obligate tetramers), inhibits their activity, or causes their selective degradation. Any of these mechanisms could provide a means to rapidly divert more GA3P into DHAP for glycerol production. Consistent with a role for Hog1-mediated phosphorylation in modulating their activity, we found that, compared with HOG1+ TDH1+ TDH2+ TDH3+ cells, HOG1+ Tdh1(T139A S302A) Tdh2(S302A) Tdh3(S302A) cells (carrying nonphosphorylatable alleles at all three loci) grow significantly more slowly on glucose-containing medium after (but not before) challenge with 2 M sorbitol, albeit not as slowly as hog1Δ TDH1+ TDH2+ TDH3+ cells (data not shown). However, we did not expect dramatic effects because the demands of glycerol formation require that Hog1 must also act at another level. Conversion of GA3P to 1,3-bisphosphoglycerate produces the NADH derived from the breakdown of glucose and provides the reducing power for the reduction of DHAP to glycerol 3-phosphate (34). Hence, Hog1-mediated inhibition of Tdh1, Tdh2, and/or Tdh3 alone is not sufficient to explain the increased rate of diversion of DHAP into glycerol because total blockade of these enzymes would deplete NADH. Hence, in addition to reducing glycolytic flux of GA3P, Hog1 must also impede mitochondrial NADH oxidation and/or other enzymes whose action drains the NADH pool, possibilities we are exploring now.
In any event, given the very large body of literature that has been interpreted overwhelming by others as evidence for an essential role of Hog1 in regulating transcription, our finding that such is not the case is remarkable. Nuclear entry of Hog1, its association with chromatin (5, 6), and the ensuing transcriptional induction of at least 50 genes (7, 13–16) were all thought to be necessary for cells to survive hyperosmotic stress. Contrary to this expectation, the findings we have presented here demonstrate that the paramount role of Hog1 is not its effect on transcription, but its role in establishing the metabolic conditions for elevated glycerol production. Nonetheless, in normal cells, Hog1-dependent transcriptional changes do occur. Why? It seems likely that Hog1-mediated transcriptional induction represents a fail-safe mechanism to ensure more than adequate levels of enzymes, e.g., Gpd2, critical for glycerol production. Moreover, it should be noted that many of the most robustly induced Hog1-dependent genes, e.g., Gre2 (Fig. S6), are involved in the glutathione-dependent detoxification of methylglyoxal, an unavoidable and highly reactive side-product of the reaction catalyzed by Tpi1 (Fig. S7). Induction of such genes during sustained elevated generation of the DHAP needed for glycerol production presumably promotes better long-term survival by reducing the cumulative damage that this compound could cause. Nevertheless, as we show, such changes in gene expression are not critical to short-term survival, and it would seem to make good physiological sense that the first wave of defense to acute hyperosmotic stress should not depend on time-consuming processes, like transcription and translation, but rather should act primarily through immediate effects on preexisting proteins.
Materials and Methods
Strains and Growth Conditions.
S. cerevisiae strains (Table S2) were grown in standard rich (YPD) or a synthetic complete (SC) medium (35) at 30° with 2% glucose as the carbon source, unless otherwise indicated, and lacking appropriate nutrients to maintain selection for plasmids (Table S3). Where necessary, 5-fluoroorotic acid (36) or geneticin (Invitrogen) were added to select for integrative transformants. d-Sorbitol (Sigma) was added at 1 or 2 M final concentration.
Measurement of Reporter Gene Expression.
The integrated FUS1prom-lacZ reporter gene has been described in ref. 11. Cells carrying FUS1prom-lacZ were grown to midexponential phase, collected by centrifugation, resuspended in fresh medium with or without 1 M sorbitol and/or 12 μM 1-NM-PP1 incubated for 5 h, and the amount of β-galactosidase expressed assessed by using a colorimetic assay (37). The values given (A420 nm) represent the average (with error bars indicating the SE) of three independent trials each conducted in triplicate.
Microscopy.
Optical sections (0.2 μm) of live exponentially growing cells that were resuspended in YPD medium with or without 1 M sorbitol for 10 min were viewed by deconvolution fluorescence microscopy using a DeltaVision Spectris system (Applied Precision) under a 100× objective lens. Image capture, deconvolution, and adjustments to brightness and contrast were performed by using SoftWoRx software (Applied Precision). For clarity and to permit ready cross-comparison, raw images were cropped and adjusted to equivalent brightness and contrast by using Photoshop CS (Adobe Systems).
Cell Extracts and Immunoblotting.
Yeast cells were grown to midexponential phase in YPD, harvested by centrifugation, resuspended in lysis buffer [20 mM Tris·HCl (pH 7.5), 50 mM NaCl, 5 mM MgCl2, 1 mM β-glycerolphosphate, and the manufacturer's recommended amount of a commercial protease-inhibitor mix (Complete EDTA-free; Roche Diagnostics)], and ruptured by vigorous vortex mixing with an equivalent volume of glass beads (450–500 nm) for three 20-s intervals each separated by 3 min of cooling on ice. Unbroken cells, glass beads, and other debris were removed by centrifugation at 1,000 rpm for 5 min in a SS34 rotor (Sorvall Instruments). The resulting crude lysate (L) was subjected to centrifugation for 1 h at 43,000 rpm in the TLA-100.3 rotor of a table top ultracentrifuge (TL100; Beckman Instruments), yielding a supernatant fraction (S1) and a pellet (P1). After removal of the S1 fraction, the P1 particulate fraction was resuspended by trituration in ice-cold lysis buffer and, after removal of a small aliquot, adjusted to a final concentration of 7 M urea, incubated at room temperature with gentle agitation for 20 min, and then subjected to centrifugation again for 1 h at 43,000 rpm, yielding a second supernatant fraction (S2) and a second pellet (P2). Samples of the L, S1, P1, S2, and P2 fractions were solubilized by resuspension in 1× SDS/PAGE loading buffer [5% SDS, 0.1 M Tris·HCl (pH 7.5), 5% glycerol, 0.07 M 2-mercaptoethanol, and 0.02 mM bromophenol blue], boiled for 5 min, and clarified by centrifugation in a microcentrifuge. Samples of the resulting clarified preparations were resolved by SDS/PAGE on an 8% gel and transferred to a nitrocellulose filter, which was incubated in Odyssey blocking buffer (Li-Cor Biosciences) for 1 h at room temperature and then overnight with a mouse monoclonal antibody (mAb) against GFP (Roche). Filter-bound primary antibodies were detected by using secondary infrared dye-conjugated anti-mouse IgG antibodies (Molecular Probes) and visualized by using an Odyssey infrared imaging system (Li-Cor Biosciences).
To determine the total cellular content of Hog1-GFP or Hog1-GFP-CCAAX and the proportion that was activated (dually phosphorylated) in exponentially growing cells before and after their exposure to 1 M sorbitol, samples were withdrawn at various times and immediately frozen in liquid N2, and then lysed by a rapid alkaline lysis procedure followed by trichloroacetic acid precipitation, as described in detail in SI Materials and Methods. The resulting denatured protein was acetone-washed to remove excess trichloroacetic acid, neutralized, solubilized in SDS/PAGE sample buffer, boiled, resolved by SDS/PAGE, and transferred to nitrocellulose filters, as above. Total Hog1-GFP or Hog1-GFP-CCAAX were decorated with the anti-GFP mouse mAb (Roche) and activated forms with a rabbit anti-phospho-p38 MAPK mAb (Cell Signaling Technology) that specifically detects dually phosphorylated Hog1 (4). Filter-bound primary antibodies were then visualized by using as the secondary antibodies infrared dye-conjugated anti-mouse IgG and anti-rabbit IgG antibodies, respectively, and quantified by infrared imaging, as above. The primary antibody for Rck2-(HA)3 detection was a mouse anti-HA epitope mAb (6E2; Cell Signaling Technology).
mRNA Hybridization Analysis.
HOG1+-, hog1Δ-, and Hog1-GFP-CCAAXRas2-expressing cells were grown to midexponential phase in YPD, collected by centrifugation, and resuspended in YPD + 1 M sorbitol. At the indicated time points, samples (≈3 × 108 cells) were recollected and frozen in liquid N2. Total RNA was extracted from each sample by using the RiboPure-Yeast total RNA isolation kit (Ambion), and then the mRNA fraction of each sample was purified by using the MicroPoly(A) Purist kit (Ambion). The nucleotide concentration of the resulting preparations was measured by using a Nanodrop spectrophotometer (NanoDrop Technologies). The mRNA samples (≈2 mg) were denatured with formaldehyde, resolved by electrophoresis in a 1.5% denaturing agarose gel (38), transferred to Hybond-N+ membrane filters (GE Healthcare), and the resulting replicas were washed by using NorthernMax reagents (Ambion), following the manufacturer's instructions. The filters were blocked with Odyssey blocking buffer and incubated with biotinylated DNA probes for the genes of interest, which were prepared by PCRs containing biotin-16-dUTP (Roche). Filter-bound probes were detected by using streptavidin-IRDye 800CW (Rockland Immunochemical) as described under “Applications” for “Northern blots” at http://biosupport.licor.com/support (Li-Cor), and signal intensities were quantified by infrared imaging.
Supplementary Material
Acknowledgments.
We thank Adam Carroll and M. Paige Nittler (University of California, San Francisco) and Jess Leber (University of California, Berkeley) for materials and advice on microarrays, and all members of the Thorner laboratory for helpful comments. This work was supported by National Institutes of Health (NIH) Kirschstein–National Research Service Award Postdoctoral Fellowship GM68343 (to P.J.W.), by NIH Predoctoral Traineeship GM07232 (to J.C.P. and R.E.C.), and by NIH Research Grant GM21841 (to J.T.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE8703).
This article contains supporting information online at www.pnas.org/cgi/content/full/0805797105/DCSupplemental.
References
- 1.Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: Lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1773:1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westfall PJ, Ballon DR, Thorner J. When the stress of your environment makes you go HOG wild. Science. 2004;306:1511–1512. doi: 10.1126/science.1104879. [DOI] [PubMed] [Google Scholar]
- 3.Ferrigno P, Posas F, Koepp D, Saito H, Silver PA. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J. 1998;17:5606–5614. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westfall PJ, Thorner J. Analysis of MAPK signaling specificity in response to hyperosmotic stress: Use of an analog-sensitive HOG1 allele. Eukaryot Cell. 2006;5:1215–1228. doi: 10.1128/EC.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- 6.Proft M, et al. The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol Cell. 2006;23:241–250. doi: 10.1016/j.molcel.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 7.O'Rourke SM, Herskowitz I. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol Biol Cell. 2004;15:532–542. doi: 10.1091/mbc.E03-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheikh-Hamad D, Gustin MC. MAP kinases and the adaptive response to hypertonicity: Functional preservation from yeast to mammals. Am J Physiol. 2004;287:F1102–F1110. doi: 10.1152/ajprenal.00225.2004. [DOI] [PubMed] [Google Scholar]
- 9.Zapater M, Sohrmann M, Peter M, Posas F, De Nadal E. Selective requirement for SAGA in Hog1-mediated gene expression depending on the severity of the external osmostress conditions. Mol Cell Biol. 2007;27:3900–3910. doi: 10.1128/MCB.00089-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiser V, Ruis H, Ammerer G. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:1147–1161. doi: 10.1091/mbc.10.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Rourke SM, Herskowitz I. The Hog1 MAPK prevents cross-talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 1998;12:2874–2886. doi: 10.1101/gad.12.18.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mettetal JT, Muzzey D, Gómez-Uribe C, van Oudenaarden A. The frequency dependence of osmo-adaptation in Saccharomyces cerevisiae. Science. 2008;319:482–484. doi: 10.1126/science.1151582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasch AP, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirasawa T, et al. Comparison of transcriptional responses with osmotic stresses induced by NaCl and sorbitol additions in Saccharomyces cerevisiae using DNA microarray. J Biosci Bioeng. 2006;102:568–571. doi: 10.1263/jbb.102.568. [DOI] [PubMed] [Google Scholar]
- 15.Proft M, Gibbons FD, Copeland M, Roth FP, Struhl K. Genomewide identification of Sko1 target promoters reveals a regulatory network that operates in response to osmotic stress in Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:1343–1352. doi: 10.1128/EC.4.8.1343-1352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rep M, et al. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol Cell Biol. 1999;19:5474–5485. doi: 10.1128/mcb.19.8.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reneke JE, Blumer KJ, Courchesne WE, Thorner J. The carboxyl-terminal segment of the yeast α-factor receptor is a regulatory domain. Cell. 1988;55:221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- 18.Wurgler-Murphy SM, Maeda T, Witten EA, Saito H. Regulation of the. Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol Cell Biol. 1997;17:1289–1297. doi: 10.1128/mcb.17.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts C, et al. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science. 2000;287:873–880. doi: 10.1126/science.287.5454.873. [DOI] [PubMed] [Google Scholar]
- 20.Bilsland-Marchesan E, Arino J, Saito H, Sunnerhagen P, Posas F. Rck2 kinase is a substrate for the osmotic stress-activated mitogen-activated protein kinase Hog1. Mol Cell Biol. 2000;20:3887–3895. doi: 10.1128/mcb.20.11.3887-3895.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teige M, Scheikl E, Reiser V, Ruis H, Ammerer G. Rck2, a member of the calmodulin-protein kinase family, links protein synthesis to high osmolarity MAP kinase signaling in budding yeast. Proc Natl Acad Sci USA. 2001;98:5625–5630. doi: 10.1073/pnas.091610798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alepuz PM, de Nadal E, Zapater M, Ammerer G, Posas F. Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 2003;22:2433–2442. doi: 10.1093/emboj/cdg243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rep M, Krantz M, Thevelein JM, Hohmann S. The transcriptional response of Saccharomyces cerevisiae to osmotic shock: Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J Biol Chem. 2000;275:8290–8300. doi: 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- 24.de Nadal E, Casadome L, Posas F. Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol Cell Biol. 2003;23:229–237. doi: 10.1128/MCB.23.1.229-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proft M, Struhl K. Hog1 kinase converts the Sko1–Cyc8–Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol Cell. 2002;9:1307–1317. doi: 10.1016/s1097-2765(02)00557-9. [DOI] [PubMed] [Google Scholar]
- 26.Proft M, Struhl K. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell. 2004;118:351–361. doi: 10.1016/j.cell.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson N, Filipsson C, Becit E, Brive L, Hohmann S. Aquaporins in yeasts and filamentous fungi. Biol Cell. 2005;97:487–500. doi: 10.1042/BC20040144. [DOI] [PubMed] [Google Scholar]
- 29.Mollapour M, Piper PW. Hog1 MAP kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol. 2007;27:6446–6456. doi: 10.1128/MCB.02205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorsen M, et al. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol Biol Cell. 2006;17:4400–4410. doi: 10.1091/mbc.E06-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlgren S, et al. Identification of residues controlling transport through the yeast aquaglyceroporin Fps1 using a genetic screen. Eur J Biochem. 2004;271:771–779. doi: 10.1111/j.1432-1033.2004.03980.x. [DOI] [PubMed] [Google Scholar]
- 32.Joseph-McCarthy D, Rost LE, Komives EA, Petsko GA. Crystal structure of the mutant yeast triosephosphate isomerase in which the catalytic base glutamic acid 165 is changed to aspartic acid. Biochemistry. 1994;33:2824–2829. doi: 10.1021/bi00176a011. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Shah K. Dissecting yeast Hog1 MAP kinase pathway using a chemical genetics approach. FEBS Lett. 2007;581:1209–1216. doi: 10.1016/j.febslet.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Rigoulet M, et al. Organization and regulation of the cytosolic NADH metabolism in the yeast Saccharomyces cerevisiae. Mol Cell Biochem. 2004;256–257:73–81. doi: 10.1023/b:mcbi.0000009888.79484.fd. [DOI] [PubMed] [Google Scholar]
- 35.Rose MD, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1990. [Google Scholar]
- 36.Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- 37.Stern M, Jensen R, Herskowitz I. Five SWI genes are required for expression of the HO gene in yeast. J Mol Biol. 1984;178:853–868. doi: 10.1016/0022-2836(84)90315-2. [DOI] [PubMed] [Google Scholar]
- 38.Skopp RN, Lane LC. Agarose gel electrophoresis of denatured RNA with silver staining. Anal Biochem. 1988;169:132–137. doi: 10.1016/0003-2697(88)90263-1. [DOI] [PubMed] [Google Scholar]
- 39.Shaner NC, et al. Improved monomeric red, orange, and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.