Abstract
BCR-ABL is proposed to impair cell-cycle control by disabling p27, a tumor suppressor that inhibits cyclin-dependent kinases. We show that in cell lines p27 expression is inversely correlated with expression of SKP2, the F-box protein of SCFSKP2 (SKP1/Cul1/F-box), the E3 ubiquitin ligase that promotes proteasomal degradation of p27. Inhibition of BCR-ABL kinase causes G1 arrest, down-regulation of SKP2, and accumulation of p27. Ectopic expression of wild-type SKP2, but not a mutant unable to recognize p27, partially rescues cell-cycle progression. A similar regulation pattern is seen in cell lines transformed by FLT3-ITD, JAK2V617F, and TEL-PDGFRβ, suggesting that the SKP2/p27 conduit may be a universal target for leukemogenic tyrosine kinases. Mice that received transplants of BCR-ABL–infected SKP2−/− marrow developed a myeloproliferative syndrome but survival was significantly prolonged compared with recipients of BCR-ABL-expressing SKP2+/+ marrow. SKP2−/− leukemic cells demonstrated higher levels of nuclear p27 than SKP2+/+ counterparts, suggesting that the attenuation of leukemogenesis depends on increased p27 expression. Our data identify SKP2 as a crucial mediator of BCR-ABL–induced leukemogenesis and provide the first in vivo evidence that SKP2 promotes oncogenesis. Hence, stabilization of p27 by inhibiting its recognition by SCFSKP2 may be therapeutically useful.
Introduction
Chronic myeloid leukemia (CML) is caused by BCR-ABL, a constitutively active tyrosine kinase that results from the t(9;22)(q34;q11) translocation, karyotypically evident as the Philadelphia chromosome (Ph).1 CML cells are characterized by increased proliferation, reduced apoptosis, and a perturbation of their interaction with the bone marrow stroma.2,3 BCR-ABL activates multiple signaling pathways, including MAPK, JNK, STAT5, PI3K, and SRC, but the precise contribution of the various activated pathways to the biologic features of CML cells and the key molecules that mediate them are not well defined.4–8 In contrast to their normal counterparts, CML CD34+ cells are capable of entering into the S phase in the absence of cytokines, consistent with impaired cell-cycle control.9 There is evidence that BCR-ABL exerts its cell-cycle effects by altering the function of p27, a key regulator of cell-cycle progression.10–13 Thus in cell lines, BCR-ABL kinase activity is correlated with increased expression of SKP2, the F-box protein of the E3 ligase SCFSKP2, which promotes ubiquitination and proteasomal degradation of p27.14 This phenomenon is not restricted to CML, as advanced solid tumors also exhibit a high rate of p27 degradation concomitant with high SKP2 levels are correlated.15–18 The fact that SKP2 and activated RAS cooperate in vitro in fibroblast transformation assays has led to the notion that SKP2 is an oncogene.19,20 However, the role of SKP2 as a tumor promoter has not been validated in vivo. Here we show that induction of a murine myeloproliferative disease (MPD) by BCR-ABL is partially dependent on SKP2. Our data implicate SKP2 as a crucial downstream mediator of BCR-ABL transformation in vivo and provide in vivo evidence that SKP2 promotes leukemogenesis.
Methods
Cell lines
The Mo7ep210BCR-ABL, Ba/F3p210BCR-ABL, and 32Dp210BCR-ABL cell lines were maintained in RPMI1640 media supplemented with 200 μM l-glutamine, 10% FCS, penicillin (200 U/mL), and streptomycin (200 μg/mL).21,22 For cell-cycle analysis, 106 cells were fixed in 70% ethanol and stained with propidium iodide (Roche Applied Science, Indianapolis, IN). Data were collected on a FACScalibur flow cytometer (BD Biosciences, San Jose, CA) using the CellQuest software (BD Biosciences) and analyzed using ModFit LT software (Verity Software, Topsham, ME).
Western blot analysis, immunoprecipitation, and kinase assays
Whole cell lysates from Mo7ep210BCR-ABL cells, cultured in the presence or absence of 2.5 μM imatinib (Oregon Health & Science University pharmacy), were prepared in NP-40 lysis buffer (1% NP40, 150 mM NaCl, 20 mM Tris, pH 8.0, 10% glycerol, 1 mM EDTA and 1% protease inhibitor [Sigma-Aldrich, St Louis, MO]). For separating nuclear and cytoplasmic fractions, cells were lysed in ice-cold hypotonic buffer A (0.2% NP-40, 10 mM HEPES [pH 7.9], 15 mM KCl, 0.1 mM EDTA, 2 mM MgCl2, 0.3 M sucrose, 1 mM DTT, 1% protease inhibitor). The supernatant containing cytoplasmic proteins was separated after centrifugation and the nuclear pellet was lysed in ice-cold hypertonic buffer B (50 mM HEPES [pH 7.9], 50 mM KCl, 0.1 mM EDTA, 25% glycerol, 1 mM DTT, 1% protease inhibitor) for 30 minutes. The nuclear extract was centrifuged at 48 000g for 15 minutes. Nuclear proteins were precipitated with 3 M ammonium sulfate, pelleted by centrifugation at 135 000g for 10 minutes and resuspended in hypertonic buffer. Proteins (50-100 μg) were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Antibodies used for immunoblotting were p27, cyclin E, CDK2, Jak2, Flt3 (Santa Cruz Biotechnology, Santa Cruz, CA), SKP2, CKS1 (Zymed, San Francisco, CA), pJak2, pFlt3, PDGFR-β (Cell Signaling, Danvers, MA), SP1 (Upstate, Charlottesville, VA), and α-tubulin (Sigma-Aldrich). KPC1/2 antibodies were generously provided by Dr K. I. Nakayama (Kyushu University, Fukuoka, Japan). Kinase assays were performed using histone H1 as a substrate.23,24
Quantitative RT-PCR analysis
RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesised using Oligo(dT)-primers with SuperScript-First-Strand Synthesis kit (Invitrogen, Carlsbad, CA). cDNA was amplified with Opticon-3 thermal cycler (MJ Research, Waltham, MA) using 2xSYBR Green (Invitrogen). The primers for human SKP2 were TCCACGGCATACTGTCTCAG (sense) and GGGCAAATTCAGAGAATCCA (antisense). GAPDH was amplified using TCCTGCACCACCAACTGCTTAG (sense) and GGCATGGACTGTGGTCATGAG (antisense) primers.
Retroviral and lentiviral infection
Full-length murine Skp2 was cloned into MSCV-IRES-GFP. Retrovirus was generated by transfecting (Fugene, Roche) 293T17 cells (ATCC) with EcoPac helper virus (a kind gift of Dr Rick Van Etten, Tufts University, Boston, MA) and MSCV constructs. Viral supernatant (1 mL) was used to infect 3 × 106 Ba/F3p210BCR-ABL cells.25,26 Cells were cultured for several days, followed by selection of green fluorescent protein (GFP)-positive cells by FACSaria (BD Biosciences, San Jose, CA) to obtain stable cell lines.
A shRNA library targeting human SKP2 was obtained from Open Biosystems (Huntsville, AL). Lentivirus was generated by transfecting 293FT cells using Vira-Power packaging system (Invitrogen) and fugene. For stable expression of shRNA Mo7ep210BCR-ABL, cells were puromycin-selected for 2 weeks.
Murine leukemia model
C57BL/6 Skp2−/− mice were maintained under standard conditions at the OHSU animal care facility. All animal experiments were performed according to OHSU guidelines and received Institutional Review Board approval. Genotyping was done by PCR amplification of tail DNA using 2 primers sets to amplify Skp2 from the wild-type and deletion alleles. Wild-type: AGAGTGGAA-GAACCCAGGCAGGAC (sense) and CCCGTGGAGGGAAAAAGAGGGACG (antisense); deletion allele: GCATCGCCTTCTATCGCCTTCTTG (sense) and TTCCCACCCCCACATCCAGTCATT (antisense).
Retroviral constructs and in vitro transformation assays
p210BCR-ABL and control retroviruses were generated as described above. For titration of retrovirus, serial dilutions of each retroviral supernatant were used to infect NIH-3T3 cells and after 48 hours, the cells were analyzed for GFP expression by FACSaria. Volumes of supernatant containing equal number of infectious particles were used to infect bone marrow cells.
For myeloid progenitor colony formation assays, bone marrow was harvested from 6- to 10-week-old mice not treated with 5-fluorouracil (5-FU), subjected to red cell lysis in NH4Cl solution (0.8% NH4Cl with 0.1 mM EDTA) and prestimulated at 37°C in Iscove modified Dulbecco medium (IMDM) with 15% FCS, 5% WEHI-3B conditioned media as a source of interleukin (IL)-3, murine IL-3 (6 ng/mL), IL-6 (10 ng/mL), and stem cell factor (SCF; 50 ng/mL; StemCell Technologies, Vancouver, BC). After 24 hours the cells were transferred into 6-well plates with viral supernatant (p210BCR-ABL or empty vector) in prestimulation medium in the presence of 2 μg/mL polybrene.26 This was repeated after 48 hours. After an additional 4-hour adsorption period, 5 × 104 cells/35-mm dish were plated in triplicate in methylcellulose medium in the presence and absence of cytokines (MethoCult M3534, MethoCult M3234, StemCell Technologies). Plates were incubated at 37°C, 5% CO2 and colonies were scored on day 8.
For primary B lymphoid transformation assays, bone marrow cells from non–5-FU–treated mice were subjected to a single round of viral transduction in Dulbecco modified Eagle media (DMEM) supplemented with 10% FBS with 2 μg/mL polybrene. After overnight adsorption at 37°C, cells were plated in Whitlock/Witte cultures in RPMI1640 supplemented with 10% FBS, 200 μM l-glutamine, 50 μM 2-mercaptonoethanol and 1% penicillin/streptomycin as described.27,28 Cells were plated in triplicate in serial dilutions at 105, 3 × 104, 104, 3 × 103 and 103 cells/mL, along with 106 untransduced bone marrow cells as feeders.29 Cells were cultured for 4 weeks and fed twice weekly. Cultures were scored as positive for transformation when the number of nonadherent cells exceeded 106/mL of culture medium.
Bone marrow transplantation assay
Murine bone marrow transplantation was performed as previously described.26,30 To determine whether the number of target cells for transformation was comparable, marrow from 5-FU–treated and –untreated Skp2+/+ and Skp2−/− mice was immunophenotyped using PE-Cy7–conjugated CD3ϵ, B220, Gr-1, CD4, CD8a, Ter-119, CD19, IgM, CD127, (BD Pharmingen) for lineage-positive cells. Antibodies for stem and progenitor cells staining were APC-Cy7–conjugated CD117; PerCP-Cy5.5–conjugated CD45, APC-Cy7–conjugated CD19 and PE-conjugated B220 (eBioscience); pacific blue–conjugated Sca-1 and PE-Cy5–conjugated CD34 (BioLegend, San Diego, CA); APC-conjugated Gr-1 and Thy1.2, PE-Cy7–conjugated Mac-1 (BD Pharmingen).31 Briefly, bone marrow cells were harvested from 6- to 8-week-old donor mice (Skp2+/+ and Skp2−/−) 4 days after 5-FU treatment and infected with either MIG-p210BCR-ABL or control MIG retroviral supernatant (matched by titrating in NIH-3T3 cells as described above) in DMEM, containing 1 U/mL penicillin, 1 μg/mL streptomycin, 2 mM l-glutamine, 15% FBS, 15% WEHI-3B 7 ng/mL murine IL-3, 12 ng/mL IL-6, 56 ng/mL SCF, and 5 μg/mL polybrene, by 2 rounds of spinoculation.32 4 × 105 cells were injected into the retro-orbital vein of lethally irradiated (2 doses of 6 Gy administered 4 hours apart) recipient mice. After transplantation, mice were monitored by daily inspection. White blood cell counts (WBC) and 3-part differential blood counts were analyzed twice weekly using a Vet ABC blood analyzer (Heska, Fort Collins, CO). Diseased mice were subjected to histopathologic analysis. Immunohistochemical analysis of spleen tissue for p27 was performed using anti-p27 antibody (C19; Santa Cruz Biotechnology). Southern blot analysis was performed as described earlier.33 Bone marrow and spleen cells of each group were also analyzed by FACSaria using above described antibodies.31
Results
Inhibiting BCR-ABL kinase activity blocks cell-cycle progression and up-regulates nuclear p27
We examined the effect of BCR-ABL kinase inhibition on cell- cycle kinetics in Mo7e cells expressing p210BCR-ABL. Exposure to 2.5 μM imatinib led to the expected complete inhibition of BCR-ABL kinase (Figure 1A). After 8 hours, imatinib-treated cells started to accumulate in G0/G1 with a concomitant reduction in S phase and G2/M fractions. At 16 hours, 78.9% (± 3.3%) of cells were in G0/G1, 16.3% (± 1.2%) were in S, and 4.7% (± 3.8%) were in G2/M, with little change at later time points (Figure 1B; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). As expected, no changes were observed in Mo7e cells or mock-treated Mo7ep210BCR-ABL cells (Figure 1B). Consistent with the cell-cycle analysis, total p27 levels started to rise at 8 hours and reached a plateau at 16 hours in Mo7ep210BCR-ABL, whereas no changes were seen in Mo7e cells (Figure 1C). The absolute increase of p27 in imatinib-treated cells was due to nuclear accumulation (Figures 1C panel 1, 1D; Figure S1B). These data suggest that BCR-ABL promotes cell-cycle progression by down-regulation of nuclear p27. Given that effects of BCR-ABL inhibition on cell-cycle progression and p27 accumulation were maximal at 16 hours, this time point was chosen for subsequent experiments.
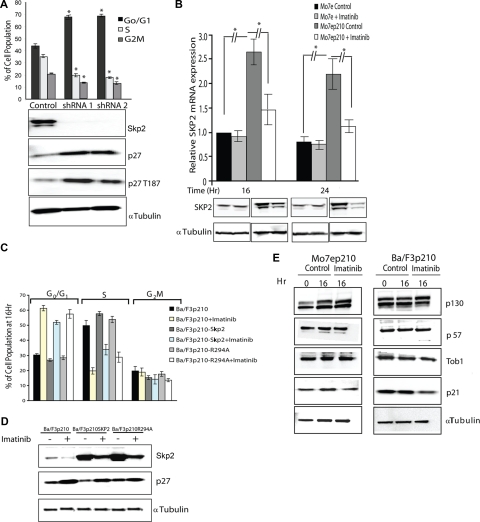
Figure 1.
Inhibiting BCR-ABL kinase activity up-regulates p27 and down-regulates SKP2. Mo7e cells expressing p210BCR-ABL were treated with imatinib (2.5 μM) for 16 hours. (A) Immunoblot analysis of whole-cell lysates with antiphosphotyrosine antibodies. (B) The cell-cycle profile was analyzed by propidium iodide (PI) staining. Mo7e cells treated with imatinib (2.5 μM) for 16 hours were used as a control. (C) Immunoblot analysis of the effect of BCR-ABL kinase inhibition on p27, cyclin E, CDK2, pT187p27, SKP2, CKS1, KPC1, KPC2 expression levels in nuclear (N) and cytoplasmic extracts (C). Sp1 and α-tubulin were used to assess the purity of the nuclear and cytoplasmic fractions, respectively, and actin was used as a loading control. No effect on p27 and SKP2 expression was observed in Mo7e cells treated with imatinib under similar conditions. (D) Analysis of p27 in nuclear (N) and cytoplasmic (C) lysates of Mo7e p210BCR-ABL cells shows accumulation of p27 predominantly in the nucleus compared with the cytoplasm. Densitometry was performed to quantitate p27 levels from 3 independent experiments. All densitometry values are given in Boehringer light units (BLU). (E) Inhibition of BCR-ABL kinase activity with imatinib reduces CDK2 activity toward histone H1. Mo7e cells expressing p210BCR-ABL were treated with 2.5 μM imatinib for 16 hours and lysates from whole cells as well as from nuclear and cytoplasmic fractions were immunoprecipitated with an antibody against CDK2. Immunoprecipitates were incubated in kinase buffer in the presence of [γ32P]-ATP and histone H1 as a substrate. CDK2 kinase activity toward histone H1 was measured by scintillation counting in 3 independent experiments. Error bars represent SD.
p27 phosphorylated at T187 accumulates in the nucleus despite reduced CDK2 activity
To determine the precise mechanism of imatinib-induced cell-cycle arrest, we investigated the effect of BCR-ABL inhibition on the expression of cyclin E and CDK2 and no differences were observed in imatinib-treated Mo7ep210BCR-ABL cells (Figure 1C panels 2,3). However, the kinase activity of anti-CDK2 immunoprecipitates from whole cell lysates was significantly reduced at 16 hours (32% ± 14.1%, P = .001; Figure 1E). As expected, 90% or more of CDK2 activity was nuclear, and a significant reduction was demonstrable at 16 hours (31.4% ± 13.7% of controls, P < .001), while cytoplasmic kinase activity was barely detectable (Figure 1E). To ensure that the activity of anti-CDK2 immunoprecipitates was indeed CDK2-specific, we treated anti-CDK2 immunoprecipitates with a CDK2 inhibitor and observed a dose-dependent reduction of activity (Figure S2).
p27 is phosphorylated by cyclin E/CDK2 on threonine 187, generating a recognition site for SKP2, the F-box protein of the SCFSKP2 E3 ubiquitin ligase, which targets p27 for proteasomal degradation. Given the reduction in nuclear CDK2 activity, we hypothesized that T187 phosphorylation of p27 may be reduced. However, despite the reduced CDK2 kinase activity, most p27 was phosphorylated on T187 in imatinib-treated cells, but not in controls (Figure 1C and Figure S3A panel 4). No T187 phosphorylated p27 was detected in the cytoplasm (Figure 1C panel 4), suggesting that p27 accumulation is due to impaired degradation and not to an inability to be recognized by SKP2.
Inhibition of BCR-ABL kinase leads to the down-regulation of SKP2
Nuclear p27 phosphorylated on T187 is a target for the SCFSKP2 complex, whereas cytoplasmic p27 is degraded by KPC1/2 in a phosphorylation-independent manner. We therefore examined the expression of SKP2 and CKS1 (2 key components of SCFSKP2) and KPC1/2 in imatinib-treated Mo7ep210BCR-ABL cells. Compared with untreated controls, SKP2 expression was reduced by 68.5% plus or minus 8.4% (P = .001), whereas expression of CKS1 and KPC1/2 were not altered (Figure 1C and Figure S3A panels 5,6). Identical effects were observed in murine cell lines (Ba/F3 and 32D) engineered to express p210BCR-ABL and human cell lines K562 and KYO-1 (Figure S3B,C). To validate that SKP2 was indeed responsible for the cell-cycle effects, we used shRNA to down-regulate SKP2 in Mo7ep210BCR-ABL cells. Down-regulation of SKP2 led to accumulation of p27 phosphorylated at T187, which was associated with a G0/G1 block (67% and 69% cells in G0/G1 phase with SKP2 shRNA-1 and -2, respectively, compared with 44% with control shRNA; Figure 2A). Quantitative RT-PCR showed a 2.5-fold increase in SKP2 mRNA expression in Mo7ep210BCR-ABL cells compared with Mo7e cells (P < .001). Imatinib treatment reduced SKP2 mRNA and protein expression in Mo7ep210BCR-ABL, but not in parental Mo7e cells, consistent with transcriptional regulation of SKP2 by BCR-ABL (Figure 2B). However, despite the complete inhibition of BCR-ABL kinase activity, some SKP2 expression was maintained, indicating that Skp2 expression is not entirely under the control of BCR-ABL. Overall, these data suggest that p27 down-regulation by BCR-ABL is partially but not completely mediated by SCFSKP2. To address the question whether SKP2 was indeed regulating p27 levels and cell-cycle effects in BCR-ABL–expressing cells, we expressed murine Skp2 in Ba/F3p210BCR-ABL cells (Ba/F3p210BCR-ABL-Skp2). The cells were treated with 2.5 μM imatinib and cell-cycle analysis was performed. Exogenous Skp2 expression, in the absence of imatinib did not affect cell-cycle distribution of exponentially growing cells (Figure 2C). However, upon treatment with imatinib, accumulation in G0/G1 was significantly reduced in Ba/F3p210BCR-ABL-Skp2 cells compared with Ba/F3p210BCR-ABL cells (51.9% ± 1.3% vs 61.4% ± 1.8%; P < .001) and conversely more cells remained in S phase (33.8% ± 3.5% vs 19.7% ± 2.1%; P < .004). Immunoblot analysis showed reduced accumulation of p27 (Figure 2D, compare lane 4 with lane 2) that did not quite reach the levels of untreated Ba/F3p210BCR-ABL cells (Figure 2D lane 1), consistent with cell- cycle analysis that showed partial but not complete rescue of cell- cycle progression (Figure 2C). In contrast, expression of a Skp2 mutant (R294A) which is unable to promote p27 ubiquitination34,35 failed to reverse the effects of imatinib treatment (Figure 2C), further supporting that SKP2 is partially responsible for the cell- cycle effects. Besides p27, SCFSKP2 ubiquitinates other proteins with potential cell-cycle effects, including p130, p57, Tob1, and p21. p21 levels were reduced in Ba/F3p210BCR-ABL cells but not in Mo7ep210BCR-ABL cells treated with imatinib, suggesting BCR-ABL regulates p21 in a cell line–dependent manner. No significant changes in expression were observed for p130, p57, and Tob1 (Figure 2E), suggesting that p27 is the key target of SCFSKP2.
Figure 2.
p27 levels are regulated by SKP2 in BCR-ABL-positive cell lines. (A) Mo7ep210BCR-ABL cells were infected with lentivirus-producing SKP2 shRNA-1 and -2 or control (scramble) shRNA construct for stable knockdown of SKP2. The effect on cell-cycle distribution (PI staining) as well as on SKP2, p27, and pT187p27 expression was analyzed by immunoblot analysis. The differences in cell-cycle progression after knockdown of SKP2 are significant compared with control with P values less than .001 from 3 independent experiments. (B) SKP2 mRNA levels were assessed in Mo7e and Mo7ep210BCR-ABL cells by quantitative RT-PCR after treatment with 2.5 μM imatinib for 16 and 24 hours. (C) Ba/F3 cells expressing p210BCR-ABL were stably transduced with a Skp2 expression vector and treated with 2.5 μM imatinib for 16 hours. Cell-cycle distribution was analyzed by PI staining in 3 independent experiments, and (D) p27 expression was analyzed by immunoblot. (E) Effect of BCR-ABL kinase inhibition on the expression of SKP2 substrates was analyzed. Cells were treated with 2.5 μM imatinib and the expression of p130, p57, Tob1, and p21 was measured by immunoblot analysis in Mo7ep210BCR-ABL and Ba/F3p210BCR-ABL cells. Error bars represent SE.
Growth factor deprivation leads to the down-regulation of SKP2
To investigate the effect of GM-CSF signaling on p27 and SKP2 expression, Mo7e and Mo7ep210BCR-ABL cells were cultured in the presence or absence of GM-CSF and/or serum. Propidium iodide staining indicated that deprivation of GM-CSF in Mo7e cells for 16 hours leads to cell-cycle block in G0/G1, with accumulation of p27 and down-regulation of SKP2 (Figure 3A, compare lanes 3 and 4), similar results were seen with Mo7ep210BCR-ABL cells treated with imatinib (Figure 3A, compare lanes 5 and 6). To determine whether cytokine signaling may be able to rescue cell-cycle progression upon inhibition of BCR-ABL with imatinib, we stimulated the cells with GM-CSF and found that the cells still arrested in G0/G1, with down-regulation of SKP2 and up-regulation of p27 (Figure 3A, compare lanes 5 and 6 with lanes 7 and 8). These data suggest that the pathway activated by GM-CSF and BCR-ABL only partially overlap. Alternatively, imatinib-inhibited BCR-ABL could exert a dominant negative effect upon GM-CSF signaling. These effects were specific for Mo7e cells, because cell-cycle progression was largely rescued by IL-3 (WEHI conditioned media) in Ba/F3 cells expressing BCR-ABL (Figure 3B). Serum deprivation had no significant effects in any condition.
Figure 3.
Effect of growth factor and serum deprivation or stimulation on BCR-ABL–positive cells. Parental and BCR-ABL–positive cell lines were deprived or stimulated with growth factor and/or serum for 16 hours. Effect on cell-cycle progression (PI staining) as well as p27 and Skp2 expression was analyzed in 3 independent experiments. BCR-ABL–positive cells were also treated with 2.5 μM imatinib. (A) Mo7e and Mo7ep210BCR-ABL cells. (B) Ba/F3 and Ba/F3p210BCR-ABL cells. Error bars represent SE. (C) Effect on Skp2 and p27 expression of inhibiting constitutively active tyrosine kinases in leukemia cell lines. Cells were incubated with the indicated concentrations of inhibitors for 24 hours and immunoblot analysis was performed on whole cell lysates. Left panel shows MOLM14 cells expressing a FLT3 internal tandem duplication (ITD) treated with 500 nM MLN518. Middle panel shows HEL cells expressing JAK2 V617F treated with 50, 75, 100 μM of AG490. Right panel shows Ba/F3 cells expressing TEL-PDGFRβ treated with 2.5, 5.0, 10 μM imatinib. Skp2 and p27 expression levels were analyzed after inhibition of tyrosine kinase activity. α-Tubulin was used as a loading control.
SKP2 expression is regulated by oncogenic tyrosine kinases other than BCR-ABL
To determine whether SKP2 is a universal target for oncogenic tyrosine kinases in leukemia, we treated various leukemia lines with specific pharmacologic inhibitors. MOLM14 cells expressing FLT3 with an internal tandem duplication were treated with 500 nM MLN518, an inhibitor of FLT3 kinase36,37; HEL cells expressing JAK2V617F were treated with AG490 (50, 75, 100 μM), an inhibitor of JAK2 kinase38; and Ba/F3 cells engineered to express TEL-PDGFRβ39 were treated with imatinib (2.5, 5, and 10 μM). After 24 hours incubation the levels of p27 and SKP2 were determined by immunoblot analysis (Figure 3C). Analogous to BCR-ABL, inhibition of FLT3-ITD, JAK2V617F, and TEL-PDGFRβ was associated with reduced expression of SKP2 and accumulation of p27. This suggests that the SKP2-p27 axis is commonly activated in leukemias driven by constitutively active tyrosine kinases.
In vitro transformation of primary bone marrow cells by BCR-ABL in SKP2+/+ versus SKP2−/− bone marrow
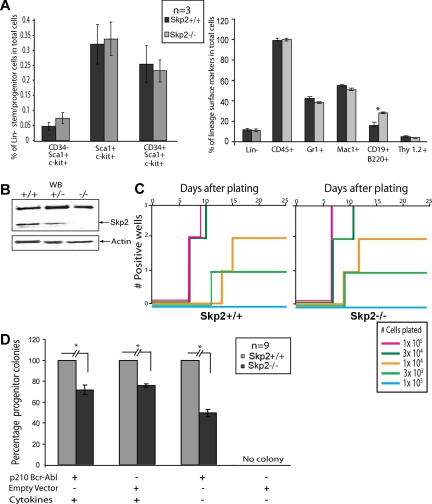
To determine whether SKP2 is a crucial downstream target of BCR-ABL in primary hematopoietic cells, we tested the ability of BCR-ABL to transform cells in the absence of SKP2. Mice null for Skp2 are viable and fertile and have normal body proportions, although their size is reduced.40 In preliminary experiments, we determined whether the distribution of immunophenotypically defined subsets of hematopoietic cells is altered in Skp2−/− bone marrow cells.31,41 No significant differences were observed in stem cells (Lin−/CD34−/c-kit+/Sca−I+), multipotent progenitor cells (Lin−CD34+/c-kit+/Sca−I+) or differentiated cells defined by expression of Gr-1, Mac-1 and Thy-1.2 except for a slight increase of B cells (CD19+B220+; Figure 4A). Immunoblot analysis of bone marrow cells confirmed the absence of Skp2 in the Skp2−/− mice (Figure 4B). To assess whether lack of SKP2 may affect the ability of BCR-ABL to transform primary bone marrow B-lymphoid progenitors, freshly harvested bone marrow from non–5-FU–treated Skp2+/+ and Skp2−/− mice was transduced with BCR-ABL or empty vector retroviruses and plated in triplicate in Whitlock-Witte conditions in the presence of stromal cells, using different serial dilutions of cells (Figure 4C). In 3 independent experiments, no significant differences were seen between Skp2+/+ and Skp2−/− marrow, whereas no outgrowth was observed with empty vector transduced cells. This indicates that Skp2 is not required for BCR-ABL–mediated in vitro transformation of primary bone marrow B cells. To determine whether Skp2 is required for transformation of primary murine myeloid cells by BCR-ABL, we assayed colony formation in semisolid media by Skp2+/+ and Skp2−/− bone marrow infected with BCR-ABL or empty vector retrovirus. In 3 independent experiments, we assayed myeloid colony formation in the presence of cytokines. In comparison with Skp2+/+ marrow, colony formation by BCR-ABL and empty vector-transduced Skp2−/− marrow was reduced to 71.6% plus or minus 4.7% and 75.7% plus or minus 5%, respectively (P < .001; Figure 4D). In the absence of cytokines, colony formation by BCR-ABL–infected Skp2−/− marrow was reduced to 49.5% plus or minus 3.4% of BCR-ABL–infected Skp2+/+ marrow (P < .001). No colonies were recovered from the cells transduced with empty vector and grown in absence of cytokines. These results suggest that myeloid but not lymphoid transformation by BCR-ABL is partially dependent on SKP2.
Figure 4.
In vitro transformation of primary bone marrow cells by BCR-ABL in Skp2+/+ versus Skp2−/− bone marrow. (A) Flow cytometric analysis of non–5-FU-treated Skp2+/+ and Skp2−/− mice bone marrow cells for stem/multipotent progenitor population (left) and lineage surface markers (right). Significant differences (P < .05) are indicated by an asterisk. (B) Immunoblot showing Skp2 expression from bone marrow cells of Skp2+/+, Skp2+/−, and Skp2−/− littermates. Actin was used as a loading control. (C) Comparison of B-cell transformation by BCR-ABL between Skp2+/+ and Skp2−/− bone marrow cells. C57/BL6 bone marrow cells were transduced with p210BCR-ABL or empty vector retroviral supernatant and the indicated numbers of transduced viable cells were plated in Whitlock-Witte culture medium in triplicate on stromal cells (106 cells/well) derived from untransduced marrow. Wells were scored as positive when the numbers of viable nonadherent cells reached 106/well. No growth was observed in Skp2+/+ or Skp2−/− bone marrow cells transduced with empty vector retrovirus. Data shown here are representative of 3 independent experiments. (D) Comparison of myeloid colony formation between Skp2+/+ and Skp2−/− bone marrow cells. Bone marrow cells were transduced with p210BCR-ABL or empty vector retrovirus and plated in methylcellulose in the presence or absence of cytokines. The histogram shows the average percentage of remaining colonies for Skp2−/− marrow cells (considering Skp2+/+ as 100%) from triplicate assays from 3 independent experiments. The difference between Skp2+/+ and Skp2−/− mice is significant (P < .001). No colonies were recovered from cells transduced with empty vector and grown in the absence of cytokines. Mice used for in vitro assays are not treated with 5-FU. Error bars represent SE.
Absence of Skp2 decreases the leukemogenicity of BCR-ABL in a murine model of CML
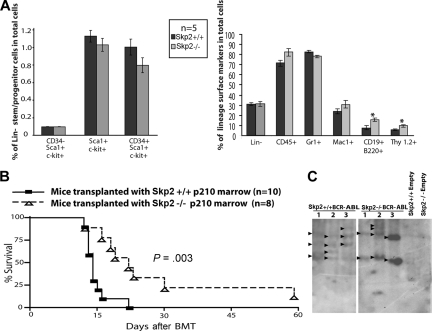
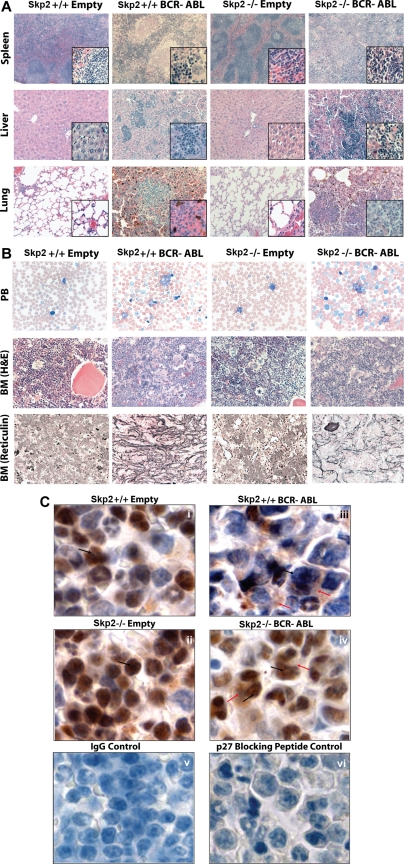
To establish whether the defect in myeloid transformation observed in vitro would lead to decreased leukemogenicity in vivo, bone marrow from 5-FU–treated Skp2+/+ and Skp2−/− mice was infected with BCR-ABL retrovirus and transplanted into lethally irradiated Skp2+/+ recipients. To control for a potential bias due to differences in the target cell populations, we immunophenotypically compared the bone marrow cells of 5-FU–treated Skp2+/+ and Skp2−/− mice and found a similar distribution of stem and multipotent progenitors cells in Skp2+/+ versus Skp2−/− marrow (Figure 5A). All mice developed a CML-like MPD. However, animals that received Skp2−/− marrow (n = 8) survived significantly longer than mice that received Skp2+/+ marrow (n = 10; median 19 days, range 12-60 days compared with median 13 days, range 12-22 days, P = .003; Figure 5B). Consistent with the more aggressive leukemia phenotype, white blood cell counts were higher in mice that received Skp2+/+ marrow compared with mice that received Skp2−/− marrow (median 59.1 × 103/μL vs median 7.9 × 103/μL). Spleen weight was also significantly higher (0.42 ± 0.07 g vs 0.28 ± 0.09 g; Table 1). Southern blot analysis from spleen of leukemic Skp2+/+ and Skp2−/− mice suggests that disease is oligoclonal in both groups (Figure 5C). Histopathology revealed a CML-like MPD characterized by trilineage extramedullary hematopoiesis in the spleen and liver. In the lungs, infiltration of granulocytes in interstitial spaces resulted in the formation of focal hemorrhages (Figure 6A). Peripheral blood smears showed mainly mature neutrophils with few myeloid and erythroid precursors and some reticulocytes. Blasts were rare and when present did not account for more than 3% of the WBC (Figure 6B). The bone marrow was hypercellular, with maturing granulocytes and clusters of megakaryocytes, which were more pronounced in mice that received Skp2+/+ cells. Compared with recipients of Skp2+/+ cells, reticulin fibrosis was much less pronounced in recipients of Skp2−/− marrow (Figure 6B). Immunohistochemical analysis of spleens revealed higher levels of p27 in recipients of Skp2−/− marrow transduced with empty vector or BCR-ABL compared with recipients of Skp2+/+ marrow (Figure 6C, compare i with ii and iii with iv). Immunophenotyping of bone marrow and spleen cells showed that the great majority of GFP-positive cells expressed the myeloid markers Gr1 and Mac1, whereas fewer cells expressed erythroid (Ter119), T-cell (Thy1.2), or B-cell (CD19) antigens. Antigens present on more primitive cells (CD34, Sca-1, c-Kit, and Thy1.1) were expressed by only a minority of cells, consistent with the mature cellular infiltrate. With the exception of Ter119-positive cells, which were reduced by approximately 3-fold in the spleens of mice receiving Skp2−/− marrow, only minor differences were observed between recipients of Skp2−/− and Skp2+/+ marrow (Figure S4).
Figure 5.
In vivo evidence for decreased leukemogenicity in Skp2−/− mice. (A) Flow cytometric analysis of 5-FU–treated Skp2+/+ and Skp2−/− mice bone marrow cells for stem/multipotent progenitor population (left) and lineage surface markers (right). Significant differences (P < .05) are indicated by an asterisk. Error bars represent SE. (B) Kaplan-Meier survival curve for mice transplanted with Skp2+/+ and Skp2−/− marrow transduced with p210BCR-ABL. The number of individual mice in each arm is indicated. Mice transplanted with Skp2−/− marrow survived significantly longer (P = .003). (C) Southern blot analysis for proviral integration from BCR-ABL or empty vector transduced Skp2+/+ and Skp2−/− mice. Genomic DNA from spleen tissue (15 μg) was digested with restriction enzyme BglII, resolved on a 1.5% agarose gel, and transferred to Hybond-N+ membrane. The blots were hybridized with a 32P-labeled GFP probe and exposed to autoradiography film.
Table 1.
Autopsy findings and blood counts in leukemic and control mice
| Donor marrow | Spleen wgt, g | Liver wgt, g | WBC, 103/mm3 | HGB, g/dL | PLT, 103/mm3 |
|---|---|---|---|---|---|
| Skp2+/+, p210BCR-ABL (n = 10) | 0.43 ± 0.07 | 1.65 ± 0.19 | 59.1 (9.6-142.6) | 17.7 (4.6-21) | 543 (260-806) |
| Skp2−/−, p210BCR-ABL (n = 8) | 0.29 ± 0.09 | 1.42 ± 0.32 | 7.9 (0.8-87) | 16.8 (10-19.8) | 262 (72-822) |
| Skp2+/+, empty vector (n = 4) | 0.08 ± 0.01 | 1.20 ± 0.34 | 11.7 (7.8-22.6) | 23.3 (22-25.6) | 598 (422-928) |
| Skp2−/−, empty vector (n = 2) | 0.07, 0.09 | 1.3, 1.1 | 8.8, 10.6 | 15.6, 16 | 357, 754 |
| P | .004 | NS | .02 | NS | .05 |
Data were recorded on the day mice died or were killed due to blood counts in excess of 200 × 103/mm3 or 20% loss in body weight. P values reflect the comparison between mice transplanted with Skp2+/+ and Skp2−/− marrow infected with p210BCR-ABL.
HGB indicates hemoglobin; PLT, platelets; WBC, white blood count; wgt, weight; and NS, not significant.
Figure 6.
Representative histology of mice transplanted with Skp2+/+ and Skp2−/− marrow transduced with p210BCR-ABL or empty vector. (A) Spleen, liver, and lung [hematoxylin and eosin (H&E) stain, original magnifications are ×5, ×20, and ×20, respectively]. Higher magnification ×40 is also shown in the lower right corners. (B) Peripheral blood smear (Wright-Giemsa stain, original magnification ×63), and bone marrow (H&E stain and reticulin fiber stain, original magnification ×40). (C) p27 Immunohistochemistry of spleen of mice that received Skp2+/+ and Skp2−/− marrow transduced with empty vector (i, ii) or p210BCR-ABL (iii, iv). p27 Staining was performed using polyclonal anti-p27 primary antibody (C19; Santa Cruz Biotechnology) and biotinylated anti–rabbit IgG (H&L) secondary antibody (Vector Laboratories, Burlingame, CA) using Vectastain Elite Universal ABC Kit (Vector Laboratories) and DAB Chromogen (Dako, Carpinteria CA). Nuclear and cytoplasmic p27 are indicated by black and red arrows, respectively. For negative controls, staining with IgG antibody (v) and with anti-p27 antibody including p27-specific blocking peptide (Santa Cruz Biotechnology) showing practically complete block of p27 staining in cells with some residual background staining (vi). Original magnifications, ×100. Images were viewed with a Leica DM LB2 microscope (Leica, Wetzlar, Germany) equipped with Leica N Plan 5×/0.12 (for 5× magnification), HC PL Fluotar 20×/0.50 (20×), HCX PL Fluotar 40×/0.75 (40×), C Plan 63×/0.75 (63×), and N Plan 100×/1.25 oil (100×) objective. Images were captured with a Leica DC300 camera running IM50 Image Manager software (v5 release 222; Heerbrugg, Switzerland) and processed with Adobe Photoshop CS2 9.0.2 (Adobe Systems, San Jose, CA).
Discussion
The chronic phase of CML is characterized by expansion of the myeloid cell compartment with preserved terminal differentiation capacity. It is thought that reduced apoptosis and increased proliferation underlie this phenotype, which in many ways resembles the adenoma stage of solid tumors. In normal hematopoietic progenitor cells, S phase entry is tightly regulated by exogenous cytokines and, consistent with this, normal CD34+ cells arrest in G0/G1 after cytokine withdrawal.9 In contrast, a much larger fraction of CD34+ cells isolated from CML patients remains in S phase under the same conditions, suggesting that BCR-ABL kinase activity promotes cell-cycle progression by mimicking cytokine stimulation. The effector molecules mediating this process are partially cell type–specific. For example, cyclin D2 expression is induced by BCR-ABL in B-lymphoblasts, but not myeloblasts.42 In contrast, down-regulation of p27 appears to be a consistent effect of BCR-ABL kinase activity. One potential explanation is that the reduced p27 levels facilitate G1-S progression by relieving p27 inhibition of CDK2/CyclinE, the net result being increased cell proliferation. There is evidence that BCR-ABL regulates p27 through the PI3K pathway. PI3K signaling has been shown to reduce p27 levels by 2 mechanisms. Firstly, PI3K activates AKT, which phosphorylates Forkhead/FoxO transcription factors, leading to reduced p27 transcription.43 Secondly, PI3K induces transcription of SKP2, the F-box protein of the SCFSKP2 complex, which targets p27 for proteasomal degradation.14 SKP2 is a bona fide oncogene, a notion based on the inverse correlation between SKP2 and p27 expression observed in many advanced solid tumors,19 the poor prognosis of tumors with high SKP2 expression,15–18 and in vitro cooperation between SKP2 and activated RAS in fibroblast transformation assays.20 However, neither the ability of SKP2 to contribute to or induce carcinogenesis nor its role for BCR-ABL–induced leukemogenesis have been validated in vivo. Here we demonstrate that ablation of SKP2 attenuates BCR-ABL–mediated MPD and may play a similar role in other types of leukemias caused by constitutively active tyrosine kinases.
In a first series of experiments we analyzed the role of SKP2 in a BCR-ABL–expressing myeloid cell line (Mo7ep210BCR-ABL). Inhibition of BCR-ABL with imatinib induced a G0/G1 arrest that coincided with up-regulation of nuclear p27 and a reduction of nuclear CDK2-associated kinase activity (Figure 1A-D, Figure 7). Because phosphorylation of p27 on T187 by CDK2 is required for its recognition by SCFSKP2, we reasoned that the accumulation of nuclear p27 may be due to reduced T187 phosphorylation as a result of reduced CDK2 activity. In addition, it was recently shown that BCR-ABL phosphorylates tyrosine 88 of p27 when p27 is in complex with CDK2. This results in partial activation of CDK2, which then phosphorylates p27 on T187, promoting its ubiqitination by SCFSKP2 and subsequent degradation in the sense of a positive feedback loop.44 However, using a pT187-specific antibody, we found a marked increase of pT187p27, suggesting that p27 accumulation was caused by a block of degradation at the level of SCFSKP2. Consistent with this hypothesis SKP2 levels decreased upon imatinib exposure (Figure 1C). In contrast, the levels of CKS1, another essential component of SCFSKP2 known to be up-regulated in some types of cancer,45–47 remained unchanged. As expected from the minimally altered levels of cytoplasmic p27, there was no change in the expression of KPC1/2, the ubiquitin ligase responsible for degradation of cytoplasmic p27.48 The central role of SKP2 for the cell-cycle dysregulation of BCR-ABL expressing cells was further supported by the fact that G0/G1 accumulation upon imatinib exposure was reduced in Ba/F3p210BCR-ABL cells ectopically expressing wild-type Skp2 (Figure 2C,D). Contrary to expectations, we observed an increase of cells in S phase upon expression of a Skp2 mutant (R294A) that is unable to recognize p27 for its ubiquitintion, albeit somewhat less than upon expression of the wild-type construct.34,35 This suggests that Skp2 has cell-cycle effects that are not impacted by the R294A mutation, for example through targeting proteins other than p27 for degradation. The identity of these substrates is currently unknown, because the levels of the known SCFSKP2 targets p130, p57 and Tob1 remained constant upon imatinib treatment of Mo7ep210BCR-ABL and Ba/F3p210BCR-ABL cells. In contrast to published work,14 no change of p21 levels was seen in Mo7ep210BCR-ABL cells, and levels were decreased in Ba/F3p210BCR-ABL cells, suggesting that p21 is not essential for the cell-cycle block. The precise mechanism by which BCR-ABL regulates SKP2 remains to be established. In accord with a previous study14 we found that SKP2 mRNA levels are reduced in Mo7ep210BCR-ABL cells exposed to imatinib (Figure 2B), consistent with transcriptional regulation. However, in Ba/F3p210BCR-ABL cells engineered to express exogenous Skp2, the degree of down-regulation of SKP2 upon imatinib treatment exceeded what would be expected from suppression of endogenous Skp2, suggesting that additional mechanisms may be operational (Figure 2D), a finding that is currently under investigation. Moreover, Skp2 expression is not completely dependent on BCR-ABL activity, as complete inhibition of BCR-ABL (Figure 1A) did not completely abolish Skp2 expression (Figure 1C).
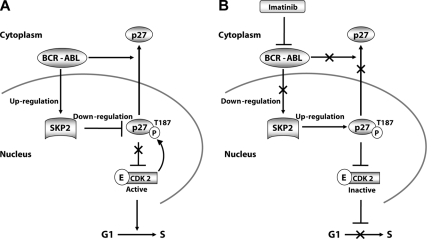
Figure 7.
Schematic representation of SKP2-mediated regulation of p27 in BCR-ABL–positive cell lines. (A) BCR-ABL kinase activity induces expression of SKP2, which increases the activity of SCFSKP2, which in turn promotes p27 degradation. This releases CDK2 from p27 inhibition and stimulates cell-cycle progression in BCR-ABL positive cell lines. In primary cells, BCR-ABL additionally promotes cytoplasmic localization of p27. (B) Inhibition of BCR-ABL kinase activity by imatinib decreases SKP2 expression, which leads to accumulation of pT187p27, which restores inhibition of CDK2.
To address the question whether SKP2 is a specific target of BCR-ABL, we assessed SKP2 and p27 expression upon growth factor withdrawal in Mo7e and Ba/F3 cells. We found that SKP2 is down-regulated with corresponding accumulation of p27 in GM-CSF– or IL-3–starved cells. However, Skp2 expression upon imatinib exposure was rescued only in Ba/F3p210BCR-ABL cells (Figure 3B), but not in Mo7ep210BCR-ABL cells (Figure 3A). The failure of GM-CSF to substitute for BCR-ABL could have 2 explanations. Firstly, kinase inactive BCR-ABL may block GM-CSF signal transduction in a dominant-negative fashion. Secondly, BCR-ABL may activate unique pathways to promote SKP2 expression. Studies in 32D cells have in fact shown that BCR-ABL and IL-3 have overlapping as well as unique targets.49 Furthermore, in Ba/F3 cells, BCR-ABL–mediated up-regulation of Skp2 does not effect cell-cycle progression (Figure 3B), in contrast to Mo7e cells, suggesting cell line–specific SKP2 substrates. Taken together, our data point to a central role for SKP2 as a regulator of p27 levels and G1/S phase progression in BCR-ABL–expressing cells. Moreover, up-regulation of p27 and down-regulation of SKP2 was consistently observed when FLT3-ITD, JAK2V617F, and TEL-PDGFRβ–expressing cells were treated with selective tyrosine kinase inhibitors, suggesting that the SKP2/p27 axis may be a universal target of leukemogenic tyrosine kinases (Figure 3C).
The importance of Skp2 for BCR-ABL–mediated leukemogenesis was confirmed by studies using bone marrow from Skp2−/− mice infected with BCR-ABL retrovirus. Compared with wild-type marrow, formation of myeloid colonies by Skp2 null marrow in the presence and absence of cytokines was reduced by 28.3% plus or minus 4.7% and 50.4% plus or minus 3.4%, respectively. This difference is not caused by reduced numbers of target cells for transformation, as FACS analysis of bone marrow cells from SKP2−/− and SKP2+/+ cells revealed no significant differences in expression of stem/progenitor cell markers, with the exception of a slight increase in CD19+/B220+ B cells. In contrast to myeloid colony formation, no consistent differences were observed in B-cell transformation assays. In light of the limited capacity of this assay to quantify transformation potency, it is possible that the differences may have been too small to be detected. Alternatively, there could be a genuine difference in the requirement of Skp2 for the transformation of myeloid versus lymphoid cells, which would not be unprecedented. For example, Gab-1 is essential for myeloid leukemogenesis but not B-cell leukemia,50 whereas SRC kinases may be required for B-cell transformation but not for MPD.51 The myeloid transformation defect of Skp2−/− marrow was confirmed in vivo. Mice transplanted with BCR-ABL–infected Skp2−/− marrow had lower white cell and platelet counts, smaller spleens and significantly increased survival compared with recipients of wild-type marrow (Figure 5, Table 1). Standard histology was similar, apart from a markedly reduced reticulin fibrosis in recipients of Skp2−/− marrow. p27 levels were increased in leukemic Skp2−/− cells compared with wild-type cells. In BCR-ABL–expressing cells, more p27 seemed to localize to the cytoplasm (Figure 6C, compare i with iii and ii with iv), which is consistent with observations in human CD34+ CML cells.10 However, a direct comparison is difficult due to the different cell types in leukemic versus nonleukemic spleens. The presence or absence of Skp2 had no clear-cut effect on the distribution of p27 between cytoplasm and nucleus. Overall, the immunohistochemistry findings suggest that the attenuation of leukemogenesis in the Skp2-null background may be mediated by increased p27 levels.
In summary, we provide evidence that Skp2 is required for full phenotypic penetration of BCR-ABL–induced MPD. Since the structure of p27 bound to SKP2 and CKS1 has been solved,34 it may be possible to design compounds that stabilize p27 by blocking its binding to the SCFSKP2 complex. Such compounds may be effective in treating leukemias caused by constitutively active tyrosine kinases.
Supplementary Material
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute (Bethesda, MD) grant HL082978-01 (M.W.D.), by the Leukemia & Lymphoma Society (White Plains, NY; M.W.D.), and by the Department of Defense CML Research Program (M.W.D.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contributions: A.A. performed the research, analyzed the data, drafted the paper and helped design the research; T.G.P.B., A.S.C., T.O., M.L., J.V.D., S.G.W., and J.D. participated in performing the research; K.I.N. provided reagents, B.J.D. provided resources and advice; and M.W.D. designed the research, supervised the project and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Corresponding author: Michael W. Deininger, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, L592, Portland, OR 97239; e-mail: deininge@ohsu.edu.
References
- 1.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 2.Clarkson B, Strife A, Wisniewski D, Lambek CL, Liu C. Chronic myelogenous leukemia as a paradigm of early cancer and possible curative strategies. Leukemia. 2003;17:1211–1262. doi: 10.1038/sj.leu.2402912. [DOI] [PubMed] [Google Scholar]
- 3.Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18:1760–1768. doi: 10.1038/sj.leu.2403515. [DOI] [PubMed] [Google Scholar]
- 4.Skorski T, Kanakaraj P, Nieborowska-Skorska M, et al. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86:726–736. [PubMed] [Google Scholar]
- 5.Cortez D, Reuther G, Pendergast AM. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene. 1997;15:2333–2342. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]
- 6.Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers CL. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–254. [PubMed] [Google Scholar]
- 7.Li S. Src kinase signaling in leukaemia. Int J Biochem Cell Biol. 2007;39:1483–1488. doi: 10.1016/j.biocel.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raitano AB, Halpern JR, Hambuch TM, Sawyers CL. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc Natl Acad Sci U S A. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonuleit T, Peschel C, Schwab R, et al. Bcr-Abl kinase promotes cell cycle entry of primary myeloid CML cells in the absence of growth factors. Br J Haematol. 1998;100:295–303. doi: 10.1046/j.1365-2141.1998.00564.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Zhao RC, Verfaillie CM. Abnormal integrin-mediated regulation of chronic myelogenous leukemia CD34+ cell proliferation: BCR/ABL up-regulates the cyclin-dependent kinase inhibitor, p27Kip, which is relocated to the cell cytoplasm and incapable of regulating cdk2 activity. Proc Natl Acad Sci U S A. 2000;97:10538–10543. doi: 10.1073/pnas.190104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer A, Horner S, Willer A, et al. Adhesion to fibronectin stimulates proliferation of wild-type and bcr/abl-transfected murine hematopoietic cells. Proc Natl Acad Sci U S A. 1999;96:2087–2092. doi: 10.1073/pnas.96.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parada Y, Banerji L, Glassford J, et al. BCR-ABL and interleukin 3 promote haematopoietic cell proliferation and survival through modulation of cyclin D2 and p27Kip1 expression. J Biol Chem. 2001;276:23572–23580. doi: 10.1074/jbc.M101885200. [DOI] [PubMed] [Google Scholar]
- 13.Jonuleit T, van der Kuip H, Miething C, et al. Bcr-Abl kinase down-regulates cyclin-dependent kinase inhibitor p27 in human and murine cell lines. Blood. 2000;96:1933–1939. [PubMed] [Google Scholar]
- 14.Andreu EJ, Lledo E, Poch E, et al. BCR-ABL induces the expression of Skp2 through the PI3K pathway to promote p27Kip1 degradation and proliferation of chronic myelogenous leukemia cells. Cancer Res. 2005;65:3264–3272. doi: 10.1158/0008-5472.CAN-04-1357. [DOI] [PubMed] [Google Scholar]
- 15.Lim MS, Adamson A, Lin Z, et al. Expression of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood. 2002;100:2950–2956. doi: 10.1182/blood.V100.8.2950. [DOI] [PubMed] [Google Scholar]
- 16.Gstaiger M, Jordan R, Lim M, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci U S A. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, Ayala G, De Marzo A, et al. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res. 2002;8:3419–3426. [PubMed] [Google Scholar]
- 18.Ma XM, Liu JH, Guo JW, Liu Y, Zuo LF. [Correlation of Skp2 expression in gastric carcinoma to expression of P27 and PTEN]. Ai Zheng. 2006;25:56–61. [PubMed] [Google Scholar]
- 19.Hershko DD, Shapira M. Prognostic role of p27(Kip1) deregulation in colorectal cancer. Cancer. 2006;107:668–675. doi: 10.1002/cncr.22073. [DOI] [PubMed] [Google Scholar]
- 20.Latres E, Chiarle R, Schulman BA, et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci U S A. 2001;98:2515–2520. doi: 10.1073/pnas.041475098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Rosee P, Johnson K, O'Dwyer ME, Druker BJ. In vitro studies of the combination of imatinib mesylate (Gleevec) and arsenic trioxide (Trisenox) in chronic myelogenous leukemia. Exp Hematol. 2002;30:729–737. doi: 10.1016/s0301-472x(02)00836-6. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui M, Shimokawa H, Yoshihara S, et al. Intracellular magnesium deficiency in acute myocardial infarction. Jpn Heart J. 1993;34:391–401. doi: 10.1536/ihj.34.391. [DOI] [PubMed] [Google Scholar]
- 23.O'Hare T, Pollock R, Stoffregen EP, et al. Inhibition of wild-type and mutant Bcr-Abl by AP23464, a potent ATP-based oncogenic protein kinase inhibitor: implications for CML. Blood. 2004;104:2532–2539. doi: 10.1182/blood-2004-05-1851. [DOI] [PubMed] [Google Scholar]
- 24.Corbin AS, Buchdunger E, Pascal F, Druker BJ. Analysis of the structural basis of specificity of inhibition of the Abl kinase by STI571. J Biol Chem. 2002;277:32214–32219. doi: 10.1074/jbc.M111525200. [DOI] [PubMed] [Google Scholar]
- 25.Vallera DA, Jin N, Baldrica JM, Panoskaltsis-Mortari A, Chen SY, Blazar BR. Retroviral immunotoxin gene therapy of acute myelogenous leukemia in mice using cytotoxic T cells transduced with an interleukin 4/diphtheria toxin gene. Cancer Res. 2000;60:976–984. [PubMed] [Google Scholar]
- 26.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–3840. [PubMed] [Google Scholar]
- 27.Million RP, Aster J, Gilliland DG, Van Etten RA. The Tel-Abl (ETV6-Abl) tyrosine kinase, product of complex (9;12) translocations in human leukemia, induces distinct myeloproliferative disease in mice. Blood. 2002;99:4568–4577. doi: 10.1182/blood-2001-12-0244. [DOI] [PubMed] [Google Scholar]
- 28.Roumiantsev S, de Aos IE, Varticovski L, Ilaria RL, Van Etten RA. The src homology 2 domain of Bcr/Abl is required for efficient induction of chronic myeloid leukemia-like disease in mice but not for lymphoid leukemogenesis or activation of phosphatidylinositol 3-kinase. Blood. 2001;97:4–13. doi: 10.1182/blood.v97.1.4. [DOI] [PubMed] [Google Scholar]
- 29.Smith KM, Yacobi R, Van Etten RA. Autoinhibition of Bcr-Abl through its SH3 domain. Mol Cell. 2003;12:27–37. doi: 10.1016/S1097-2765(03)00274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotani H, Newton PB, Zhang S, 3rd, et al. Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther. 1994;5:19–28. doi: 10.1089/hum.1994.5.1-19. [DOI] [PubMed] [Google Scholar]
- 33.Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66:11156–11165. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 34.Hao B, Zheng N, Schulman BA, et al. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol Cell. 2005;20:9–19. doi: 10.1016/j.molcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Ungermannova D, Gao Y, Liu X. Ubiquitination of p27Kip1 requires physical interaction with cyclin E and probable phosphate recognition by SKP2. J Biol Chem. 2005;280:30301–30309. doi: 10.1074/jbc.M411103200. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo Y, MacLeod RA, Uphoff CC, et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia. 1997;11:1469–1477. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- 37.Walters DK, Stoffregen EP, Heinrich MC, Deininger MW, Druker BJ. RNAi-induced down-regulation of FLT3 expression in AML cell lines increases sensitivity to MLN518. Blood. 2005;105:2952–2954. doi: 10.1182/blood-2004-07-2758. [DOI] [PubMed] [Google Scholar]
- 38.Koschmieder S, Hofmann WK, Kunert J, et al. TGF beta-induced SMAD2 phosphorylation predicts inhibition of thymidine incorporation in CD34+ cells from healthy donors, but not from patients with AML after MDS. Leukemia. 2001;15:942–949. doi: 10.1038/sj.leu.2402119. [DOI] [PubMed] [Google Scholar]
- 39.Carroll M, Tomasson MH, Barker GF, Golub TR, Gilliland DG. The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways. Proc Natl Acad Sci U S A. 1996;93:14845–14850. doi: 10.1073/pnas.93.25.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama K, Nagahama H, Minamishima YA, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deininger MW, Vieira SA, Parada Y, et al. Direct relation between BCR-ABL tyrosine kinase activity and cyclin D2 expression in lymphoblasts. Cancer Res. 2001;61:8005–8013. [PubMed] [Google Scholar]
- 43.Komatsu N, Watanabe T, Uchida M, et al. A member of Forkhead transcription factor FKHRL1 is a downstream effector of STI571-induced cell cycle arrest in BCR-ABL-expressing cells. J Biol Chem. 2003;278:6411–6419. doi: 10.1074/jbc.M211562200. [DOI] [PubMed] [Google Scholar]
- 44.Grimmler M, Wang Y, Mund T, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 45.Kitajima S, Kudo Y, Ogawa I, et al. Role of Cks1 overexpression in oral squamous cell carcinomas: cooperation with Skp2 in promoting p27 degradation. Am J Pathol. 2004;165:2147–2155. doi: 10.1016/S0002-9440(10)63264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shapira M, Ben-Izhak O, Bishara B, et al. Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer. 2004;100:1615–1621. doi: 10.1002/cncr.20172. [DOI] [PubMed] [Google Scholar]
- 47.Masuda TA, Inoue H, Nishida K, et al. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res. 2003;9:5693–5698. [PubMed] [Google Scholar]
- 48.Kamura T, Hara T, Matsumoto M, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 49.Matulonis U, Salgia R, Okuda K, Druker B, Griffin JD. Interleukin-3 and p210 BCR/ABL activate both unique and overlapping pathways of signal transduction in a factor-dependent myeloid cell line. Exp Hematol. 1993;21:1460–1466. [PubMed] [Google Scholar]
- 50.Podar K, Mostoslavsky G, Sattler M, et al. Critical role for hematopoietic cell kinase (Hck)-mediated phosphorylation of Gab1 and Gab2 docking proteins in interleukin 6-induced proliferation and survival of multiple myeloma cells. J Biol Chem. 2004;279:21658–21665. doi: 10.1074/jbc.M305783200. [DOI] [PubMed] [Google Scholar]
- 51.Hu Y, Liu Y, Pelletier S, et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet. 2004;36:453–461. doi: 10.1038/ng1343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.