Abstract
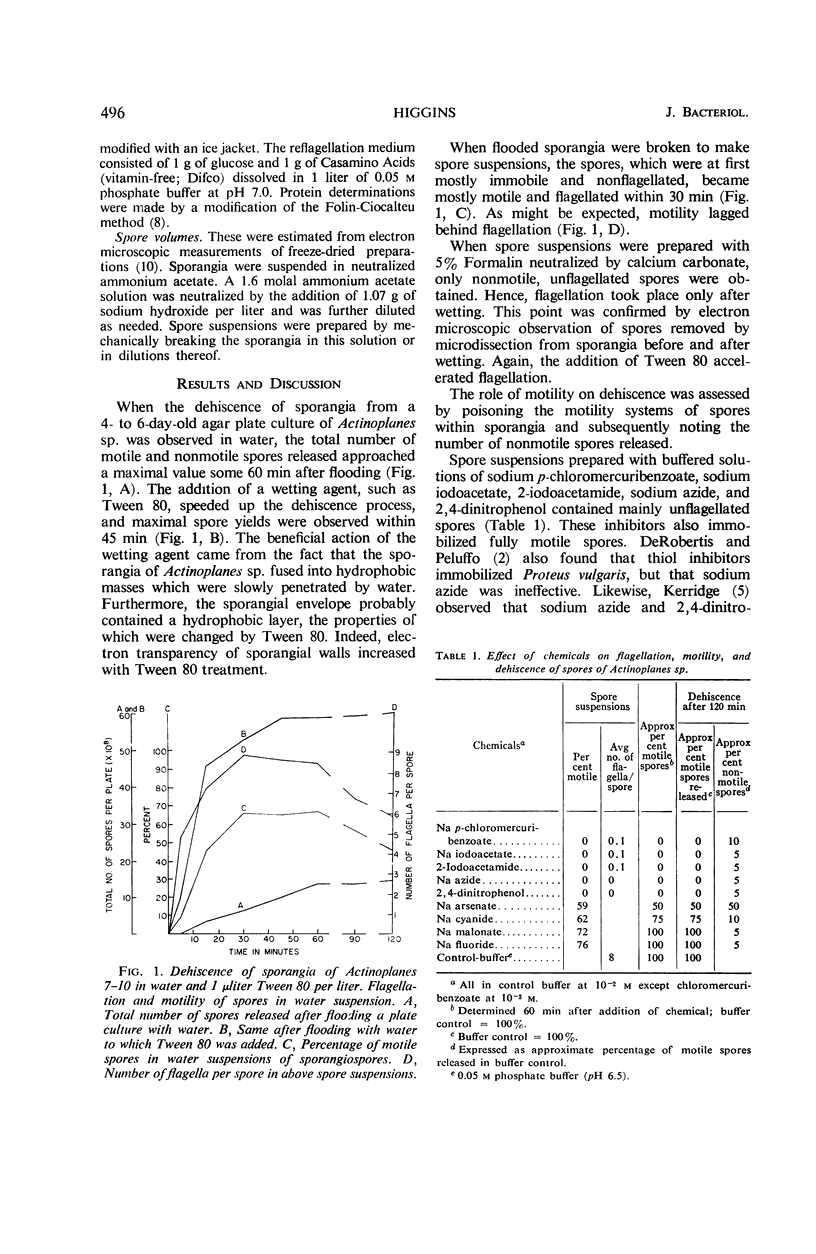
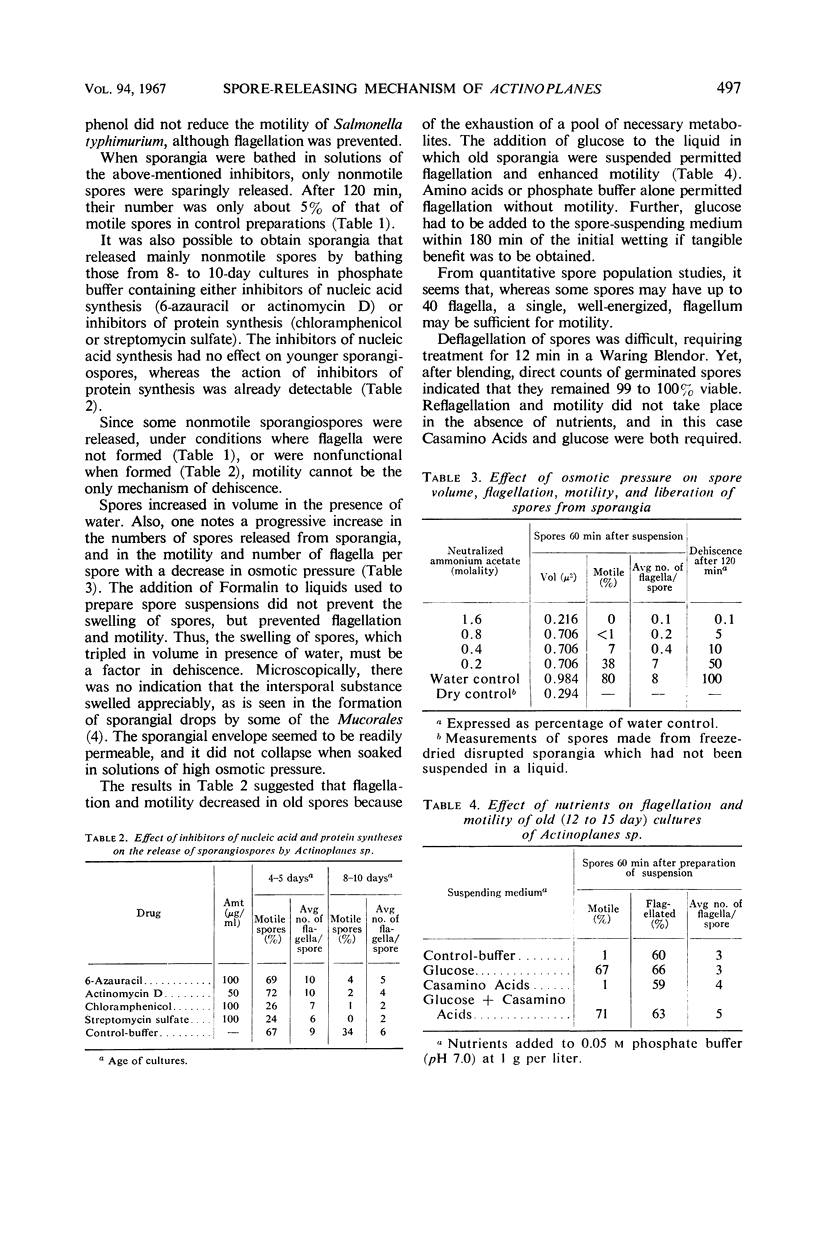
Dehiscence of Actinoplanes sp. 7–10 sporangia is triggered by wetting of the spores. This process requires time because of the hydrophobic nature of the sporangial envelope; it can be speeded up and enhanced by a wetting agent. Once wetted, the spores swell, usually ripping the sporangial wall, and escape as motile elements when functional flagella are synthesized. Flagellation and motility are separate phenomena, both of which lose intensity with age. Spores from old sporangia can regain motility when supplied with an exogenous carbon source, but, when provided only with water, phosphate buffer, or amino acids, flagellation takes place without motility. Deflagellation-reflagellation experiments indicated that functional flagella can be reformed only in presence of both amino acids and glucose which must be added within 180 min of deflagellation. Inoperative flagella were formed in the presence of inhibitors of nucleic acid synthesis, such as 6-azauracil, but inhibitors of protein synthesis, such as chloramphenicol, did not interfere with reflagellation. Flagellated spores remained so after germination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DE ROBERTIS E., PELUFFO C. A. Chemical stimulation and inhibition of bacterial motility studied with a new method. Proc Soc Exp Biol Med. 1951 Nov;78(2):584–589. doi: 10.3181/00379727-78-19148. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Lechevalier M. P., Lechevalier H. A. Flagellated actinomycetes. J Bacteriol. 1967 Apr;93(4):1446–1451. doi: 10.1128/jb.93.4.1446-1451.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of inhibitors on the formation of flagella by Salmonella typhimurium. J Gen Microbiol. 1960 Dec;23:519–538. doi: 10.1099/00221287-23-3-519. [DOI] [PubMed] [Google Scholar]
- LECHEVALIER H., HOLBERT P. E. ELECTRON MICROSCOPIC OBSERVATION OF THE SPORANGIAL STRUCTURE OF A STRAIN OF ACTINOPLANES. J Bacteriol. 1965 Jan;89:217–222. doi: 10.1128/jb.89.1.217-222.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lechevalier H. A., Lechevalier M. P., Holbert P. E. Electron microscopic observation of the sporangial structure of strains of Actinoplanaceae. J Bacteriol. 1966 Oct;92(4):1228–1235. doi: 10.1128/jb.92.4.1228-1235.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J., Gordee E. Z. Formation of bacterial flagella. I. Demonstration of a functional flagellin pool in spirillum serpens and bacillus subtilis. J Bacteriol. 1966 Feb;91(2):870–875. doi: 10.1128/jb.91.2.870-875.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


