Abstract
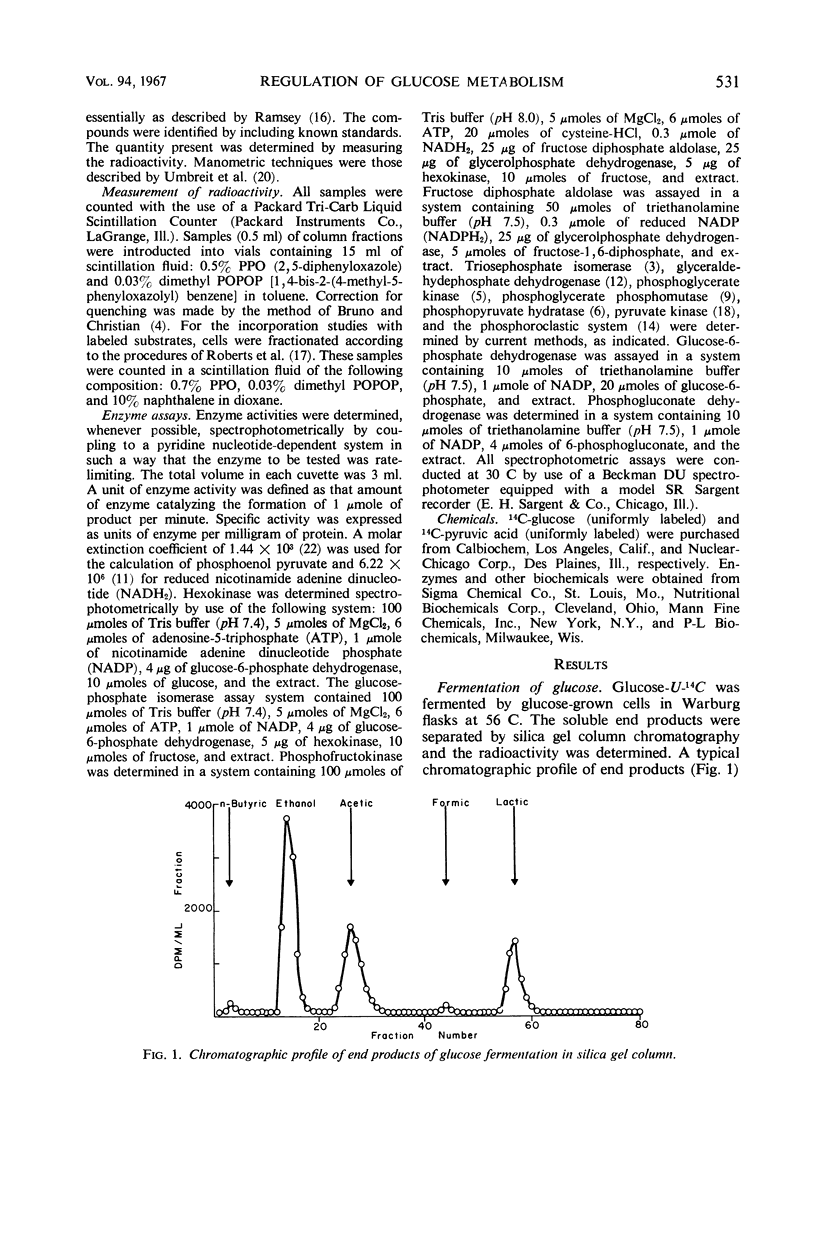
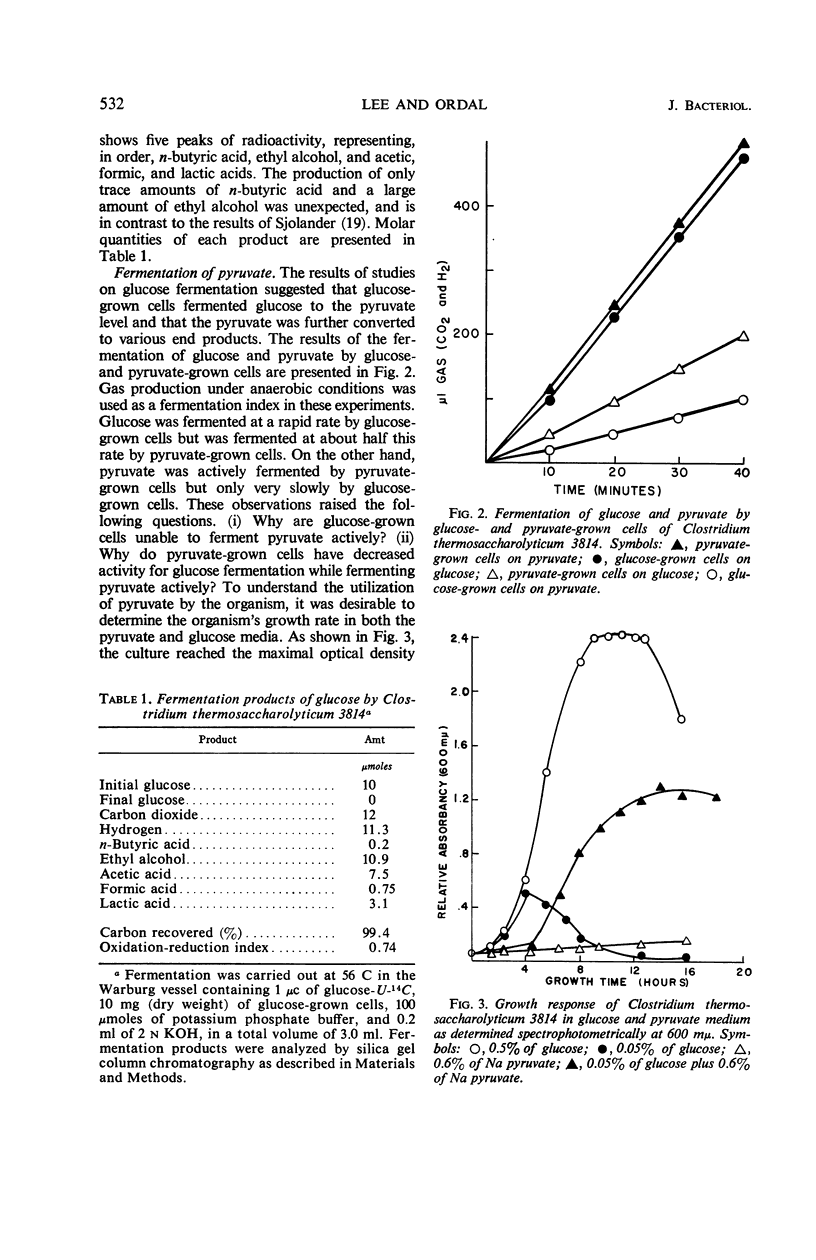
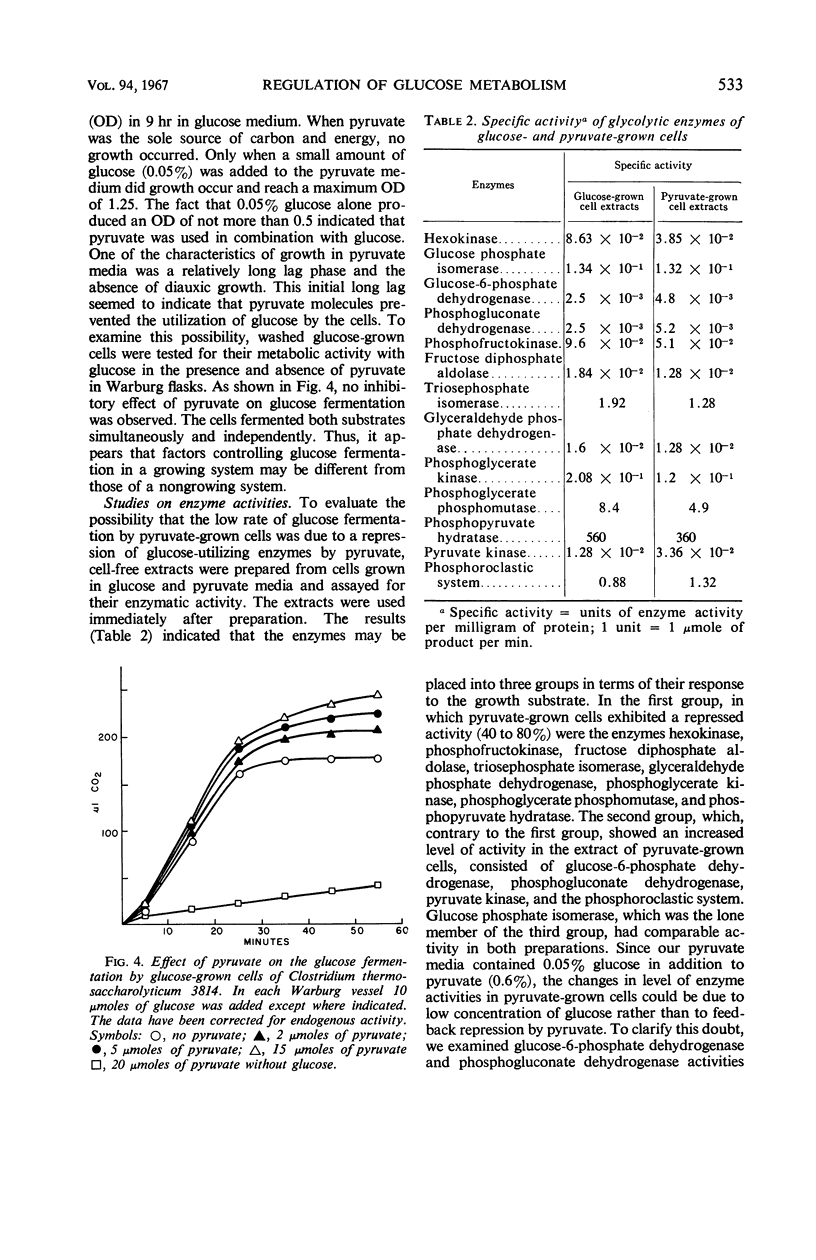
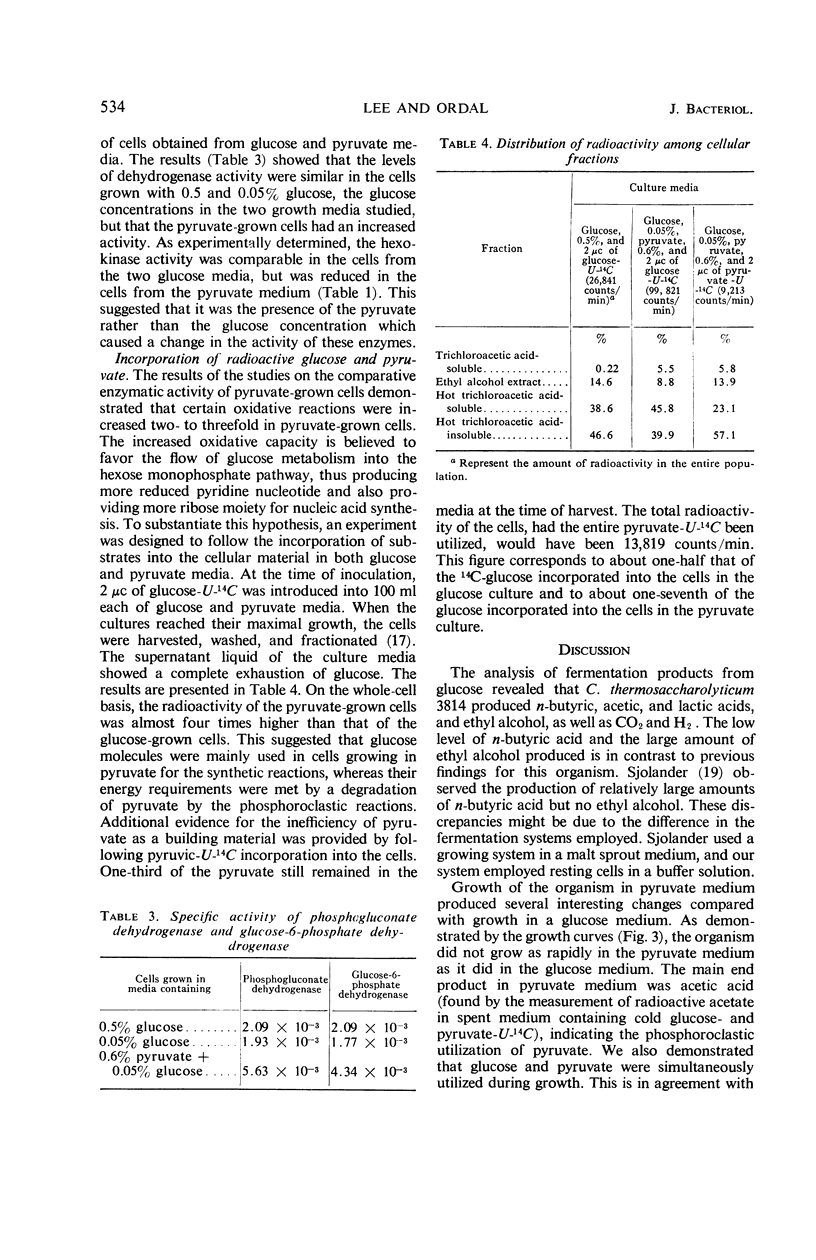
Whole cells and cell-free extracts of Clostridium thermosaccharolyticum 3814 grown in media containing 0.5% glucose or 0.6% pyruvate were evaluated for their metabolic activities toward these compounds. Glucose-grown cells rapidly fermented glucose with the production of gases (CO2 and H2), acids, and alcohol, but they did not ferment pyruvate well. Pyruvate-grown cells, on the other hand, readily fermented pyruvate, while fermenting glucose at a rate of one-half that of pyruvate. An analysis of the enzyme levels in the two cell culture conditions revealed that pyruvate-grown cells had lower levels of most of the glycolytic enzymes and increased levels of the hexose monophosphate pathway enzymes. Incorporation studies with the use of labeled glucose demonstrated that cells do have a control mechanism(s) whereby they can discriminate between a carbon (glucose) and an energy (pyruvate) source, selectively utilizing glucose in the synthetic pathway while obtaining energy from the phosphoroclastic degradation of pyruvate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNETT J. A., KORNBERG H. L. The utilization by yeasts of acids of the tricarboxylic acid cycle. J Gen Microbiol. 1960 Aug;23:65–82. doi: 10.1099/00221287-23-1-65. [DOI] [PubMed] [Google Scholar]
- BARRETT J. T., LARSON A. D., KALLIO R. E. The nature of the adaptive lag of Pseudomonas fluorescens toward citrate. J Bacteriol. 1953 Feb;65(2):187–192. doi: 10.1128/jb.65.2.187-192.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S. The synthesis of nucleic acid by virus-infected bacteria. Bacteriol Rev. 1951 Sep;15(3):131–146. doi: 10.1128/br.15.3.131-146.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes F. A. Effect of glucose on the level of glycolytic enzymes in yeast. Arch Biochem Biophys. 1966 Apr;114(1):231–233. doi: 10.1016/0003-9861(66)90325-0. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Model P., Rittenberg D. Measurement of the activity of the hexose monophosphate pathway of glucose metabolism with the use of [18O]glucose. Variations in its activity in Escherichia coli with growth conditions. Biochemistry. 1967 Jan;6(1):69–80. doi: 10.1021/bi00853a013. [DOI] [PubMed] [Google Scholar]
- ROSE I. A. Studies on the enolization of pyruvate by pyruvate kinase. J Biol Chem. 1960 Apr;235:1170–1177. [PubMed] [Google Scholar]
- Sjolander N. O. Studies on Anaerobic Bacteria: XII. The Fermentation Products of Clostridium Thermosaccharolyticum. J Bacteriol. 1937 Oct;34(4):419–428. doi: 10.1128/jb.34.4.419-428.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDER M., VALENTINE R. C., AKAGI J. M. FERREDOXIN OF CLOSTRIDIUM THERMOSACCHAROLYTICUM. J Bacteriol. 1963 Oct;86:861–865. doi: 10.1128/jb.86.4.861-865.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLD F., BALLOU C. E. Studies on the enzyme enolase. I. Equilibrium studies. J Biol Chem. 1957 Jul;227(1):301–312. [PubMed] [Google Scholar]


